Abstract
Reactive oxygen species-induced cyclophilin A (CyPA) release from vascular smooth muscle cells (VSMCs) may be inhibited by simvastatin in vitro. The present study aimed to further examine the effect of simvastatin on serum CyPA levels and the basigin (CD147)-extracellular signal-regulated kinase (ERK) 1/2-cyclin pathway during thoracic aorta remodeling. The mechanisms through which simvastatin may inhibit CyPA secretion from VSMCs were further investigated. Serum CyPA levels and the expression kinetics of CyPA-associated signaling pathways were examined following simvastatin treatment in rat thoracic aortas during hypertension. Cell lysates were prepared from middle layer of thoracic aortas at 1, 4, 8 and 12 weeks subsequent to surgery. ELISA analysis revealed that serum CyPA levels were gradually increased with the progression of thoracic aorta remodeling. Western blotting demonstrated that the expression of CD147, phosphorylated-ERK1/2, cyclin D1, cyclin A, and cyclin E were increased with the progression of thoracic aorta remodeling. Simvastatin administration for 4, 8 and 12 weeks diminished all these changes, as observed in the hypertensive group. VSMCs from simvastatin-treated rats secreted a decreased amount of CyPA compared with VSMCs from hypertensive rats. In addition, pretreatment with geranylgeraniol partly reversed the inhibitory effect of simvastatin on LY83583-induced CyPA secretion in cultured VSMCs, whereas GGTI-298 and KD025 [a selective Rho-associated protein kinase 2 (ROCK2) inhibitor] mimicked the inhibitory effect of simvastatin. The present study demonstrated that simvastatin alleviated thoracic aorta remodeling by reducing CyPA secretion and expression of the CD147-ERK1/2-cyclin signaling pathway. In addition, the results of the present study demonstrated that the Rho-ROCK2 pathway mediated CyPA secretion from VSMCs.
Keywords: Rho-associated protein kinase 2, basigin, cyclophilin A, simvastatin, aorta remodeling, hypertension
Introduction
Abnormal vascular smooth muscle cell (VSMC) growth contributes to the pathogenesis of vascular remodeling of large arteries during hypertension. Autocrine and paracrine growth factors may modulate VSMC growth, partly by promoting the secretion of growth factors (1), including platelet-derived growth factor (2,3). Cyclophilin A (CyPA) has been identified to be a growth factor secreted from VSMCs and has a direct mitogenic effect on the growth of human aorta smooth muscle cells (4) and rat VSMCs (5). Additionally, CyPA mediates vascular remodeling by promoting vascular smooth muscle cell proliferation and inflammation (6). Previous studies demonstrated that CyPA levels in rat serum gradually increased with the development of hypertension (7), and were positively correlated with systolic and diastolic blood pressure in untreated patients (8). The extracellular matrix metalloproteinase inducer basigin (CD147) was reported to be a surface receptor for extracellular CyPA (9) and mediates CyPA-specific signaling events. CyPA activates extracellular signal-regulated kinase (ERK) 1/2 via binding with CD147, leading to upregulation of cyclin D1 expression in cholangiocarcinoma cells (10). However, the kinetics of the alterations in serum CyPA and its downstream signaling cascade (CD147-ERK1/2-cyclin) during thoracic aorta remodeling have not been completely elucidated. Therefore, the present study was performed to further examine the kinetics of alterations in serum CyPA and its downstream signaling cascade (CD147-ERK1/2-cyclin), and to clarify the potential of CyPA to be a biomarker of the extent of thoracic aorta remodeling.
