Abstract
Methyl tert-butyl ether (MTBE) is widely used as an oxygenating agent in gasoline to reduce harmful emissions. However, previous studies have demonstrated that MTBE is a cytotoxic substance that has harmful effects in vivo and in vitro. Although remarkable progress has been made in elucidating the mechanisms underlying the MTBE-induced reproductive toxicological effect in different cell lines, the precise mechanisms remain far from understood. The present study aimed to evaluate whether mammalian ovary cells were sensitive to MTBE exposure in vitro by assessing cell viability, lactate dehydrogenase (LDH) leakage, malondialdehyde (MDA) content and antioxidant enzyme activities. In addition, the effect of MTBE exposure on differential protein expression profiles was examined by two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry. MTBE exposure induced significant effects on cell viability, LDH leakage, plasma membrane damage and the activity of antioxidant enzymes. In the proteomic analysis, 24 proteins were demonstrated to be significantly affected by MTBE exposure. Functional analysis indicated that these proteins were involved in catalytic activity, binding, structural molecule activity, metabolic processes, cellular processes and localization, highlighting the fact that the cytotoxic mechanisms resulting from MTBE exposure are complex and diverse. The altered expression levels of two representative proteins, heat shock protein family A (Hsp70) members 8 and 9, were further confirmed by western blot analysis. The results revealed that MTBE exposure affects protein expression in Chinese hamster ovary cells and that oxidative stress and altered protein levels constitute the mechanisms underlying MTBE-induced cytotoxicity. These findings provided novel insights into the biochemical mechanisms involved in MTBE-induced cytotoxicity in the reproductive system.
Keywords: methyl tert-butyl ether, Chinese hamster ovary cells, oxidative stress, cytotoxicity, proteomics
Introduction
Methyl tert-butyl ether (MTBE) is a gasoline oxygenate additive that is used to increase the oxygen content of fuel, decreasing carbon monoxide and reducing air pollution (1). MTBE enters the environment during all phases of the fuel cycle (2). Due to its high volatility, high solubility and low biodegradation, MTBE moves through the soil and into the ground water more rapidly and remains there more persistently compared with other gasoline-related chemicals, resulting in groundwater contamination and global environmental pollution (3). The US Geological Survey reported that MTBE is one of the most frequently detected chemicals in shallow urban monitoring wells (4). High levels of MTBE are encountered during motor vehicle refueling, where attendants and customers are exposed to evaporated emissions (5,6). MTBE is readily absorbed when inhaled or ingested (7) with the metabolic pathways underlying this involving oxidative demethylation of MTBE to formaldehyde and tert-butanol by microsomal cytochrome P450 enzymes (8,9).
The effect of MTBE exposure on health has been assessed using a variety of study designs, including epidemiological investigations of large human populations and laboratory studies (3). Acute MTBE exposure causes a variety of symptoms, including headaches, and eye, nose and throat irritation (10). Previous studies in experimental rodent bioassays revealed that the incidence of three tumor types increased following chronic exposure to MTBE: Renal tubule cell tumors, testicular Leydig interstitial cell tumors and liver tumors (11–13). MTBE exposure also exerts toxic effects on cultured cell lines (14,15), affecting cell proliferation and cell transformation (16). DNA damage in the form of fragmentation of the single and double helix has been reported in human lymphocytes exposed to MTBE (17). General stress is suspected to be the underlying reason behind the induction of toxicity (18). Increased MTBE-induced reactive oxygen species (ROS) production was also reported and revealed to be involved in its cytotoxicity in in vitro studies (14,19). These previous studies indicate that oxidative stress is one of the important mechanisms underlying MTBE-induced cytotoxicity.
Proteins are primary effectors of the response of biological systems to environmental alterations. Physiological or pathological conditions are reflected in protein expressions and enzyme activities (20). Proteomics is a high-throughput approach that has been extensively used to analyze adverse effects on cellular responses, discover novel biomarkers, and elucidate precise molecular mechanisms (21). Although remarkable progress had been made in previous studies regarding MTBE-induced reproductive toxicological effect in vitro, the precise mechanisms of MTBE-induced reproductive toxicology are far from understood. The present study aimed to evaluate whether mammalian ovary cells in vitro were sensitive to MTBE exposure, by measuring cell proliferation, lactate dehydrogenase (LDH) leakage, maleic dialdehyde (MDA) content and the enzymatic activity of antioxidant proteins. In addition, MTBE-induced effects on differential protein expression profiles were examined by two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). All identified proteins were analyzed by bioinformatics and two representative proteins, heat shock protein family A (Hsp70) member 9 (Hspa9) and heat shock protein family A (Hsp70) member 8 (Hspa8), were confirmed by western blot analysis. The present study is the first, to the best of our knowledge, to investigate MTBE-induced effects on the proteome of cultured mammalian cells. These findings provided novel insights into the biochemical mechanisms involved in MTBE-induced cytotoxicity in the reproductive system.
Materials and methods
Reagents and chemicals
MTBE (cat. no. 1634-04-4; 99.9% purity) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Chinese hamster ovary (CHO) cells were purchased from the cell bank at the Chinese Academy of Science (Shanghai, China). RPMI-1640 medium, fetal bovine serum (FBS), penicillin-streptomycin and trypsin were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit to measure cell viability was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Test kits for LDH, MDA, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). CyDye DIGE fluorescent dyes were obtained from GE Healthcare Life Sciences (Chalfont, UK). Hspa9 antibodies (cat. no. ab171089) were obtained from Abcam (Cambridge, UK). Hspa8 (cat. no. sc-7298) and β-actin (cat. no. sc-47778) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other chemicals were of analytical grade.
Cell culture and proliferation assay
CHO cells were cultured in RPMI-1640 media containing 10% FBS, 100 µg/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37°C. MTBE was administered at concentrations of 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM when cell confluence reached 80%, and the cells were treated for 6, 12 and 24 h. MTBE concentrations ranging between 0.5 and 100.0 mM were relatively high compared with expected environmental exposure (~0.45 µM) (19,22). However, 100 mM MTBE is a lower concentration compared with that used in previous studies focusing on MTBE toxicity (210 mM) (23).
CHO cell viability was assessed using an MTT assay, as described previously with minor modifications (24). Briefly, cells (100 µl) were seeded into 96-well culture plates (Corning Incorporated, Corning, NY, USA) at a density of 8.0×10−3 cells/well, and the cells were treated with MTBE at the stated concentrations and incubated at 37°C in an atmosphere of 5% CO2 for 6, 12 and 24 h. Folloiwng treatment, 20 µl MTT working solution (5 mg/ml) was added to each well and the plates were incubated at 37°C for 4 h. The MTT solution was discarded and 150 µl dimethyl sulfoxide was added to each well. The optical density was measured on a microplate reader at 490 nm. Exposure to MTBE to 12 h resulted in the greatest inhibition of cell viability, and was subsequently used for further biochemical analysis.
LDH leakage assay
The fraction of total LDH activity in the cell supernatants was used as an indicator of membrane leakage or cell lysis. Cytosolic LDH activity was estimated, as previously described (25). Following 12 h exposure to MTBE at 37°C in a 5% CO2 incubator, the medium was collected and the level of LDH released by cells was determined using an assay kit (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocol. Following the reaction, the absorbance of each sample was measured at a wavelength of 450 nm with an Infinite M1000 Microplate Reader (TECAN Group, Ltd., Männedorf, Switzerland).
