Abstract
Recurrent translocations, t(8;21) or inv(16), in core binding factor acute myeloid leukemia (CBF-AML) are amenable to monitoring for minimal residual disease (MRD) with reverse transcriptase polymerase chain reaction (RTPCR). Despite a favorable prognosis, disease relapse remains the single cause of treatment failure in CBF-AML. Fusion products of these translocations recruit epigenetic silencing complexes resulting in hematopoietic maturation arrest. We hypothesized that maintenance therapy with hypomethylating agents (HMA), including decitabine (DAC) and azacitidine (AZA) after induction/consolidation, can be used for MRD elimination to ultimately prolong relapse free survival. Real-time quantitative (RTPCR) trends were reviewed in 23 patients (median age 53 years) with CBF-AML that received HMA therapy following induction/consolidation with fludarabine, cytarabine and G-CSF (FLAG) with low dose gemtuzumab or idarubicin (NCT00801489). Of the 23 patients evaluated, 17 had a detectable RTPCR at HMA initiation. Five patients had progressive disease and a notable increase in RTPCR values over 1 to 2 cycles of HMA therapy. Twelve patients did not fail HMA and had a median RTPCR at HMA initiation of 0.06 (range, 0.01–0.91). Unlike the HMA failure subset, 11 of these patients had a reduction in RTPCR after the first or second cycle of HMA. Our data suggests that CBF-AML patients with low levels of RTPCR (between 0.01 and 0.05) at the conclusion of induction/consolidation chemotherapy benefit most from maintenance HMA, particularly those that have a reduction in the RTPCR within the first 2 cycles of HMA therapy.
Keywords: Core binding factor, acute myeloid leukemia, minimal residual disease, reverse transcriptase polymerase chain reaction, hypomethylating agent
INTRODUCTION
The presence of translocation t(8;21) (q22;q22) or inversion inv(16) (p13q22)/t(16;16) characterizes core binding factor acute myeloid leukemia (CBF-AML).1 CBF-AML, representing 15% of all acute myeloid leukemia, has a favorable prognosis when treated with intermediate to high dose cytarabine-based induction and consolidation regimens.2–4 However, disease relapse remains as a major cause of treatment failure, despite a cure rate of >65% with chemotherapy alone.5
Real-time quantitative (RTPCR) techniques allow for the detection and quantification of leukemia-associated genes to measure minimal residual disease (MRD). RTPCR based MRD monitoring provides prognostic information and guides post-remission therapy including allogeneic stem cell transplantation (SCT) in CBF-AML.5 Several prior studies have shown that routine monitoring of MRD is important in CBF-AML to monitor for relapse and provide dynamic risk stratification while on treatment, with several efforts trying to quantitatively define the MRD level that might signify overt relapse and necessitate salvage therapy.5–13
In addition to persistent elevation of RTPCR transcript, identification of concomitant mutations along with t(8;21) and inv(16) also portend a higher risk of relapse.14,15 Mutations in c-KIT have been shown to shorten relapse free survival (RFS) and event free survival (EFS) in t(8;21) patients, reduced overall survival (OS) in inv(16) patients, and when present with persistent MRD and a high white blood cell count, shorter RFS in all CBF-AML patients.14–16 FLT3-ITD mutations have been shown to shorten EFS and RFS in CBF-AML.15 RAS mutations do not seem to impact prognosis but are important in the pathogenesis of CBF-AML and are possible targets for therapy with tyrosine kinase inhibitors.17 Along with evaluation of MRD, additional mutation data was obtained for further investigation in this analysis.
One unanswered question is what strategy to adopt when a patient is in morphological remission but has persistent molecular MRD or in patients who had truncated high dose consolidation because of adverse events. Hypermethylation of certain genes while patients are in remission has been associated with an increased likelihood of relapse in AML.5,18,19 Promoter hypermethylation of tumor suppressors is also frequently seen in CBF-AML.20,21 Hypomethylating agents including decitabine (DAC) and azacitidine (AZA) can potentially reverse such epigenetic silencing.22,23 Several studies have recently explored HMA maintenance therapy in AML.24,25 Though significant survival improvement has not been shown with this strategy, some clinical benefit has been observed, and the use of MRD directed HMA maintenance has not been extensively explored.25 We hypothesized that maintenance therapy with hypomethylating agents, such as DAC and AZA, can be used to target residual low-level PCR positivity and eliminate minimal residual disease (MRD) to ultimately prolong relapse free survival in CBF-AML.