Geranylgeranyl pyrophosphate (GGPP) and farnesyl pyrophosphate (FPP) are essential for the geranylgeranylation and farnesylation of Rho and Ras family small G proteins, respectively (11). The downstream effectors of Rho include the Rho kinase family members, consisting of Rho-associated kinase 1 (ROCK1) and ROCK2, which are serine-threonine kinases that are activated by Rho GTPases (12), and recent studies have demonstrated that ROCK2 regulates the secretion of proinflammatory cytokines (13). It has been demonstrated that 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) prevent the conversion of HMG-CoA to mevalonate, and thereby inhibit the synthesis of the other products of the mevalonate pathway, including GGPP and FPP. By inhibiting the synthesis of GGPP, statins prevent the activation of Rho GTPases and Rho-associated kinases (11). Simvastatin has been observed to decrease reactive oxygen species (ROS) -mediated CyPA release in cultured VSMCs, and the pan ROCK inhibitor Y27632 has exhibited similar effects to simvastatin (14); however, it remains unclear whether ROCK1 or ROCK2 regulates the secretion of CyPA. In addition, it is uncertain whether simvastatin-inhibited CyPA secretion is mediated by the Rho-Rho kinase pathway. These uncertainties provided a basis for further investigation of whether ROCK1 or ROCK2 is involved in CyPA secretion from VSMCs. Previous studies have demonstrated that CyPA levels in rat serum gradually increase with the development of hypertension (7) and contribute to vascular remodeling (6). Atorvastatin was reported to improve thoracic aortic remodeling in spontaneously hypertensive rats (15). However, the novel underlying mechanisms have not been completely elucidated. Available data about the effect of simvastatin on thoracic aorta remodeling during hypertension are limited. The above previous studies led to the question of whether simvastatin may improve thoracic aorta remodeling by regulating serum CyPA levels, and whether the Rho-Rho kinase signaling pathway mediates simvastatin-inhibited CyPA secretion. In addition, the present study aimed to examine whether ROCK1 or ROCK2 regulates the secretion of CyPA.
The present study investigated whether simvastatin was able to affect serum CyPA levels in hypertensive rats and downstream signaling cascades in the remodeled thoracic aorta. The mechanism underlying simvastatin-inhibited CyPA secretion was additionally examined. The results of the present study demonstrated that treatment with simvastatin diminished the time-dependent upregulation of serum CyPA levels and CD147-ERK1/2-cyclin signaling pathway in the remodeled thoracic aorta. The secretion of CyPA is ROCK2-dependent. Additionally, simvastatin-inhibited CyPA release was mediated by the Rho-Rho kinase pathway in VSMCs.
Materials and methods
Reagents and cell culture
Simvastatin and LY83583 were purchased from Calbiochem (Merck KGaA, Darmstadt, Germany). Geranylgeraniol (GGOL) was purchased from Sigma-Aldrich (Merck KGaA). Geranylgeranyltransferase inhibitor (GGTI) 298 and KD025 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The above reagents were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. The final concentration of DMSO in the culture medium was <0.1%. VSMCs were isolated from the thoracic aorta media of male Sprague-Dawley rats (80–100 g) and maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere of 5% CO2/95% air. VSMCs were confirmed by positive staining with α-smooth muscle actin antibody, as described previously (16).
Animal model and primary culture of VSMCs
The present study was approved by the Animal Care and Use Committee of Guangzhou Medical University (Guangzhou, China). All animal experimental procedures were performed in line with the Guide for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China. A total of 96 male adult rats were used for these experiments. Two-kidney, two clip renovascular hypertensive rats were induced as described by our previous studies (7,16). U-shaped silver clips with an internal diameter of 0.3 mm were placed around the right and left renal arteries of 8 to 10-week old healthy normotensive male Sprague-Dawley rats (80–100 g), after anaesthetized by 10% chloral hydrate (300 mg/kg; intraperitoneal injection). The criterion for hypertension was a systolic blood pressure of ≥150 mmHg. Following 3 days of adaptation, rats were randomly allocated to sham, hypertension (Htn) or simvastatin groups (Sim; hypertensive rats treated with simvastatin). Hypertension was induced using U-shaped silver clips, as described above. The Sim group received simvastatin by daily gavage (10 mg/kg/day) from the second day post-clipping. The sham-operated rats were treated in the same manner without the placement of silver clips, and served as the control. All groups were investigated for 1, 4, 8 and 12 weeks following surgery. The rats were injected with penicillin G (25,000 U intramuscular) prior to surgery to mitigate the risk of infection. Systolic blood pressure was detected preoperatively and every 3 days post-surgery under a conscious state using the indirect tail-cuff method (Powerlab 4/30; AD Instruments, Sydney, New South Wales, Australia), subsequent to warming of the rats at 35°C for 5 min under mild restraint. The average of three pressure readings was recorded for each measurement. Animals were supplied by the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China). All rats were maintained in pathogen-free facilities with a 12-h light/dark cycle at 23–25°C, with a relative humidity of 55–60% and ad libitum access to food and water.
To investigate whether CyPA secretion from VSMCs was influenced by treatment with simvastatin in rats, VSMCs of the thoracic aorta from sham, Htn and Sim rats at the 12-week point were isolated and cultured in DMEM containing 10% FBS in an incubator with 95% O2 and 5% CO2 at 37°C, as previously described (17). CyPA in conditioned medium was detected by western blotting as previously reported (14).