Lipid peroxidation assay
Lipid peroxidation was assessed by determining the levels of thiobarbituric acid reactive substances, using the method previously described by Mihara and Uchiyama (26). CHO cells were seeded into 6-well plates (1.0×106 per well) and were allowed to attach for 24 h. The medium was replaced with RPMI-1640 medium along with 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE for 12 h. The treated cells were fragmented and centrifuged at 4,000 × g at 37°C for 10 min, and the supernatants were measured using the MDA test kit (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's instructions. The absorbance was determined using an Infinite M1000 Microplate Reader (TECAN Group, Ltd.) at wavelength of 532 nm. MDA content was calculated, according to a standard curve and expressed as nmol/mg protein. The protein content was determined using a Bradford assay, with FBS used as the standard (27).
Enzyme activity assays of SOD, CAT and GSH-Px
The enzyme activity of SOD, CAT and glutathione GSH-Px were examined to investigate the effect of MTBE-induced oxidative stress. These indexes were detected with corresponding assay kits (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocol. CHO cells were seeded into 6-well plates (1.0×106 per well) and were allowed to attach for 24 h. The medium was replaced with RPMI-1640 medium along with 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE for 12 h. The medium was subsequently removed and the cells were pooled in a 1.5 ml tube. The cells were centrifuged at 3,000 × g at 4°C for 10 min, and the pellet was resuspended with 1,000 µl PBS and centrifuged at 10,000 × g at 4°C for 15 min. The supernatant was collected for use in SOD, CAT and GSH-Px assays. The activities of SOD, CAT and GSH-Px were calculated according to a standard curve and expressed as U/mg protein. Protein content was determined using the Bradford assay with FBS as the standard (27).
Protein labeling and two-dimensional difference gel electrophoresis (2-D DIGE)
Treated and untreated samples were minimally labeled (25 µg proteins per 200 pmol dyes) with cyanine (Cy)2, Cy3 or Cy5 fluorescent dyes, according to the manufacturer's protocol (GE Healthcare Life Sciences). A reference design was used, in which a 50.0 mM MTBE exposure condition and control was labeled once with Cy3 and once with Cy5, with the reference sample labeled with Cy2 to generate an internal standard for normalization. By including the internal standard on each gel, the abundance of each protein spot of the individual biological samples was measured relative to its corresponding spot in the internal standard present on the same gel.
For 2-D DIGE, immobilized pH gradient (IPG) strips were dehydrated in an Ettan IPGphor 3 isoelectric focusing system (GE Healthcare Life Sciences). Later samples were focused until a total of 8,000 Vh was achieved. Each strip was embedded on top of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and separated on an Ettan DALT electrophoresis system (GE Healthcare Life Sciences). A loading of 1,000 mg unlabeled proteins was performed in parallel for spots picking and in-gel digestion.
Image analysis
Following 2-D DIGE, the gels were scanned using a Typhoon Trio Variable Mode Imager (GE Healthcare Life Sciences) at excitation/emission wavelengths of 532(blue)/580, 532(green)/425 and 633(red)/425 nm for Cy2, Cy3 and Cy5, respectively. The resultant maps were analyzed using Decyder 2D v6.5 (GE Healthcare Life Sciences). Gel images were imported into the software, and the protein spots were analyzed with the differential in-gel analysis and biological analysis modules. To compare protein expression levels, the spot volume of the Cy3 and Cy5 samples was normalized to the corresponding spot volume of the Cy2 internal standard samples from the same gel. Student's t-test and one-way analysis of variance (ANOVA) were used to calculate the statistical significance. The spots exhibiting statistically significant differences (P<0.05) were further analyzed.
Protein identification and bioinformatics analysis
The spots of interest detected by Decyder software analysis were manually excised from the 2-D DIGE gel and stained using Coomassie Brilliant Blue G-250 staining solution (0.12% Coomassie Brilliant Blue G-250, 20% ethanol, 10% phosphoric acid and 10% ammonia sulfate). The protein spots were washed three times with Milli-Q Ultrapure water (Merck Millipore, Darmstadt, Germany) and were subsequently destained with destaining solution (25 mM ammonium bicarbonate/50% acetonitrile). Gel pieces were dehydrated with 100% acetonitrile and incubated at 37°C for 30 min. Each gel piece was digested overnight at 37°C with 0.03 µg sequencing-grade trypsin (Promega, Madison, WI, USA) in 15 ml of 25 mM ammonium bicarbonate buffer. The tryptic peptides were used for MALDI-TOF-MS/MS analysis.
All mass spectrums were acquired on an AutoFlex MALDI-TOF/TOF with LIFT technology (Bruker Corporation, Billerica, MA, USA). Protein identification by PMF and MS/MS spectra was performed using the MASCOT search engine 2.2 (Matrix Science, Inc., Boston, MA, USA) and BioTools software (version 3.2 SR4; Bruker Corporation). Search parameters were set as follows: Taxonomy: Mus musculus; enzyme: Trypsin; missed cleavage: 1; fixed modification: Carbamidomethyl (C); variable modification: Oxidation (M); peptide tolerance: ±100 ppm; mass tolerance: ±0.3 kDa; ions: [M+H]. Confident matches in the SwissProt database (www.ebi.ac.uk/uniprot) were defined by the MASCOT score, the sequence coverage by matching peptides and statistical significance (P<0.05). Protein ontology classification was performed by importing proteins into the Protein Analysis Through Evolutionary Relationships (PANTHER) classification system (http://www.pantherdb.org/) (28). The proteins were grouped according to the association with molecular functions and biological processes.
Western blot analysis
The MTBE-induced effects on Hspa9 and Hspa8 protein expression levels were confirmed by western blot analysis. CHO cells underwent 12 h exposure to 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE and were lysed using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and collected by scraping. Protein concentration was determined using the Bradford assay, with FBS used as the standard. The resultant whole lysates (20 µg/lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrically transferred onto a polyvinylidene fluoride membrane (GE Healthcare Life Sciences). The membranes were blocked in 5% milk dissolved in Tris-buffered saline with 0.04% Tween-20 (TBST), and were subsequently incubated with Hspa9 (1:1,000), Hspa8 (1:1,000) and β-actin antibodies (1:2,000) at 4°C overnight. Following primary antibody incubation, the membranes were washed with TBST buffer three times and incubated at room temperature for 2 h with goat anti-mouse IgG-HRP (cat. no. sc-2031) or goat anti-rabbit IgG-HRP (cat. no. sc-2054) secondary antibodies at 1:4,000 dilutions (Santa Cruz Biotechnology, Inc.). The blots were incubated in Super signal®West Dura Extended Duration Substrate (Thermo Fisher Scientific, Inc.) and detected using the ECL™ western blotting detection system (Image-Quant™ RT; GE Healthcare Life Sciences).