METHODS
An analysis of 23 patients with CBF-AML who received HMA after fludarabine, cytarabine and G-CSF (FLAG) with low dose gemtuzumab or idarubicin induction/consolidation (NCT00801489) was performed between August 2008 and April 2015. Serial RTPCR from peripheral blood or bone marrow was obtained approximately every 3 months per protocol. The methodology of our RTPCR analysis panel for CBF-AML has been previously published and is in line with the Europe Against Cancer (EAC) Program.26,27 The sensitivity of detection for transcript RTPCR at the time of this analysis was between 1 in 10,000 and 1 in 100,000. Patient characteristics and outcomes were obtained from chart review and departmental database. Mutations in KIT, FLT3ITD, FLT3D835 and RAS genes were tested at baseline. In addition, most patients were evaluated for NPM1, TP53, IDH1 and IDH2 mutations at baseline.
Objectives
The primary objectives of this analysis were to monitor MRD status by serial RTPCR response to HMA therapy after the completion of induction/consolidation and to determine RTPCR baseline values and trends that predict for a higher likelihood of achieving durable remission on HMA maintenance.
Patient Selection
All patients ≥18 years old with a diagnosis of AML with t(8;21), inv(16), or t(16;16), with or without additional cytogenetic abnormalities, who received at least 1 cycle of HMA maintenance therapy following completion of FLAG-based induction or consolidation were included in this analysis. No patients were in morphologic relapse at time of enrollment.
Treatment Regimen
The induction regimen included fludarabine (FL) 30 mg/m2 on Days 1–5, cytarabine (A) 2 g/m2 IV on Days 1–5, gemtuzumab ozogamicin (GO) 3 mg/m2 on Day 1 or idarubicin (Ida) 6 mg/m2 on days 3–4, and G-CSF (G) 5 mcg/kg on Day −1 until neutrophil recovery. FLAG for 3 days with GO on day 1 in Cycle 2/3 and 5/6 or with Ida (Dose: 6 mg/m2 on Days 2 and 3 in one post remission cycle) was the consolidation regimen utilized, and the target was to complete approximately 6 cycles.28 Patients transitioned to HMA therapy received monthly maintenance therapy with DAC 20 mg/m2 on Days 1–5 or AZA 75 mg/m2 on Days 1–5 repeated every 4–5 weeks based on count recovery and toxicity.
Mutation Analysis
Mutation analysis was carried out in exons 8 and 17 in KIT gene, in codons 12, 14, and 61 in NRAS and KRAS genes using PCR-based DNA sequencing methods, and for internal tandem duplications (ITD) or D835 mutations in FLT3 gene according to published methods.29
Statistical Analysis
Median RTPCR values between HMA responders and HMA failures were compared using the non-parametric Mann-Whitney test. OS was calculated using Kaplan-Meier estimates, and survival estimates were compared by using the log-rank test.
RESULTS
Patient Characteristics
Between August 2008 and April 2015, a total of 23 patients [t(8;21)=8 and inv(16)=15] received maintenance HMA. Patient characteristics are summarized in Table 1. The reason for transition to HMA was persistent PCR positivity in 13 patients (57%), prolonged myelosuppression precluding chemotherapy based consolidation in 9 patients (39%), and molecular relapse after SCT in 1 patient (4%). Three of these patients received salvage clofarabine, cytarabine and idarubicin (CIA) for relapsed CBF-AML after front-line FLAG based therapy, achieved morphologic remission, and then went on HMA maintenance for persistent molecular MRD. Decision to pursue HMA therapy was at the discretion of the treating physician. There were patients with persistent PCR positivity and prolonged myelosuppression (N=4). In these cases, the reason for pursuing HMA reported depicts what was documented in the patient’s chart by the treating physician. Last follow up was in April 2015.
Table 1.