Histological analysis of thoracic aorta specimens, and hematoxylin and eosin (H&E) staining
Following anesthetization at 1, 4, 8 and 12 weeks with 10% chloral hydrate, the thorax was opened, perfusion commenced at a pressure of 55±5 mmHg, and the right atrium incised to allow drainage of blood and perfusate. The same pressure was used in all animals so that all aortas would be fixed under uniform tension. Perfusion with 0.1 mol/l phosphate buffer containing heparin (100 U/kg) for 5 min was followed by 4°C fixative solution containing 4% freshly depolymerized paraformaldehyde in 0.1 mol/l phosphate buffer for 15 min. At a defined distance from the aortic arch (1 cm), a segment of the thoracic aorta was dissected free of perivascular tissue, cleaned and immersed in 4% fresh paraformaldehyde for an additional 3–4 h at 4°C. Following fixation, each thoracic aorta was cut transversely into 5–6 rings of 2–3-mm thickness, which were subsequently embedded in paraffin. Sections (4-µm thick) of paraffin-embedded thoracic aortas that included the entire circumference of each ring were sectioned from five different blocks in each animal. Sections were stained with H&E for ~2 min at room temperature for general morphology and quantitative morphometric analysis. Thoracic aortas without perfusion were rapidly frozen at −80°C for subsequent western blot analysis.
Image acquisition, methods of measurement and calculation of vascular remodeling parameters
All stained histological sections of thoracic aortas were visualized under light microscopy (Eclipse-80i; Nikon Corporation, Tokyo, Japan), and digital images of thoracic aortas were captures using the imaging software NIS-Elements F v4.00.00 (Nikon Corporation) at magnification, ×40 and ×400, marked with a micrometer scale. Integrated Performance Primitives software (version 6.0; Intel Corporation, Santa Clara, CA, USA) was used to measure the perimeter of the lumen (C) and media (without adventitia) of sections from each thoracic aorta at magnification, ×40. The diameter of lumen (the internal diameter, Di) and external diameter (De) were calculated using the formula: D=C/π, according to a previously-published method with minor modifications (18,19). The media thickness was calculated by subtraction of Di/2 from De/2. The cross-sectional area occupied by the lumen was calculated from its mean diameter (πD2/4). Medial area was calculated by subtraction of the inner outlined area from the outer outlined area according to the formula: Media CSA=π(De2-Di2)/4. Remodeling was assessed by media thickness and media area in paraffin-embedded tissue sections of thoracic aortas with H&E staining.
ELISA for measurement of rat serum CyPA
Rat serum CyPA was assayed as our previous experiment (7), using a rat cyclophilin A ELISA kit (cat. no. CSB-E14928r; Cusabio, Wuhan, China). A total of 3–5 ml blood was collected with a serum vacuumed blood collection tube, clotted overnight at 4°C, and centrifuged for 15 min at 1,000 × g at 4°C. The separated serum was stored in fresh tubes in aliquots at −80°C. Fresh samples were used for the ELISA, according to the manufacturer's protocol. The optical density of each well was read at 450 nm using a Multiskan GO 1.00.38 microplate reader (Thermo Fisher Scientific, Inc.). A standard curve was produced and the CyPA concentration of each sample was calculated.
Western blot analysis of the expression of CyPA, CD147, phosphorylated-ERK1/2 and cyclins
Western blot analysis was performed as described previously (20). Total proteins were extracted from the media of rat thoracic aortas. Equal amounts of total protein (20–30 µg) were loaded on an 8–12% SDS-PAGE gel and transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked at room temperature for 1 h in 5% non-fat milk in TBST buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 0.1% Tween-20], incubated overnight at 4°C with the following primary antibodies: Phospho (p)-ERK1/2 (cat. no. 4370; 1:1,000), ERK1/2 (cat. no. 9102; 1:1,000), cyclin D1 (cat. no. 2978; 1:500), CyPA (cat. no. 2175; 1:1,000), β-actin (cat. no. 8457; 1:2,000; all from Cell Signaling Technology, Inc., Danvers, MA, USA), CD147 (cat no. SAB4502896; 1:500; Sigma-Algrich; Merck KGaA), cyclin A (cat. no. sc-239; 1:500) and cyclin E (cat. no. sc-247; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). They were subsequently incubated for 1 h at room temperature with the appropriate anti-rabbit (cat. no. 7074) and anti-mouse (cat. no. 7076) secondary horseradish peroxidase-conjugated antibodies (1:2,000; Cell Signaling Technology, Inc.). Blots were incubated with chemiluminescence substrates (cat. no. 34079; Pierce; Thermo Fisher Scientific, Inc.) and were visualized using a gel image system. The integrated optical density of target bands was determined using Gel-Pro analyzer software v6.3 (Media Cybernetics, Inc., Rockville, MD, USA). β-actin was used as an internal control to guarantee equal loading and transfer of protein.