Statistical analysis
All experiments were replicated ≥3 independent times and the data are expressed as the mean ± standard deviation. The data were analyzed using one-way ANOVA tests followed by Dunnett's tests to determine the statistical difference between treatment groups. These tests were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxic effect of MTBE exposure in CHO cells
To study the cytotoxic effect of MTBE exposure in CHO cells, cell viability was assessed using an MTT assay following treatment with 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE for different durations: 6, 12 and 24 h. Incubation of CHO cells for 6 h with 50.0 and 100.0 mM of MTBE resulted in a significant decrease in cell viability compared with the control cells (P=0.0086 and P=0.001, respectively; Fig. 1). When CHO cells were incubated with MTBE for 12 h cell viability significantly decreased at concentrations ≥5.0 mM compared with control cells (5.0 mM, P=0.036; 25.0 mM, P=0.042; 50.0 mM, P=0.023; 100.0 mM, P=0.026; Fig. 1). When CHO cells were incubated with MTBE for 24 h, cell viability significantly decreased at 50.0 and 100.0 mM compared with the control cells (P=0.028 and P=0.025, respectively; Fig. 1). By comparing cell viability rates at the different exposure times, 12 h MTBE exposure was selected for further analysis of MTBE-induced cytotoxicity and oxidative stress in CHO cells as it resulted in the highest inhibition of cell viability. The concentration of 50.0 mM was selected for 2DE analysis, and 5.0 and 50.0 mM were selected for western blot analysis.
Figure 1.
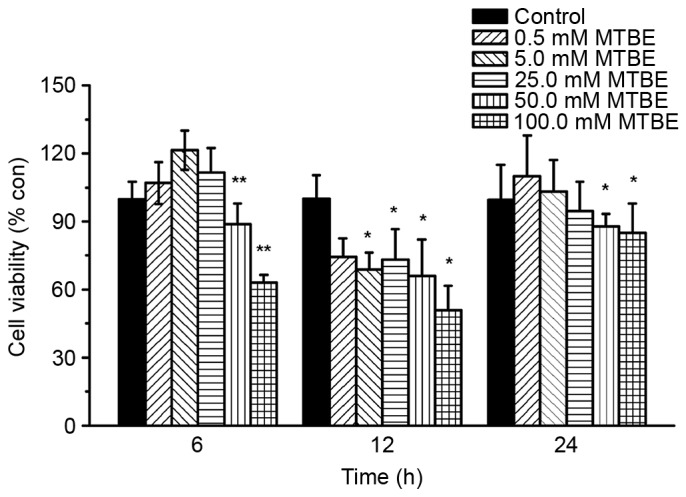
Effect of MTBE on Chinese hamster ovary cell viability. Cell viability was assessed at 6, 12 and 24 h following various treatments with MTBE. The data are presented as the mean ± standard deviation (*P<0.05 and **P<0.01 vs. control). MTBE, methyl tert-butyl ether; con, control.
MTBE exposure leads to plasma membrane damage in CHO cells
Plasma membrane damage was assessed by monitoring LDH release following 12 h exposure to 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE. MTBE exposure induced a significant increase in LDH leakage at concentrations ≥25.0 mM compared with the control group (25.0 mM, P=0.001; 50.0 mM, P=0.002; 100.0 mM, P=0.042; Fig. 2).
Figure 2.
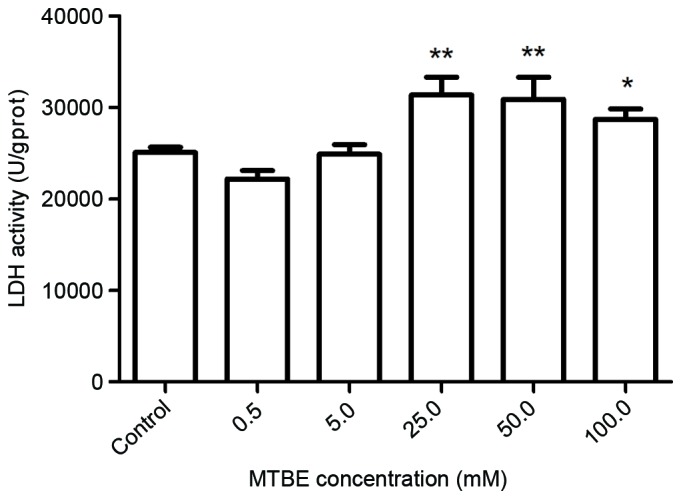
Effect of MTBE on LDH activity in Chinese hamster ovary cells. The LDH activity was assessed following treatment with various concentrations of MTBE. The data are presented as the mean ± standard deviation (*P<0.05 and **P<0.01 vs. the control). MTBE, methyl tert-butyl ether; LDH, lactate dehydrogenase.
MTBE exposure induces oxidative stress in CHO cells
The effect of 0, 0.5, 5.0, 25.0, 50.0 or 100.0 mM MTBE on lipid peroxidation, as indicated by MDA content, was estimated. MDA is one of the end products of lipid peroxidation and the MDA assay has previously been used for assessing oxidative damage (29). Exposure to MTBE for 12 h induced a significant increase in MDA levels in CHO cells, at all concentrations ≥5.0 mM, compared with control cells 5.0 mM, P<0.001; 25.0 mM, P<0.001; 50.0 mM, P<0.001; 100.0 mM, P<0.001; Fig. 3), indicating an increase in lipid peroxidation.
Figure 3.
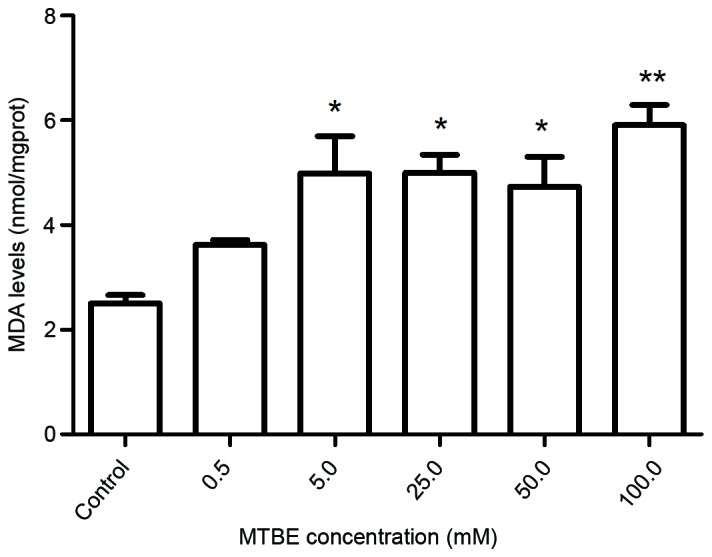
Effect of MTBE on lipid peroxidation in Chinese hamster ovary cells. Following 12 h exposure to various concentrations of MTBE, the MDA levels were assessed. The data are presented as the mean ± standard deviation (*P<0.05 and **P<0.01 vs. control). MTBE, methyl tert-butyl ether; MDA, maleic dialdehyde.
SOD, CAT and GSH-Px are three important antioxidative enzymes, and their activity levels following MTBE exposure were assessed. Exposure to 50.0 mM or 100.0 mM MTBE for 12 h induced a significant increase of SOD activity compared with the control cells (P=0.035 and P=0.002, respectively; Fig. 4). Exposure to 25.0, 50.0 or 100.0 mM MTBE induced a significant increase of CAT activity in CHO cells compared with control cells (P<0.001, P<0.001 and P<0.001, respectively; Fig. 5) and a significant increase of GSH-Px activity compared with control cells (P<0.001, P<0.001 and P<0.001, respectively; Fig. 6).
Figure 4.