Baseline characteristics, N=23.
| Characteristic | N (%)/Median [Range] |
|---|---|
| Age | 53 [23–70] |
|
| |
| Gender | |
|
| |
| Male | 12 (52) |
|
| |
| Cytogenetics | |
| Inv(16) | 15 (65) |
| t(8;21) | 8 (35) |
|
| |
| WBC (K/μL) × 109/L at start of HMA | 2.8 [0.9–7.3] |
|
| |
| Platelets × 109/L at start of HMA | 67 [9–227] |
|
| |
| BM Blasts at start of HMA | 1% [0%–2%] |
|
| |
| Cycles of FLAG-Based Induction/Consolidation | 6 [1–7] |
|
| |
| Reason for Transition to HMA Therapy | |
| Prolonged myelosuppression | 9 (39) |
| Persistent PCR positivity | 13 (57) |
| Molecular relapse after transplant | 1 (4) |
|
| |
| Hypomethylating Maintenance Therapy | |
| DAC | 21 (91) |
| AZA | 2 (9) |
|
| |
| Detectable PCR at start of HMA | |
| PCR ≥ 0.01 | 17 (74) |
| PCR < 0.01 | 6 (26) |
|
| |
| Median PCR at start of HMA | 0.06 [0–0.3.84] |
|
| |
| Cycles of HMA Maintenance | 6 [1–17] |
|
| |
| Median Follow-Up | 11.3 [2.9–67.7] |
Abbreviations: WBC = white blood count; BM = bone marrow; HMA = hypomethylating agents; PCR = polymerase chain reaction; DAC = decitabine; AZA = azacitidine.
Response
Patients received a median of 6 cycles of FLAG based induction/consolidation (range, 1–7) prior to transitioning to HMA. The median number of HMA cycles received was 6 (range, 1–17). Seventeen patients had detectable MRD (RTPCR ≥ 0.01) at initiation of HMA maintenance with a median RTPCR of 0.06 (range, 0.01–3.84). All 17 patients were in morphologic complete remission (CR) when HMA was initiated. FISH was performed at HMA initiation for 7 patients and was negative in all cases. Responses for these patients are summarized in Table 2.
Table 2.
Current status of MRD positive patients, N=17.
| Continued Remission on HMA | HMA Failure | |||
|---|---|---|---|---|
|
| ||||
| N=12 | (%) | N=5 | (%) | |
| Morphologic Complete Remission | 12 | 100% | 5 | 100% |
| Cytogenetic Complete Remission | 12 | 100% | 5 | 100% |
| RTPCR at Initiation, median (range) | 0.06 (0.01–0.91) |
0.06 (0.01–3.84) |
||
| RTPCR Increase after initial 2 cycles HMA | 1 | 8% | 5 | 100% |
| RTPCR Decrease after initial 2 cycles HMA | 11 | 92% | 0 | 0% |
Of these 17 patients, 5 patients (29%) had progressive disease post HMA initiation, including 1/7 with t(8:21) and 4/10 with inv(16). The RTPCR values preceding HMA in these 5 patients were 3.84, 0.06, 0.08, 0.01, and 0.01, respectively (median=0.06). All 5 patients had a log increase in the RTPCR value after one to two cycles of HMA as noted on sequential RTPCR done after first or second cycle of HMA. RTPCRs for these 5 patients following the first or second cycle of HMA were 51.67, >100, 77.48, 1.33, and 0.17, respectively (shown in Figure 1). RTPCR transcript levels measured after first or second cycle of HMA maintenance in the HMA failure group were significantly greater than transcript values in the HMA responders (p=0.01) based on the Mann-Whitney test. All five patients failing HMA proceeded to SCT, and 3 are alive and disease free post-SCT with a median follow up of 11 months (range 3–13 months). Two of the 3 surviving HMA failure patients had undetectable MRD following SCT. As of last follow up, 1 surviving patient from the HMA failure group had a detectable RTPCR of 0.02 following SCT, and re-initiation of maintenance therapy with AZA was planned.
Figure 1.
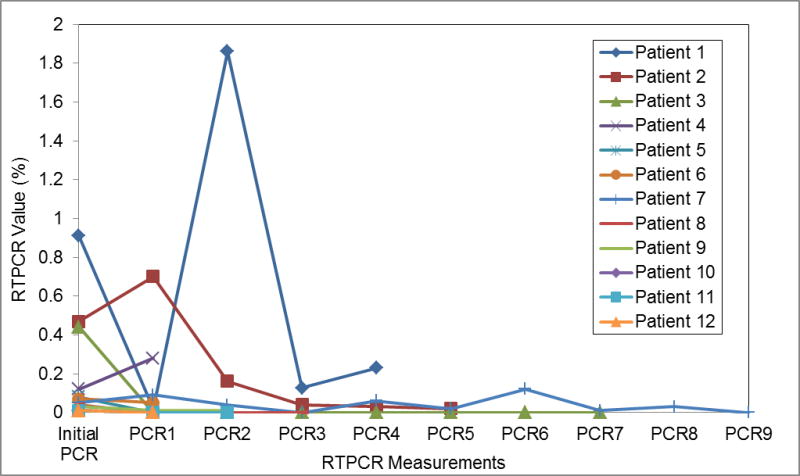
RTPCR values over serial measurements for patients with HMA failure.