Assay of medium CyPA concentration
CyPA in the medium was assayed as previously reported (14). Following pretreatment with simvastatin (5 µM), GGOL (10 µM), GGTI 298 (10 µM) and KD025 (10 µM) for 30 min at 37°C, VSMCs were exposed to 1 µmol/l LY83583 for 2 h at 37°C in a CO2 incubator. Conditioned medium (CM) from VSMCs in each group was collected and centrifuged at 4°C for 10 min at 800 × g to remove cell debris. The medium was subsequently concentrated 100-fold using a Centricon Plus-20 filter (EMD Millipore). CyPA in CM and total cell lysate were detected using western blot analysis, as aforementioned.
Statistical analysis
Quantitative results were expressed as the mean ± standard deviation of at least 3 independent experiments. Statistical significance of differences between means was assessed by one-way analysis of variance using SPSS 19.0 software (IBM Corp., Armonk, NY, USA), and post hoc multiple comparisons were analyzed using Bonferroni testing. P<0.05 was considered to indicate a statistically significant difference.
Results
Simvastatin alleviates hypertension-induced remodeling of the rat thoracic aorta
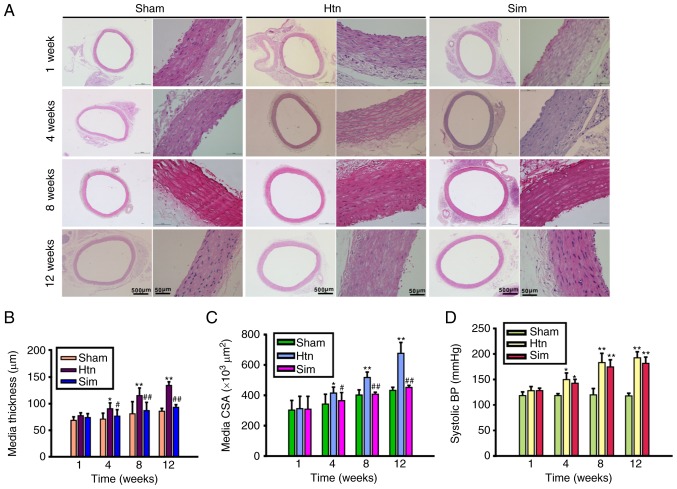
The present study evaluated the effect of simvastatin on hypertension-induced remodeling of the rat thoracic aorta. Representative images of transverse sections of H&E-stained thoracic aorta and morphometric analysis of thoracic aortic remodeling are presented in Fig. 1A-C. The thoracic aorta media thickness (Fig. 1B), and the media cross sectional area (Fig. 1C) of hypertensive rats were significantly increased compared with those of sham controls at 4, 8 and 12 weeks post-clipping. The results of the present study indicated that the rat thoracic aortas from the Htn group had undergone remodeling. However, treatment with simvastatin ameliorated hypertension-induced thoracic aorta remodeling at 4, 8 and 12 weeks, in a time-dependent manner. Treatment with simvastatin slightly, although not significantly, decreased the elevated systolic blood pressure at 4, 8 and 12 weeks post-surgery (Fig. 1D). The results of the present study suggested that treatment with simvastatin attenuated thoracic aorta remodeling without a significant lowering of blood pressure, indicating that the beneficial effect of simvastatin on thoracic aorta remodeling involves other mechanisms likely not associated with blood pressure.
Figure 1.
Simvastatin improves the remodeling of the rat thoracic aorta. (A) Representative images of cross sections of hematoxylin and eosin stained rat thoracic aortas. Scale bar, 500 µm (whole aorta ring; original magnification, ×40) and 50 µm (aorta section; original magnification, ×400). The bar graphs indicate the results of the analysis of the following parameters: (B) Media thickness; (C) media CSA; (D) systolic BP. Data are expressed as the mean ± standard deviation from 8 rats/group. *P<0.05, **P<0.01 vs. sham; #P<0.05, ##P<0.01 vs. Htn. Htn, hypertension; Sim, simvastatin; CSA, cross-sectional area; BP, blood pressure.