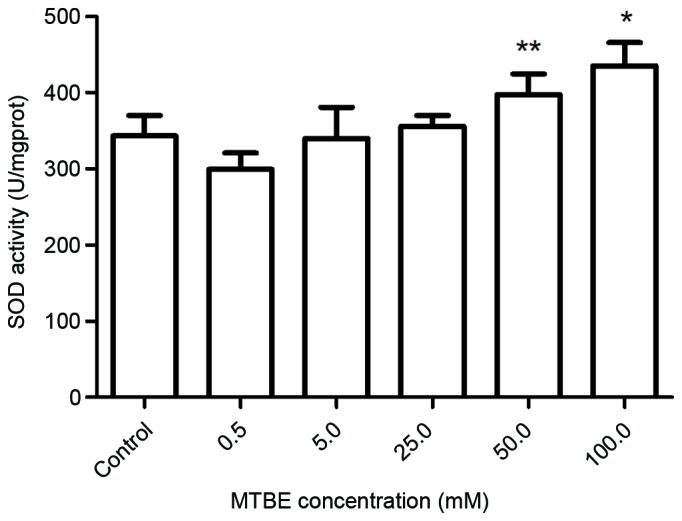
Effect of MTBE on SOD activity in Chinese hamster ovary cells. Following 12 h exposure to various concentrations of MTBE, the SOD activity was assessed. The data are presented as the mean ± standard deviation (*P<0.05 and **P<0.01 vs. the control). MTBE, methyl tert-butyl ether; SOD, superoxide dismutase.
Figure 5.
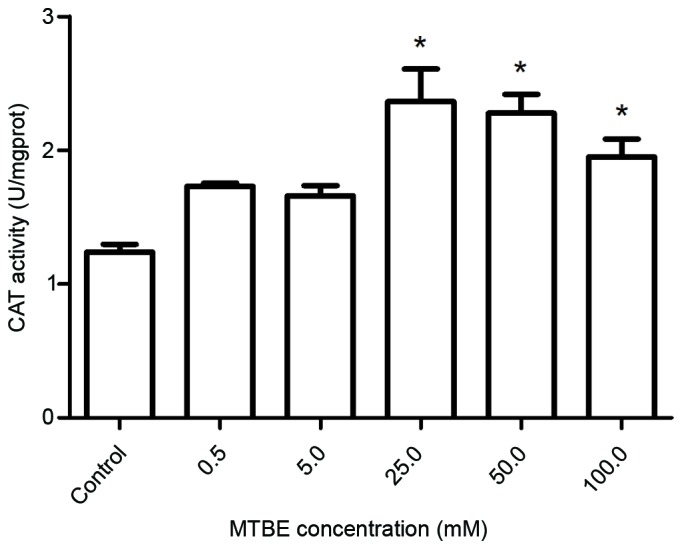
Effect of MTBE on CAT activity in Chinese hamster ovary cells. Following 12 h exposure to various concentrations of MTBE, the CAT activity was assessed. The data are presented as the mean ± standard deviation (*P<0.05 vs. control). MTBE, methyl tert-butyl ether; CAT, catalase.
Figure 6.
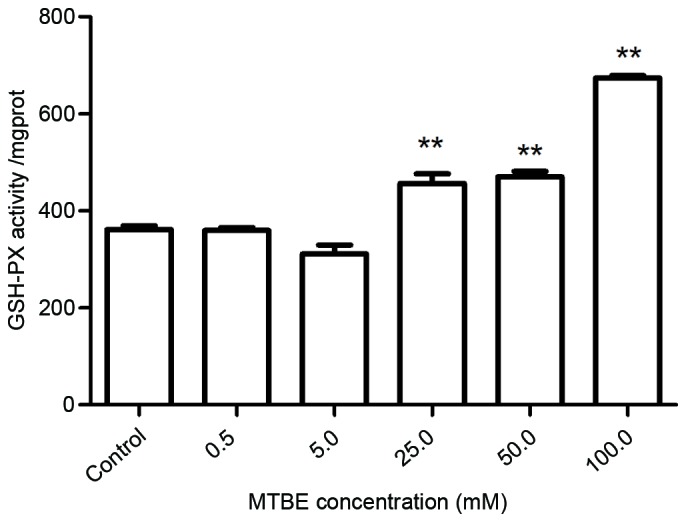
Effect of MTBE on GSH-Px activity in Chinese hamster ovary cells. Following 12 h exposure to various concentrations of MTBE, the GSH-PX activity was assessed. The data are presented as the mean ± standard deviation (**P<0.01 vs. control). MTBE, methyl tert-butyl ether; GSH-Px, glutathione peroxidase.
Effect of MTBE exposure on the CHO cell proteome
To assess the effect of MTBE exposure on protein profiles, a quantitative proteomic analysis by 2DE was performed between 50.0 mM MTBE-treated CHO cells and the untreated control cells. Comparative analysis of the protein profiles was performed between the four test groups using DeCyder software (version 6.5; Fig. 7). The matching of protein spots was corrected by inserting landmarks. With this labeling strategy, it is possible to analyze all biological replicates in a single experimental run. Following 2-D DIGE analysis, protein spots demonstrating ≥1.2-fold differential expression were selected for MS/MS identification (30).
Figure 7.
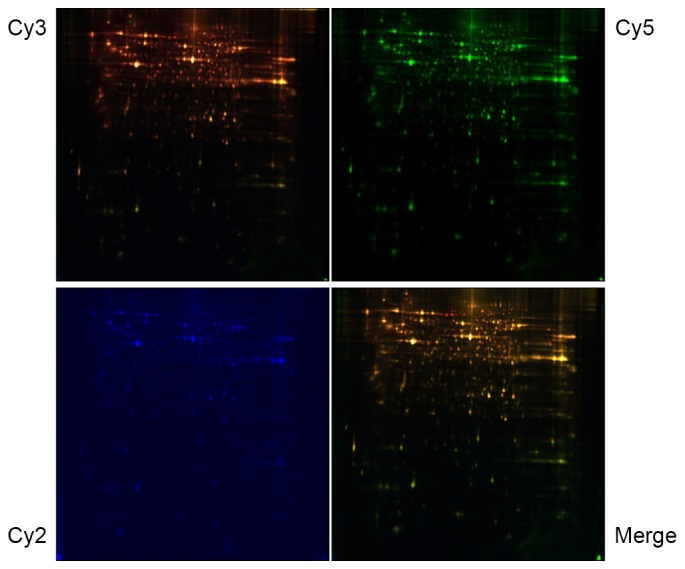
Representative two-dimensional difference gel electrophoresis (DIGE) images. Samples treated with 50.0 mM methyl tert-butyl ether or control samples were labeled with Cy3 and Cy5, respectively, while the protein reference pool (internal standard) was labeled with Cy2. The images were scanned at excitation/emission wave length, 488/520 (Cy2), 532/580 (Cy3), and 633/670 (Cy5) nm. The green spots represent upregulated proteins, while the red spots represent downregulated proteins in treated groups. Cy, cyanine.
A total of 27 protein spots demonstrated statistically significant differences between the 50.0 mM MTBE-exposed CHO cells and untreated control cells, with 19 spots were downregulated and 8 spots were upregulated in 50.0 mM MTBE-exposed CHO cells compared with the control cells (P<0.05; Table I; Fig. 8). The spots were digested with trypsin and MALDI-TOF/TOF mass spectrometery was applied to obtain high-resolution peptide mass fingerprints and peptide fragment fingerprints. The proteins were subsequently identified by searching the SwissProt database. Of the upregulated spots, 2 were identified as ATP synthase subunit β (Atp5b; Table I), 2 were identified as heat shock protein family A (Hsp70) member 8 (Hspa8; Table I) and 2 were identified as β-actin (Actb; Table I).