Of the 23 patients reviewed in this analysis only 2 deaths occurred between August 2008 and April 2015. These 2 deaths occurred in the HMA failure group, 1 from leukemia relapse, and 1 from SCT complications. OS difference between two groups is shown in Supplemental Figure S1 and S2.
For the 12 patients who did not fail HMA, the median RTPCR at HMA initiation was also 0.06 (range, 0.01–0.91). Unlike the HMA failure subset described above, 11 of these patients had a reduction in RTPCR after the first or second cycle of HMA. One patient was in early follow up. These 12 patients remained in morphologic and cytogenetic complete remission as of last follow up. RTPCR values and trends over time are shown in Figure 2.
Figure 2.
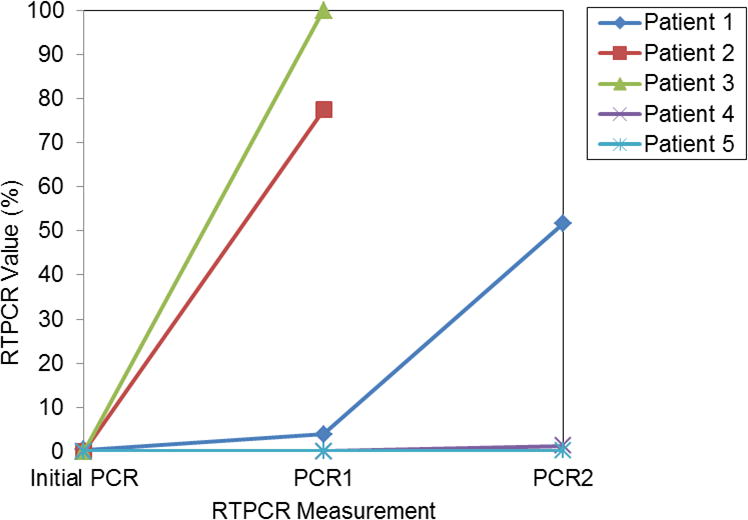
RTPCR values over serial measurements for HMA responders.
As of last follow up, all 6 patients who started HMA with undetectable MRD remained MRD negative (RTPCR < 0.01) and in remission, and 8 out of the total 23 patients continued on maintenance therapy. Median follow up was 11.3 months (range, 2.9–67.7).
Mutational Status
Of the 5 patients failing HMA, 4 [inv(16)=3 and t(8;21)=1] had a RAS mutation detected, with the t(8;21) patient also having a concomitant KIT mutation, and 1 inv(16) patient with a co-existing FLT3D835 mutation. Of the 12 patients who responded to HMA, 4 [inv(16)=2 and t(8;21)=2] had a RAS mutation, one t(8;21) had a co-existing FLT3ITD, 3 [inv(16)=1 and t(8;21)=2] had a sole KIT mutation, and 1 inv(16) patient had a FLT3D835 mutation. Of the 6 patients who proceeded to HMA maintenance without detectable MRD, only 1 inv(16) patient had a co-existing FLT3D835 mutation. All other mutations (NPM1=20 evaluated, TP53=13 evaluated, IDH1 and IDH2=16 evaluated) were negative at diagnosis.