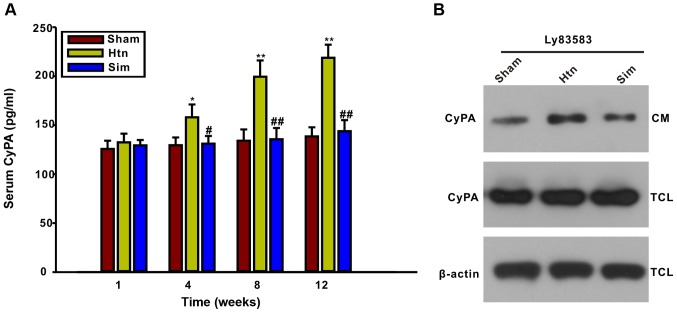
Treatment with simvastatin significantly decreases serum CyPA and CyPA secretion from primary VSMCs of thoracic aortas
It has been reported that pretreatment with simvastatin inhibited CyPA secretion from cultured VSMCs induced by ROS (14). The present study further examined whether the rat serum CyPA concentration may be influenced by treatment with simvastatin. The ELISA results illustrated that serum CyPA levels were significantly elevated at 4, 8 and 12 weeks post-surgery, concomitant with the progress of thoracic aorta remodeling, compared with the sham group (Fig. 2). The results of the present study indicated that serum CyPA levels may be an indicator of the degree of thoracic aorta remodeling. However, serum CyPA was decreased by treatment with simvastatin for 4, 8 and 12 weeks, suggesting that simvastatin may inhibit CyPA secretion in hypertensive rats. In order to confirm further whether simvastatin may affect CyPA secretion from VSMCs, VSMCs from the thoracic aortas of sham, Htn and simvastatin-treated rats at the 12-week point were isolated and cultured. The level of CyPA in the CM was determined by western blotting. Notably, the LY83583-induced CyPA secretion from VSMCs of Htn (12 weeks) rats was increased compared with that of sham (12 weeks) rats, suggesting that CyPA secretion from VSMCs increased with the progress of thoracic aorta remodeling. Additionally, LY83583-induced CyPA secretion from VSMCs of simvastatin-treated rats (12 weeks) was decreased compared with that of Htn rats (12 weeks). These results further demonstrated that treatment with simvastatin inhibited the secretion of CyPA from VSMCs during remodeling of the thoracic aorta.
Figure 2.
Rat serum CyPA and CyPA secretion in primary VSMCs from thoracic aortas is markedly decreased by treatment with simvastatin. (A) Serum CyPA levels gradually increased during the development of thoracic aorta remodeling, as assayed using a rat CyPA ELISA kit. Treatment with simvastatin for 4, 8 and 12 weeks reduced the serum CyPA concentration. (B) LY83583-induced CyPA secretion from primary VSMCs of simvastatin-treated rats was decreased compared with that from VSMCs of Htn rats at 12 weeks. Primary VSMCs were isolated from sham, Htn and simvastatin-treated rats at 12 weeks and cultured in vitro. Primary VSMCs were exposed to 1 µmol/l LY83583 for 2 h. The CyPA level in the CM and TCL were detected by western blotting. Data are expressed as mean ± standard deviation from 3 independent experiments. *P<0.05, **P<0.01 vs. sham; #P<0.05, ##P<0.01 vs. Htn. Htn, hypertension; Sim, simvastatin; CM, conditioned medium; TCL, total cell lysate; CyPA, cyclophilin A.
Treatment with simvastatin reduces upregulation of the CD147-ERK1/2-cyclin pathway with the extent of thoracic aorta remodeling
In order to determine the association between the CD147-ERK1/2-cyclin pathway and the progression of remodeling during hypertension, protein levels of CD147, phosphorylated-ERK1/2, cyclin D1, cyclin A and cyclin E in rat thoracic aortas were detected using western blotting. As presented in Fig. 3, CD147, phosphorylated-ERK1/2, cyclin D1, cyclin A and cyclin E were markedly increased in thoracic aortas with the progression of remodeling. The results of the present study indicated that the CD147-ERK1/2-cyclin pathway was activated and upregulated with the process of remodeling of the rat thoracic aorta. Treatment with simvastatin for 4, 8 and 12 weeks significantly diminished these alterations. The results of the present study implied that simvastatin may improve remodeling of the rat thoracic aorta by diminishing the upregulation and activation of the CD147-ERK1/2-cyclin pathway.