Table I.
Differentially expressed proteins of membrane fractions from CHO cells following MTBE exposure.
| Entry | Protein name | Gene name | Accession no. | Score | Mw/PI | AV ratio | P-valuea |
|---|---|---|---|---|---|---|---|
| 1 | 40S ribosomal protein SA | Rpsa | RSSA_MOUSE | 254 | 32,931/4.80 | 2.02 | 0.03000 |
| 2 | ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide | Atp5b | ATPB_MOUSE | 878 | 56,265/5.19 | 1.80 | 0.04400 |
| ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide | Atp5b | ATPB_MOUSE | 407 | 56,265/5.19 | 1.72 | 0.01500 | |
| 3 | Heat shock protein family A member 9 | Hspa9 | GRP75_MOUSE | 88 | 73,768/5.91 | 1.77 | 0.04700 |
| 4 | Eukaryotic translation elongation factor 1 delta | Eef1d | EF1D_MOUSE | 107 | 31,388/4.91 | 1.70 | 0.04400 |
| 5 | Heat shock protein family A (Hsp70) member 8 | Hspa8 | HSP7C_MOUSE | 100 | 71,055/5.37 | 1.61 | 0.01100 |
| Heat shock protein family A (Hsp70) member 8 | Hspa8 | HSP7C_MOUSE | 171 | 71,055/5.37 | 1.52 | 0.04400 | |
| 6 | Beta-actin n, cytoplasmic | Actb | ACTB_MOUSE | 103 | 42,052/5.29 | 1.52 | 0.04400 |
| Beta-actin, cytoplasmic | Actb | ACTB_MOUSE | 384 | 42,052/5.29 | 1.25 | 0.02300 | |
| 7 | Beta-actin -like protein 2 | Actbl2 | ACTBL_MOUSE | 53 | 42,319/5.30 | 1.52 | 0.04400 |
| 8 | Heterogeneous nuclear ribonucleoprotein K | Hnrnpk | HNRPK_MOUSE | 261 | 51,230/5.39 | 1.48 | 0.01200 |
| 9 | T-complex protein 1 subunit α | Tcp1 | TCPA_MOUSE | 72 | 60,867/5.82 | 1.31 | 0.03900 |
| 10 | Ubiquinol-cytochrome c reductase core protein I | Uqcrc1 | QCR1_MOUSE | 189 | 53,446/5.81 | 1.29 | 0.03500 |
| 11 | FK506 binding protein 4 | Fkbp4 | FKBP4_MOUSE | 133 | 51,939/5.54 | 1.27 | 0.00210 |
| 12 | Pyruvate kinase, muscle, isozymes M1/M2 | Pkm | KPYM_MOUSE | 31 | 58,378/7.18 | 1.26 | 0.04400 |
| 13 | Heat shock protein family D (Hsp60) member 1 | Hspd1 | CH60_MOUSE | 121 | 61,088/5.91 | 1.25 | 0.03600 |
| 14 | Enolase 1 | Eno1 | ENOA_MOUSE | 206 | 47,453/6.37 | 1.22 | 0.00740 |
| 15 | Eukaryotic translation initiation factor 4E | Eif4e | IF4E_MOUSE | 201 | 25,266/5.79 | 1.22 | 0.03900 |
| 16 | Protein disulfide isomerase family A member 3 | Pdia3 | PDIA3_MOUSE | 207 | 57,099/5.88 | 1.21 | 0.04800 |
| 17 | Actin, α2, smooth muscle, aorta | Acta2 | ACTA_MOUSE | 117 | 42,381/5.23 | −7.30 | 0.02300 |
| 18 | Peptidylprolyl isomerase A | Ppia | PPIA_MOUSE | 91 | 18,131/7.74 | −2.18 | 0.00073 |
| 19 | Calponin 3 | Cnn3 | CNN3_MOUSE | 142 | 36,577/5.46 | −1.29 | 0.02700 |
| 20 | ENY2, transcription and export complex 2 subunit | Eny2 | ENY2_MOUSE | 18 | 11,635/9.39 | −1.26 | 0.03700 |
| 21 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1, cardiac muscle | Atp5a1 | ATPA_MOUSE | 627 | 59,830/9.22 | −1.25 | 0.02200 |
| 22 | Histone cluster 1 H2B family member B | Hist1h2bb | H2B1B_MOUSE | 96 | 13,944/10.31 | −1.23 | 0.00047 |
| 23 | Receptor for activated C kinase 1 | Gnb2l1 | GBLP_MOUSE | 31 | 35,511/7.60 | −1.22 | 0.03600 |
| 24 | Nudix hydrolase 17 | Nudt17 | NUD17_MOUSE | 29 | 33,116/5.44 | −1.21 | 0.01700 |
P-value was obtained by t-test. Mw, molecular weight PI, isoelectric point; AV ratio, average ratio.
Figure 8.

Differentially expressed protein spots identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. The differentially expressed proteins were marked on the two-dimensional gels and numbered.
Functional categories of identified proteins
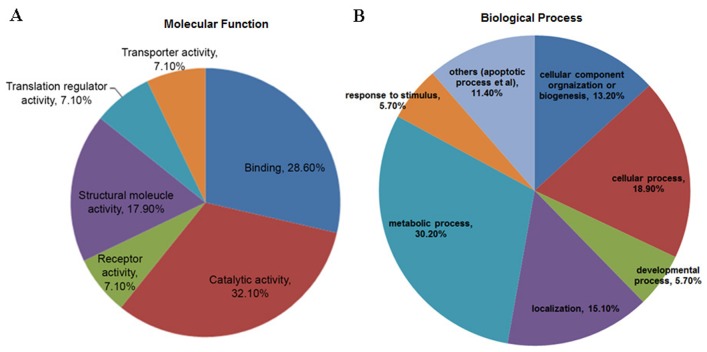
The 24 identified proteins were classified with respect to their molecular functions (Fig. 9A) and biological processes (Fig. 9B), according to PANTHER (28). The most dominant function that the identified proteins were involved in was catalytic activity (32.1%), followed by binding (28.6%) and structural molecule activity (17.9%; Fig. 9A). The most dominant biological processes included metabolic processes (30.2%), cell processes (18.9%) and localization (15.1%).
Figure 9.
Functional classifications of the identified proteins according to (A) molecular function and (B) biological processes, as determined by Protein Analysis Through Evolutionary Relationships.