DISCUSSION
Despite a favorable prognosis, relapse occurs in 20–25% of the patients with CBF and is usually preceded by a period of increased RTPCR transcripts. Strategies to intervene if a patient does not achieve appropriate reduction in RTPCR levels or shows rising trends post completion of planned induction/consolidation may avert full-blown relapses. SCT is one such option, but donor availability and patient related factors including age, performance status, and organ dysfunction may be potential barriers to timely and safe SCT.30
The importance of MRD monitoring in CBF-AML has been well defined.5,7,9,13 What has not been clearly defined, however, is what to do when MRD positivity beyond a threshold level is detected, particularly when SCT is not an option or when MRD levels are positive but overt relapse is not yet recognized. Beyond re-induction followed by SCT, other strategies for preventing relapse in CBF-AML patients with detectable MRD have not been explored extensively. Recently, results of a phase II study (Cancer and Leukemia Group B Study 10503) of maintenance DAC in AML patients younger than 60 years old were published.24 Though preliminary data and case reports of using DAC maintenance in CBF-AML were promising, maintenance DAC following complete remission after induction therapy did not provide clinical benefit in either the non CBF-AML or CBF-AML cohorts; the authors reported the study was not powered to identify small differences in survival, and minimal residual disease was not addressed.24 Boumber and colleagues explored DAC maintenance versus conventional care in AML patients in complete remission and found that DAC maintenance was safe, feasible, and led to fewer relapses; however, the trial was not powered to provide more significant conclusions.25 In Boumber’s study, MRD was assessed and associated with EFS and OS in a multivariate Cox regression model. Only 1 patient had CBF-AML, and that patient received conventional care and went on to relapse.25 With these studies in mind, using MRD to guide HMA maintenance might provide improved outcomes for CBF-AML patients. Our analysis reveals that HMA maintenance is effective at controlling MRD and prolonging remission in patients with CBF-AML with detectable or rising RTPCR at the end of induction/consolidation.
Though we have shown that HMA maintenance therapy is effective at prolonging remission in patients with low level MRD, determining the specific MRD cut-off that would benefit most from the HMA maintenance strategy and deciding when to abort such therapy and proceed to SCT remain as important concerns. Our data suggests that patients with low levels of RTPCR (between 0.01 and 0.05) residual disease following induction/consolidation chemotherapy might benefit most from maintenance HMA, particularly those that have a reduction in the RTPCR within one to two cycles following initiation of hypomethylating therapy. A structured introduction of HMAs at a pre-defined cut-off for MRD positivity post induction/consolidation in a larger cohort of CBF-AML will allow for further exploration of these conclusions and further understanding of the impact of baseline mutation status on this population. Such an effort is underway.
Co-existing mutations were identified in patients including RAS, KIT, and FLT3. In the HMA failure group, 80% of patients had a co-existing mutation. For those who responded to HMA maintenance, 67% carried a concomitant mutation. Co-existing RAS mutations were identified in highest frequency, followed by KIT, then FLT3 mutations. This is concordant with the typical distribution of co-existing mutations in CBF-AML.31 With the small number of patients in this analysis, the impact of co-existing mutations on the efficacy of HMA maintenance therapy cannot be adequately determined. In future studies, it will be important to monitor for co-existing mutations as a majority of patients with persistent MRD in our analysis had concomitant mutations. Only 1 of 6 patients (17%) who proceeded to HMA maintenance without detectable MRD had a co-existing mutation, suggesting that mutation burden might contribute to persistent MRD in CBF-AML.
There are many limitations to this analysis. The small sample size and exploratory nature of this retrospective analysis limit the conclusions that can be made. An important limitation in this analysis is that levels of MRD required for initiation of HMA therapy were not pre-defined. Subsequently, observations regarding appropriate levels of MRD that would warrant HMA maintenance can be made, but a well-designed clinical trial with pre-defined MRD levels will provide improved guidance in utilizing maintenance therapy in CBF-AML. Another possible limitation is that peripheral blood and bone marrow were used in MRD assessments for patients in this analysis. The study was not powered to recognize differences between the two sample types, but others studies suggest that either can be used for MRD assessment.32 An additional important limitation identified is that this analysis includes patients who proceeded to maintenance therapy without detectable MRD, and although these patients have successfully remained in remission, it is difficult to attribute the success completely to HMA maintenance.
Despite the limitations of this analysis, CBF-AML patients with low levels of RTPCR (between 0.01 and 0.05) at the conclusion of induction/consolidation chemotherapy derived the most benefit from maintenance HMA, particularly those with a reduction in the RTPCR within the first 2 cycles of HMA therapy. We propose this population be explored further in future studies of HMA maintenance in CBF-AML.
Supplementary Material
Acknowledgments
None
Funding Source
This study was supported in part by the MD Anderson Cancer Centre Support Grant (CCSG) CA016672
Footnotes
Conflict of Interest: No relevant COI to disclose.
Author Contributions
BKR, ND, FR, JC, TK, BO, GGM, MO, AF, NP, HK, and GB collected and reviewed the data and wrote the paper. All authors participated in the discussion, have reviewed the final manuscript, have added comments or suggestions, and approved the current version of the manuscript.