Figure 3.
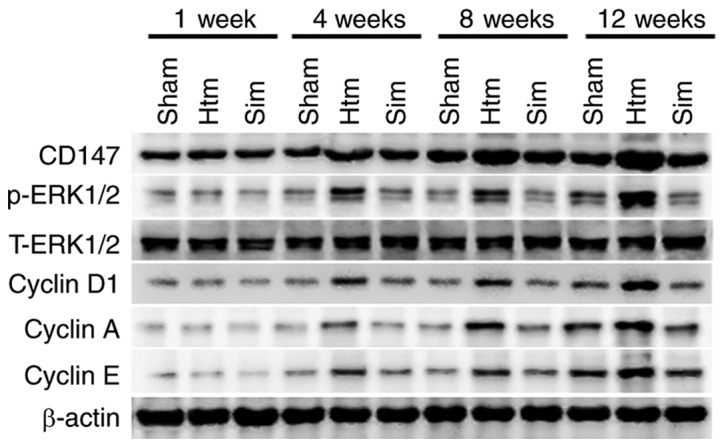
Upregulation of the CD147-ERK1/2-cyclin pathway with the extent of thoracic aorta remodeling is attenuated by treatment with simvastatin. Representative bands are from at least three independent experiments. CD147, basigin; ERK, extracellular signal-regulated kinase; p-, phosphorylated; Htn, hypertension; Sim, simvastatin.
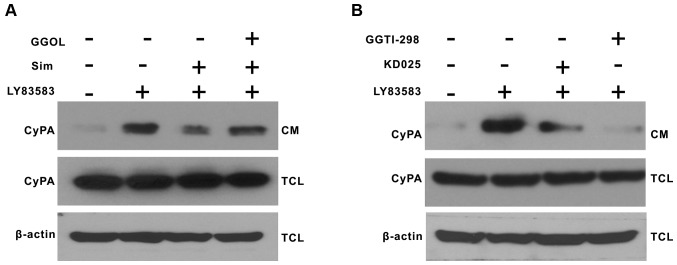
Rho-ROCK2 pathway mediates the inhibitory action of simvastatin on CyPA secretion induced by LY83583 in VSMCs
Previous studies have suggested that the pleiotropic effects of statins may be mediated through inhibition of the Rho/Rho kinase pathway (16,21). In order to further demonstrate whether Rho-ROCK2 mediates CyPA secretion from VSMCs, and whether simvastatin inhibits CyPA secretion by inhibiting the Rho-ROCK2 kinase pathway. VSMCs were pretreated with GGOL (to stimulate protein geranylgeranylation), KD025 (a selective ROCK2 inhibitor) and GGTI-298 (a geranylgeranyl transferase-I inhibitor) for 30 min prior to the addition of 1 µmol/l LY83583 for 2 h. Pretreatment with GGOL partially reversed the inhibitory effect of simvastatin on CyPA secretion induced by LY83583 (Fig. 4A). KD025 and GGTI-298 inhibited CyPA secretion, suggesting that Rho and ROCK2 may mediate CyPA secretion (Fig. 4B). The results of the present study further elucidated that the Rho-ROCK2 pathway may partially mediate the inhibitory effect of simvastatin on CyPA secretion induced by LY83583.
Figure 4.
The Rho-ROCK2 pathway mediates simvastatin-inhibited CyPA secretion. (A) Growth-arrested VSMCs were pretreated with GGOL for 30 min, and simvastatin was added for 30 min prior to exposure to 1 µmol/l LY83583 for 2 h. (B) Growth-arrested VSMCs were pretreated with KD025 and GGTI-298 for 30 min prior to exposure to 1 µmol/l LY83583 for 2 h. CyPA in the CM and TCL were detected by western blotting. All the experiments were repeated at least three times. GGOL, geranylgeraniol; Sim, simvastatin; CM, conditioned medium; TCL, total cell lysate; CyPA, cyclophilin A; GGTI, geranylgeranyltransferase inhibitor; ROCK2, Rho-associated protein kinase 2; VSMCs, vascular smooth muscle cells.