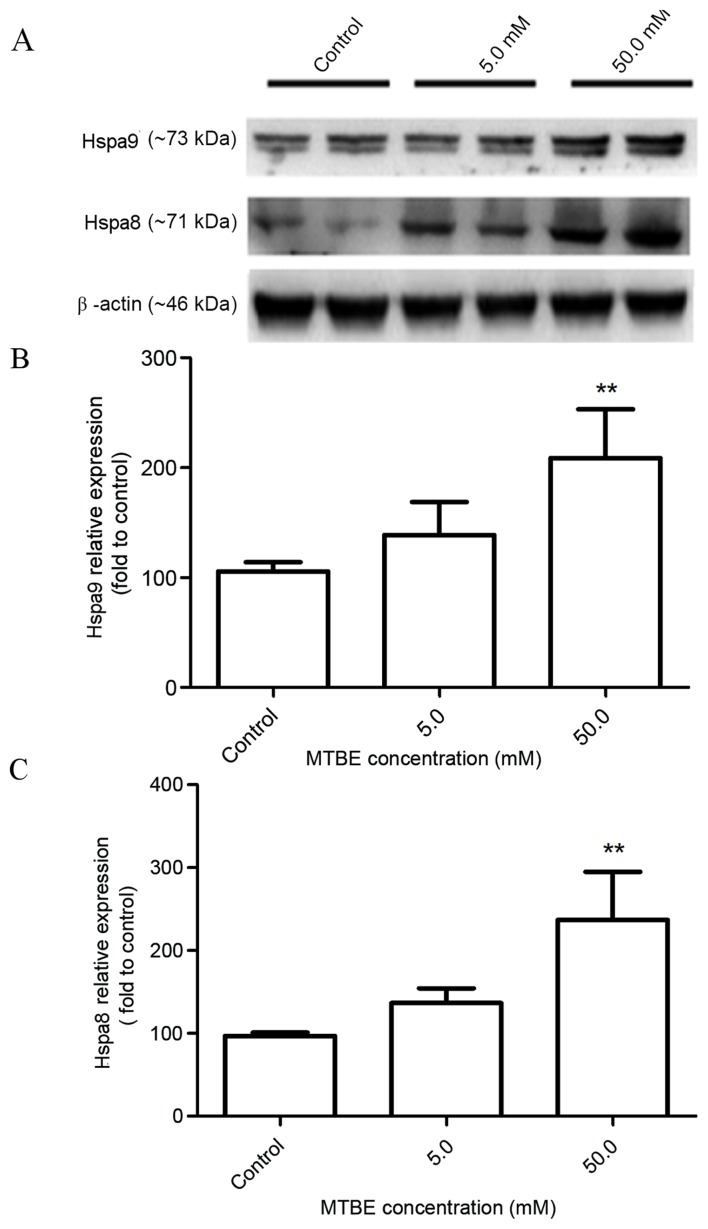
Confirmation of differential expression levels of GRP7 and Hspa8 in MTBE-induced CHO cells
Hspa9 and Hspa8 were selected as representative proteins and subjected to western blot analysis to confirm the differential expression levels (Fig. 10A). The protein expression levels of Hspa9 and Hspa8 were significantly upregulated in CHO cells following 12 h MTBE exposure (50.0. mM) compared with the control cells (P<0.01 and P<0.01, respectively, Fig. 10B and C, respectively). These results were consistent with the 2DE data (Table I).
Figure 10.
Expression levels of Hspa proteins following treatment with MTBE. (A) Differential expression of Hspa9 and Hspa8 following 12 h exposure to MTBE, as confirmed by western blot analysis. β-actin was included as an internal control. The protein expression of (B) Hspa9 and (C) Hspa8 was quantified, relative to β-actin. The data are presented as the mean ± standard deviation (n=3; **P<0.01 vs. control). Hspa, heat shock protein family A (Hsp70); MTBE, methyl tert-butyl ether.
Discussion
MTBE is primarily used as an oxygenating agent in gasoline to reduce harmful emissions. Occupational MTBE exposure affects all workers exposed to gasoline, and environmental MTBE exposure (in the air and groundwater) affects the general population (31,32). Although available data demonstrate conflicting results, there is evidence that MTBE is a toxic substance that has harmful effects in vivo and in vitro, with MTBE exposure reported to induce reproductive toxicity in multiple cell lines (14,15,33–35). In vitro cell systems are an excellent model for studying the molecular mechanisms of toxicity since they allow rapid and reliable results that can be easily confirmed without using laboratory animals. Numerous measurement endpoints for cytotoxicity have been established and the results were used to assess the basal cytotoxicity (36,37). Consistent results from previous in vitro studies led to higher tier in vivo tests and, eventually, to hazard identification and classification. In the present study, MTBE-induced effects on cytotoxicity and oxidative stress were investigated in CHO cells; an ovary cell line widely used for assessing chemical-induced cytotoxicity. Furthermore, the protein profile of MTBE-exposed and non-exposed CHO cells was analyzed using a proteomic approach, providing data at a molecular level, which is useful for more informative risk assessment and mechanism studies.
MTBE significantly inhibited CHO cell viability following 6, 12 and 24 h exposure, with the cell viability decreasing as result of cytotoxicity. In the present study, the concentration of MTBE ranged between 0.50 and 100.0 mM, with 100.0 mM being lower compared with the 210.0 mM used in previous studies on MTBE toxicity (23). By comparing cell viability following 6, 12 and 24 h exposure, 12 h MTBE exposure was selected for further analysis as it induced the highest inhibition of cell viability (Fig. 1). It is hypothesized that 12 h produced greater inhibition as MTBE is highly volatile and the concentration of MTBE is decreased in a time-dependent manner. LDH is a stable cytoplasmic enzyme that is present in all cells, and it is rapidly released into the cell culture media when the plasma membrane is damaged. Increased LDH release in CHO cells revealed that MTBE exposure alters cell membrane properties (Fig. 2) (25). Increased plasma membrane damage may directly induce cell necrosis, resulting in the significantly increased ratio of cell necrosis and apoptosis demonstrated at higher concentrations. The data obtained in the present study indicated that MTBE exposure has a direct cytotoxic effect, decreasing cell viability and inducing plasma membrane damage in CHO cells.
Oxidative stress is considered to be an important mechanism underlying MTBE-induced reproductive toxicity (14,19,38). The extent of membrane lipid peroxidation was estimated by measuring MDA formation (39), which is a byproduct of lipid peroxidation considered to be a biomarker of cellular damage (40). MTBE exposure significantly increased the MDA content, implying an increased peroxide level in CHO cells. In addition, MTBE exposure induced significant increases in antioxidant enzyme activities, including SOD, CAT and GSH-Px. The apparent reverse concentration-response association observed in CAT activity levels at higher MTBE concentrations may be due to the low solubility and high volatility of MTBE. The above results demonstrated that MTBE exposure exerted oxidative stress in CHO cells, which may be the underlying mechanism of MTBE-induced cytotoxicity. Cellular oxidative status is determined by the balance between the production of ROS and their destruction by various antioxidant enzymes. In particular, ROS are scavenged continuously by antioxidative enzymes, including SOD, CAT and GSH-Px (41). SOD is an important antioxidant that eliminates excess cellular oxidants. CAT is a key antioxidant enzyme in the body's defense against oxidative stress, converting hydrogen peroxide to water and oxygen and thereby mitigating the cytotoxic effects of hydrogen peroxide (42). GSH-Px also functions in the detoxification of hydrogen peroxide, and provides an effective protection mechanism against cytosolic injury by eliminating H2O2 and lipid peroxides (43). Therefore, by measuring the levels of these antioxidative enzymes, the status of oxidative stress in cells can be indirectly indicated. ROS production was observed to decrease following MTBE exposure (data not shown), which may be trapped and scavenged by endogenous antioxidants including SOD, CAT and GSH-Px, which may be overexpressed in response to MTBE exposure. The enhanced MDA levels and increased activities of SOD, CAT and GSH-Px indicated that increased oxidative stress contributes to the cytotoxicity of MTBE.