References
- 1.Bhatt VR, Kantarjian H, Cortes JE, Ravandi F, Borthakur G. Therapy of Core Binding Factor Acute Myeloid Leukemia: Incremental Improvements Toward Better Long-Term Results. Clinical lymphoma, myeloma & leukemia. 2013;13(2) doi: 10.1016/j.clml.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of Prolonged Remission Duration after High-Dose Cytarabine Intensification in Acute Myeloid Leukemia Varies by Cytogenetic Subtype. Cancer Research. 1998;58(18):4173–9. [PubMed] [Google Scholar]
- 3.Byrd JC, Dodge RK, Carroll A, Baer MR, Edwards C, Stamberg J, et al. Patients With t(8;21)(q22;q22) and Acute Myeloid Leukemia Have Superior Failure-Free and Overall Survival When Repetitive Cycles of High-Dose Cytarabine Are Administered. Journal of Clinical Oncology. 1999;17(12):3767–75. doi: 10.1200/JCO.1999.17.12.3767. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Kopecky KJ, Tallman MS, Slovak ML, Gundacker HM, Kim HT, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. British Journal of Haematology. 2006;135(2):165–73. doi: 10.1111/j.1365-2141.2006.06276.x. [DOI] [PubMed] [Google Scholar]
- 5.Yin JAL, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–35. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 6.Martinelli G, Rondoni M, Buonamici S, Ottaviani E, Piccaluga P, Malagola M, et al. Molecular monitoring to identify a threshold of CBFbeta/MYH11 transcript below which continuous complete remission of acute myeloid leukemia inv16 is likely. Haematologica. 2004;89(4):495–7. [PubMed] [Google Scholar]
- 7.Krauter J, Görlich K, Ottmann O, Lübbert M, Döhner H, Heit W, et al. Prognostic Value of Minimal Residual Disease Quantification by Real-Time Reverse Transcriptase Polymerase Chain Reaction in Patients With Core Binding Factor Leukemias. Journal of Clinical Oncology. 2003;21(23):4413–22. doi: 10.1200/JCO.2003.03.166. [DOI] [PubMed] [Google Scholar]
- 8.Corbacioglu A, Scholl C, Schlenk RF, Eiwen K, Du J, Bullinger L, et al. Prognostic Impact of Minimal Residual Disease inCBFB-MYH11–Positive Acute Myeloid Leukemia. Journal of Clinical Oncology. 2010;28(23):3724–9. doi: 10.1200/JCO.2010.28.6468. [DOI] [PubMed] [Google Scholar]
- 9.Tobal K, Newton J, Macheta M, Chang J, Morgenstern G, Evans PAS, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000;95(3):815–9. [PubMed] [Google Scholar]
- 10.Buonamici S, Ottaviani E, Testoni N, Montefusco V, Visani G, Bonifazi F, et al. Real-time quantitation of minimal residual disease in inv(16)-positive acute myeloid leukemia may indicate risk for clinical relapse and may identify patients in a curable state. Blood. 2002;99(2):443–9. doi: 10.1182/blood.v99.2.443. [DOI] [PubMed] [Google Scholar]
- 11.Leroy H, de Botton S, Grardel-Duflos N, Darre S, Leleu X, Roumier C, et al. Prognostic value of real-time quantitative PCR (RQ-PCR) in AML with t(8;21) Leukemia. 2005;19(3):367–72. doi: 10.1038/sj.leu.2403627. [DOI] [PubMed] [Google Scholar]
- 12.Stentoft J, Hokland P, Østergaard M, Hasle H, Nyvold CG. Minimal residual core binding factor AMLs by real time quantitative PCR—Initial response to chemotherapy predicts event free survival and close monitoring of peripheral blood unravels the kinetics of relapse. Leukemia Research. 2006;30(4):389–95. doi: 10.1016/j.leukres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Perea G, Lasa A, Aventin A, Domingo A, Villamor N, Paz Queipo de Llano M, et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [lsqb]t(8;21) and inv(16)[rsqb] Leukemia. 2005;20(1):87–94. doi: 10.1038/sj.leu.2404015. [DOI] [PubMed] [Google Scholar]
- 14.Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–23. doi: 10.1182/blood-2012-10-462879. [DOI] [PubMed] [Google Scholar]