Discussion
The primary findings of the present study were that the administration of simvastatin significantly decreased the serum levels of CyPA and ameliorated the remodeling of thoracic aortas in hypertensive rats. Upregulation of the CD147-ERK-cyclin signaling pathway in remodeled thoracic aortas was alleviated by treatment with simvastatin. The Rho-ROCK2 pathway partly mediated simvastatin-inhibited CyPA release from cultured VSMCs.
CyPA was previously demonstrated to promote vascular remodeling by inducing VSMC proliferation (6), and LY83583-induced CyPA secretion was dose-dependently inhibited by simvastatin in cultured VSMCs (14). It was previously demonstrated that serum CyPA levels were gradually increased with the development of hypertension in rats (7), and that the levels were positively correlated with systolic and diastolic blood pressure in untreated patients (8). Therefore, the present study further investigated whether serum CyPA levels may be influenced by treatment with simvastatin in hypertensive rats. The results of the present study demonstrated that CyPA levels were upregulated with the process of thoracic aorta remodeling, and that treatment with simvastatin significantly decreased the serum CyPA levels and improved thoracic aorta remodeling during hypertension. Through detection of CyPA secretion from primary VSMCs from the sham, hypertensive and simvastatin group at 12 weeks, in response to LY83583, it was observed that the CyPA secretion of primary VSMCs from the simvastatin group was decreased compared with that from the hypertensive group. This result illustrated that CyPA secretion from VSMCs was enhanced by hypertension, while this hypertension-induced CyPA secretion was inhibited by treatment with simvastatin. Since CyPA contributes to vascular remodeling (6), it is possible that simvastatin may improve thoracic aorta remodeling during hypertension via the inhibition of CyPA release from VSMCs and the downregulation of serum CyPA levels.
CyPA has been suggested to mediate vascular remodeling by stimulating ERK1/2, VSMC growth and inflammation (5,6). Secreted CyPA was recently reported to mediate the G1/S phase transition of cholangiocarcinoma cells via the CD147-ERK1/2 pathway (10). Therefore, the present study further investigated alterations to downstream signaling molecules and the influence of simvastatin in the thoracic aorta. Western blotting demonstrated that CD147, phosphorylated-ERK1/2 and certain cyclins (cyclin D1, cyclin E and cyclin A) were time-dependently upregulated with the development of thoracic aorta remodeling. Treatment with simvastatin decreased the upregulation of CD147, phosphorylated-ERK1/2 and cyclins in the thoracic aorta, and alleviated thoracic aorta remodeling during hypertension. VSMC proliferation is controlled by various cell cycle proteins. In particular, cyclin D1, cyclin E and cyclin A induce cell cycle progression from the G1 phase to the S phase (22), and VSMC proliferation following angioplasty in the rat carotid artery is associated with a temporally- and spatially-coordinated expression of cyclin E, cyclin A and proliferating cell nuclear antigen (23). These results indicated that simvastatin may improve thoracic aorta remodeling during hypertension in part by impeding the upregulation of CD147, phosphorylated-ERK1/2 and cyclins in thoracic aortas.
Due to the downregulation of serum CyPA levels by simvastatin, the present study examined the underlying mechanisms through which simvastatin may decreased CyPA release from VSMCs. GGOL, which promotes Rho geranylgeranylation and the subsequent activation of Rho kinase, prevented the inhibitory effect of simvastatin on CyPA secretion. GGTI-298 (a geranylgeranyl transferase-I inhibitor) and KD025 (a selective ROCK2 inhibitor) inhibited CyPA secretion in VSMCs. The results of the present study demonstrated that activation of the Rho-ROCK2 pathway promoted CyPA secretion from VSMCs, while inhibition of the Rho-ROCK2 pathway diminished CyPA secretion, and that simvastatin decreased CyPA secretion in VSMCs by inhibiting the Rho-ROCK2 pathway.
In conclusion, serum CyPA concentration and CD147-ERK1/2-cyclins were time-dependently upregulated with the development of thoracic aorta remodeling, whereas treatment with simvastatin decreased the upregulation of the CD147-ERK1/2-cyclin pathway in thoracic aortas and CyPA secretion from VSMCs. The Rho-ROCK2 pathway mediated the inhibitory effect of simvastatin on CyPA secretion from VSMCs.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81302771), the Natural Science Foundation of Guangdong Province (grant no. 2014A030313087), the Science and Technology Program of Guangzhou City (grant no. 201607010255), the Medical Scientific Research Foundation of Guangdong Province, China (grant no. A2016382), the National College Students' Innovation Entrepreneurship Training Plan Program of China (grant no. 201410570003), the College Students' Science and Technology Innovation Cultivation Special Funds Program of Guangdong Province (grant no. pdjh2015b0438), the Guangzhou Medical University College Students Science Technology Innovation Project (grant no. 2013A0014), the Scientific Research Foundation of Guangzhou Medical University and the Foundation for Excellent Teachers by Guangzhou Medical University, China.