High-throughput techniques, including proteomics, provide effective strategies for toxicological studies and are regarded as a powerful tool to investigate cellular responses to environmental and occupational pollutants (44). In the present study, 2-D DIGE technology, in combination with mass spectrometry, was applied to detect differentially expressed proteins following MTBE exposure. Differentially expressed proteins were subsequently analyzed by bioinformatics and confirmed by western blot analysis. As a result, 24 proteins associated with MTBE-induced toxicity were identified. Of these, 16 were upregulated and 8 were downregulated in CHO cells following MTBE exposure. Using bioinformatics analysis (28), the identified proteins were classified into different groups based on their molecular functions and biological processes. The most dominant function of the identified protein was the catalytic activity, followed by binding and structural molecule activity. The most dominant biological processes included metabolic processes, cellular process and localization. These results demonstrated the complexity of the mechanisms underlying MTBE cytotoxicity in CHO cells.
Cellular stress, including oxidative stress, activates the nuclear factor, erthryroid 2 like 2 (Nrf2) pathway. Under oxidative stress Nrf2 dissociates from kelch-like ECH associated protein 1 and translocates into the nucleus, activating target genes by heterodimerizing with small MAF transcription factors and binding to antioxidant-responsive elements (45–47). Activation of both Nrf2 and transcription factors coordinates and regulates the convergence of oxidative stress signaling (48). In the present study, transcription factors, including enhancer of yellow 2 transcription factor homolog were identified as being differentially expressed following MTBE exposure. The stress-activated mitogen activated protein kinase (MAPK) pathway, which involves c-Jun NH2-terminal kinase, p38 and MAPK2, is highly conserved and transduces cellular information in response to a variety of distinct signals (49). Cells experience an accumulation of misfolded and aggregated proteins when exposed to stress. Protective mechanisms involve the rapid induction of heat shock protein (HSP) expression, and activation of MAPK signaling pathways. Together, the activities directed by HSPs and MAPKs influence cellular responses to oxidative stress. Several previous studies have demonstrated the direct inhibition of pro-apoptotic stress signaling and promotion of pro-survival pathways by HSPs (50,51). Therefore, the present study focused on HSPs, selecting two for confirmation by western blot analysis.
Hspa9 is a highly conserved member of the heat shock cognate protein family and is observed in multiple subcellular sites (52). It is involved in multiple physiological functions, including mitochondrial import, antigen processing, and the control of cell proliferation and differentiation (53). Hspa9 overexpression was previously demonstrated to significantly inhibit H2O2 exposure-induced ROS accumulation in liver cells (54). In the present study, MTBE exposure was revealed to upregulate the expression of Hspa9 in both proteomic and western blot analysis. Hspa8 is constitutively expressed and is involved in housekeeping functions associated with chaperone-mediated autophagy and prevention from protein aggregation under stress conditions (55), and overexpression of Hspa8 is also known to be associated with necrosis (56). The majority of cells possess intrinsic defense mechanisms that protect against oxidative stress/injury. Hspa8 has been demonstrated to be involved in the signal transduction and apoptosis process, and translocates to the nucleus following exposure to heat shock (57,58). Upregulation of Hspa8 in leukemic cells was demonstrated to contribute to cell cycle disruption and lead to cell proliferation (59,60). Hspa8 was also revealed to contribute to the regulation of cancer cell growth by secretion in the extracellular environment (61). Abnormal Hspa8 expression has also been observed during stress responses following exposure to linear alkylbenzene sulfonate and other chemical pollutants (62,63). In the present study, upregulation of Hspa8 and Hspa9 revealed that MTBE exposure induced affected HSP expression. When taken together, the results of the present study, including the increase in MDA content and SOD, CAT and GSH-Px activity, suggested that a redox imbalance occurs in CHO cells following MTBE exposure.
In conclusion, the primary aim of the present study was to examine cytotoxicity and protein expression alteration in CHO cells following MTBE exposure. MTBE exposure induced significant effects on cell viability, LDH leakage, plasma membrane damage and the activities of antioxidant enzymes. In the proteomic analysis, 24 proteins were demonstrated to be significantly modulated by MTBE exposure. Functional analysis indicated that these proteins were involved in catalytic activity, binding, structural molecule activity, metabolic and cellular processes, and localization, highlighting the fact that the cytotoxic mechanisms of MTBE are complex and diverse. The altered expression of two representative proteins, Hspa9 and Hspa8, was further confirmed by western blot analysis. These results revealed the effect of MTBE exposure on protein expression in CHO cells, and the data indicated that oxidative stress and altered protein expression constituted the mechanisms underlying MTBE-induced cytotoxicity. Therefore, the present study provided useful scientific information and enhanced the understanding of the molecular mechanisms underlying MTBE-induced oxidative stress and reproductive toxicity
Acknowledgements
The present study was supported by the Upgrade Scheme of Shenzhen Municipal Key Laboratory (no. JCYJ20130401102255980).
References
- 1.Mehlman MA. Misclassification of carcinogenic methyl tertiary butyl ether (MTBE) by the National Toxicology Program Board: Smokescreen in, science out? Arch Environ Health. 2000;55:73–74. doi: 10.1080/00039890009603389. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed FE. Toxicology and human health effects following exposure to oxygenated or reformulated gasoline. Toxicol Lett. 2001;123:89–113. doi: 10.1016/S0378-4274(01)00375-7. [DOI] [PubMed] [Google Scholar]
- 3.Phillips S, Palmer RB, Brody A. Epidemiology, toxicokinetics, and health effects of methyl tert-butyl ether (MTBE) J Med Toxicol. 2008;4:115–126. doi: 10.1007/BF03160966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayotte JD, Argue DM, McGarry FJ, Degnan JR, Hayes L, Flanagan SM, Helsel DR. Methyl tert-butyl ether (MTBE) in public and private wells in New Hampshire: Occurrence, factors, and possible implications. Environ Sci Technol. 2008;42:677–684. doi: 10.1021/es071519z. [DOI] [PubMed] [Google Scholar]
- 5.Hakkola MA, Saarinen LH. Customer exposure to gasoline vapors during refueling at service stations. Appl Occup Environ Hyg. 2000;15:677–680. doi: 10.1080/10473220050110086. [DOI] [PubMed] [Google Scholar]
- 6.Lindstrom AB, Pleil JD. Alveolar breath sampling and analysis to assess exposures to methyl tertiary butyl ether (MTBE) during motor vehicle refueling. J Air Waste Manag Assoc. 1996;46:676–682. doi: 10.1080/10473289.1996.10467502. [DOI] [PubMed] [Google Scholar]