- 15.Boissel N, Leroy H, Brethon B, Philippe N, de Botton S, Auvrignon A, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML) Leukemia. 2006;20(6):965–70. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 16.Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse Prognostic Significance of KIT Mutations in Adult Acute Myeloid Leukemia With inv(16) and t(8;21): A Cancer and Leukemia Group B Study. Journal of Clinical Oncology. 2006;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 17.Boissel N, Renneville A, Leguay T, Lefebvre PC, Recher C, Lecerf T, et al. Dasatinib in high-risk core binding factor acute myeloid leukemia in first complete remission: a French Acute Myeloid Leukemia Intergroup trial. Haematologica. 2015;100(6):780–5. doi: 10.3324/haematol.2014.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal S, Unterberg M, Koschmieder S, zur Stadt U, Brunnberg U, Verbeek W, et al. DNA Methylation of Tumor Suppressor Genes in Clinical Remission Predicts the Relapse Risk in Acute Myeloid Leukemia. Cancer Research. 2007;67(3):1370–7. doi: 10.1158/0008-5472.CAN-06-1681. [DOI] [PubMed] [Google Scholar]
- 19.Kroeger H, Jelinek J, Estécio MRH, He R, Kondo K, Chung W, et al. Aberrant CpG island methylation in acute myeloid leukemia is accentuated at relapse. Blood. 2008;112(4):1366–73. doi: 10.1182/blood-2007-11-126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki R, Onizuka M, Kojima M, Shimada M, Fukagawa S, Tsuboi K, et al. Preferential hypermethylation of the Dickkopf-1 promoter in core-binding factor leukaemia. British Journal of Haematology. 2007;138(5):624–31. doi: 10.1111/j.1365-2141.2007.06702.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CK, Li L, Cheng SH, Ng K, Chan NPH, Ip RKL, et al. Secreted-frizzled related protein 1 is a transcriptional repression target of the t(8;21) fusion protein in acute myeloid leukemia. Blood. 2011;118(25):6638–48. doi: 10.1182/blood-2011-05-354712. [DOI] [PubMed] [Google Scholar]
- 22.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21(1):103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Shen T, Huynh L, Klisovic MI, Rush LJ, Ford JL, et al. Interplay of RUNX1/MTG8 and DNA Methyltransferase 1 in Acute Myeloid Leukemia. Cancer Research. 2005;65(4):1277–84. doi: 10.1158/0008-5472.CAN-04-4532. [DOI] [PubMed] [Google Scholar]
- 24.Blum W, Sanford BL, Klisovic R, DeAngelo DJ, Uy G, Powell BL, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B Study (CALGB 10503) Leukemia. 2017;31(1):34–9. doi: 10.1038/leu.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boumber Y, Kantarjian H, Jorgensen J, Wen S, Faderl S, Castoro R, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012;26(11):2428–31. doi: 10.1038/leu.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beillard E, Pallisgaard N, van der Velden VHJ, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using/‘real-time/’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474–86. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 27.Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of/‘real-time/’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - A Europe Against Cancer Program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 28.Borthakur G, Cortes JE, Estey EE, Jabbour E, Faderl S, O’Brien S, et al. Gemtuzumab ozogamicin with fludarabine, cytarabine, and granulocyte colony stimulating factor (FLAG-GO) as front-line regimen in patients with core binding factor acute myelogenous leukemia. American journal of hematology. 2014;89(10):964–8. doi: 10.1002/ajh.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Jones D, Jeffrey Medeiros L, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. British Journal of Haematology. 2005;130(5):726–8. doi: 10.1111/j.1365-2141.2005.05666.x. [DOI] [PubMed] [Google Scholar]
- 30.Kadia TM, Ravandi F, Cortes J, Kantarjian H. Toward individualized therapy in acute myeloid leukemia: A contemporary review. JAMA Oncology. 2015;1(6):820–8. doi: 10.1001/jamaoncol.2015.0617. [DOI] [PubMed] [Google Scholar]
- 31.Paschka P, Du J, Schlenk RF, Gaidzik VI, Bullinger L, Corbacioglu A, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG) Blood. 2013;121(1):170–7. doi: 10.1182/blood-2012-05-431486. [DOI] [PubMed] [Google Scholar]
- 32.Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJM, Scholten WJ, Snel AN, Veldhuizen D, et al. Peripheral blood minimal residual disease may replace bone marrow minimal residual disease as an immunophenotypic biomarker for impending relapse in acute myeloid leukemia. Leukemia. 2016;30(3):708–15. doi: 10.1038/leu.2015.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