References
- 1.Berk BC. Vascular smooth muscle growth: Autocrine growth mechanisms. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 2.Dobrian A, Wade SS, Prewitt RL. PDGF-A expression correlates with blood pressure and remodeling in 1K1C hypertensive rat arteries. Am J Physiol. 1999;276:H2159–H2167. doi: 10.1152/ajpheart.1999.276.6.H2159. [DOI] [PubMed] [Google Scholar]
- 3.Naftilan AJ, Pratt RE, Dzau VJ. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Li M, Chai H, Yan S, Lin P, Lumsden AB, Yao Q, Chen C. Effects of cyclophilin A on cell proliferation and gene expressions in human vascular smooth muscle cells and endothelial cells. J Surg Res. 2005;123:312–319. doi: 10.1016/j.jss.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.RES.87.9.789. [DOI] [PubMed] [Google Scholar]
- 6.Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang FC, Wang HY, Ma MM, Guan TW, Pan L, Yao DC, Chen YL, Chen WB, Tu YS, Fu XD. CyPA-CD147-ERK1/2-cyclin D2 signaling pathway is upregulated during rat left ventricular hypertrophy. Sheng Li Xue Bao. 2015;67:393–400. [PubMed] [Google Scholar]
- 8.Chang CS, Su SL, Chang CC, Lee KW, Kuo CL, Huang CS, Tseng WM, Liu CS. Cyclophilin-A: A novel biomarker for untreated male essential hypertension. Biomarkers. 2013;18:716–720. doi: 10.3109/1354750X.2013.847122. [DOI] [PubMed] [Google Scholar]
- 9.Yurchenko V, Zybarth G, O'Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 10.Obchoei S, Sawanyawisuth K, Wongkham C, Kasinrerk W, Yao Q, Chen C, Wongkham S. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumour Biol. 2015;36:849–859. doi: 10.1007/s13277-014-2691-5. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 12.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 13.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, Depoil D, Rao N, Liu B, Wei J, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism; Proc Natl Acad Sci USA; 2014; pp. 16814–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 15.Ge CJ, Hu SJ, Wu YS, Chen NY. Effects of atorvastatin on vascular remodeling in spontaneously hypertensive rats. J Zhejiang Univ Sci. 2003;4:612–615. doi: 10.1631/jzus.2003.0612. [DOI] [PubMed] [Google Scholar]
- 16.Ma MM, Li SY, Wang M, Guan YY. Simvastatin attenuated cerebrovascular cell proliferation in the development of hypertension through Rho/Rho-kinase pathway. J Cardiovasc Pharmacol. 2012;59:576–582. doi: 10.1097/FJC.0b013e318250ba2c. [DOI] [PubMed] [Google Scholar]
- 17.Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1–7) inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:104–108. doi: 10.1161/01.HYP.28.1.104. [DOI] [PubMed] [Google Scholar]
- 18.Short D. Morphology of the intestinal arterioles in chronic human hypertension. Br Heart J. 1966;28:184–192. doi: 10.1136/hrt.28.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener J, Loud AV, Giacomelli F, Anversa P. Morphometric analysis of hypertension-induced hypertrophy of rat thoracic aorta. Am J Pathol. 1977;88:619–634. [PMC free article] [PubMed] [Google Scholar]
- 20.Pan L, Yao DC, Yu YZ, Li SJ, Chen BJ, Hu GH, Xi C, Wang ZH, Wang HY, Li JH, Tu YS. Necrostatin-1 protects against oleic acid-induced acute respiratory distress syndrome in rats. Biochem Biophys Res Commun. 2016;478:1602–1608. doi: 10.1016/j.bbrc.2016.08.163. [DOI] [PubMed] [Google Scholar]
- 21.Laufs U, Kilter H, Konkol C, Wassmann S, Böhm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/S0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 22.Sherr CJ. G1 phase progression: Cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 23.Wei GL, Krasinski K, Kearney M, Isner JM, Walsh K, Andrés V. Temporally and spatially coordinated expression of cell cycle regulatory factors after angioplasty. Circ Res. 1997;80:418–426. [PubMed] [Google Scholar]