- 7.Prah J, Ashley D, Blount B, Case M, Leavens T, Pleil J, Cardinali F. Dermal, oral, and inhalation pharmacokinetics of methyl tertiary butyl ether (MTBE) in human volunteers. Toxicol Sci. 2004;77:195–205. doi: 10.1093/toxsci/kfh009. [DOI] [PubMed] [Google Scholar]
- 8.Hong JY, Yang CS, Lee M, Wang YY, Huang WQ, Tan Y, Patten CJ, Bondoc FY. Role of cytochromes P450 in the metabolism of methyl tert-butyl ether in human livers. Arch Toxicol. 1997;71:266–269. doi: 10.1007/s002040050386. [DOI] [PubMed] [Google Scholar]
- 9.Shamsipur M, Beigi AA Miran, Teymouri M, Poursaberi T, Mostafavi SM, Soleimani P, Chitsazian F, Tash SA. Biotransformation of methyl tert-butyl ether by human cytochrome P450 2A6. Biodegradation. 2012;23:311–318. doi: 10.1007/s10532-011-9510-0. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler N, Kelly-McNeil K, Mohr S, Lehrer P, Opiekun RE, Lee C, Wainman T, Hamer R, Weisel C, Edelberg R, Lioy PJ. Controlled human exposure to methyl tertiary butyl ether in gasoline: Symptoms, psychophysiologic and neurobehavioral responses of self-reported sensitive persons. Environ Health Perspect. 2000;108:753–763. doi: 10.1289/ehp.00108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belpoggi F, Soffritti M, Filippini F, Maltoni C. Results of long-term experimental studies on the carcinogenicity of methyl tert-butyl ether. Ann N Y Acad Sci. 1997;837:77–95. doi: 10.1111/j.1749-6632.1997.tb56865.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Yuan D, Huang G, Zhang H, Ye S. Mutagenicity of methyl tertiary butyl ether. J Environ Pathol Toxicol Oncol. 2000;19:35–39. [PubMed] [Google Scholar]
- 13.McGregor D. Methyl tertiary-butyl ether: Studies for potential human health hazards. Crit Rev Toxicol. 2006;36:319–358. doi: 10.1080/10408440600569938. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Yin D, Han X. Methyl tert-butyl ether (MTBE)-induced cytotoxicity and oxidative stress in isolated rat spermatogenic cells. J Appl Toxicol. 2007;27:10–17. doi: 10.1002/jat.1178. [DOI] [PubMed] [Google Scholar]
- 15.Li DM, Han XD. Evaluation of toxicity of methyl tert-butyl ether (MTBE) on mouse spermatogenic cells in vitro. Toxicol Ind Health. 2006;22:291–299. doi: 10.1177/0748233706070310. [DOI] [PubMed] [Google Scholar]
- 16.Iavicoli I, Carelli G, Ardito R, Cittadini A, Sgambato A. Methyl-tertiary-butyl ether (MTBE) inhibits growth and induces cell transformation in rodent fibroblasts. Anticancer Res. 2002;22:2173–2177. [PubMed] [Google Scholar]
- 17.Chen CS, Hseu YC, Liang SH, Kuo JY, Chen SC. Assessment of genotoxicity of methyl-tert-butyl ether, benzene, toluene, ethylbenzene, and xylene to human lymphocytes using comet assay. J Hazard Mater. 2008;153:351–356. doi: 10.1016/j.jhazmat.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 18.Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. Interpreting stress responses during routine toxicity studies: A review of the biology, impact, and assessment. Toxicol Pathol. 2013;41:560–614. doi: 10.1177/0192623312466452. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Liu Q, Gong Y, Huang Y, Han X. Cytotoxicity and oxidative stress study in cultured rat Sertoli cells with methyl tert-butyl ether (MTBE) exposure. Reprod Toxicol. 2009;27:170–176. doi: 10.1016/j.reprotox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Huang QY, Huang HQ. Differential expression profile of membrane proteins in zebrafish (Danio rerio) brain exposed to methyl parathion. Proteomics. 2011;11:3743–3756. doi: 10.1002/pmic.201100084. [DOI] [PubMed] [Google Scholar]
- 21.Merrick BA, Witzmann FA. The role of toxicoproteomics in assessing organ specific toxicity. EXS. 2009;99:367–400. doi: 10.1007/978-3-7643-8336-7_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nihlén A, Löf A, Johanson G. Controlled ethyl tert-butyl ether (ETBE) exposure of male volunteers. I. Toxicokinetics. Toxicol Sci. 1998;46:1–10. doi: 10.1006/toxs.1998.2516. [DOI] [PubMed] [Google Scholar]
- 23.Krayl M, Benndorf D, Loffhagen N, Babel W. Use of proteomics and physiological characteristics to elucidate ecotoxic effects of methyl tert-butyl ether in Pseudomonas putida KT2440. Proteomics. 2003;3:1544–1552. doi: 10.1002/pmic.200300477. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Grant RL, Yao C, Gabaldon D, Acosta D. Evaluation of surfactant cytotoxicity potential by primary cultures of ocular tissues: I. Characterization of rabbit corneal epithelial cells and initial injury and delayed toxicity studies. Toxicology. 1992;76:153–176. doi: 10.1016/0300-483X(92)90162-8. [DOI] [PubMed] [Google Scholar]
- 26.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013;41(Database issue):D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 30.Tonge R, Shaw J, Middleton B, Rowlinson R, Rayner S, Young J, Pognan F, Hawkins E, Currie I, Davison M. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1:377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Vainiotalo S, Ruonakangas A. Tank truck driver exposure to vapors from oxygenated or reformulated gasolines during loading and unloading. Am Ind Hyg Assoc J. 1999;60:518–525. doi: 10.1080/00028899908984473. [DOI] [PubMed] [Google Scholar]
- 32.Lin CW, Chiang SB, Lu SJ. Investigation of MTBE and aromatic compound concentrations at a gas service station. Environ Monit Assess. 2005;105:327–339. doi: 10.1007/s10661-005-4334-1. [DOI] [PubMed] [Google Scholar]
- 33.Belpoggi F, Soffritti M, Maltoni C. Methyl-tertiary-butyl ether (MTBE)-a gasoline additive-causes testicular and lymphohaematopoietic cancers in rats. Toxicol Ind Health. 1995;11:119–149. doi: 10.1177/074823379501100202. [DOI] [PubMed] [Google Scholar]
- 34.Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, Andrews LS. Oncogenicity studies of inhaled methyl tertiary-butyl ether (MTBE) in CD-1 mice and F-344 rats. J Appl Toxicol. 1997;17(Suppl 1):S45–S55. doi: 10.1002/(SICI)1099-1263(199705)17:1+<S45::AID-JAT410>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Clegg ED, Cook JC, Chapin RE, Foster PM, Daston GP. Leydig cell hyperplasia and adenoma formation: Mechanisms and relevance to humans. Reprod Toxicol. 1997;11:107–121. doi: 10.1016/S0890-6238(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 36.Knox P, Uphill PF, Fry JR, Benford J, Balls M. The FRAME multicentre project on in vitro cytotoxicology. Food Chem Toxicol. 1986;24:457–463. doi: 10.1016/0278-6915(86)90092-X. [DOI] [PubMed] [Google Scholar]
- 37.Putnam KP, Bombick DW, Doolittle DJ. Evaluation of eight in vitro assays for assessing the cytotoxicity of cigarette smoke condensate. Toxicol In Vitro. 2002;16:599–607. doi: 10.1016/S0887-2333(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Yuan C, Gong Y, Huang Y, Han X. The effects of methyl tert-butyl ether (MTBE) on the male rat reproductive system. Food Chem Toxicol. 2008;46:2402–2408. doi: 10.1016/j.fct.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Hu F, Wang C, Wang X. Enantioselective induction of oxidative stress by acetofenate in rat PC12 cells. J Environ Sci (China) 2010;22:1980–1986. doi: 10.1016/S1001-0742(09)60349-1. [DOI] [PubMed] [Google Scholar]
- 41.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Murthy MR, Reid TJ, III, Sicignano A, Tanaka N, Rossmann MG. Structure of beef liver catalase. J Mol Biol. 1981;152:465–499. doi: 10.1016/0022-2836(81)90254-0. [DOI] [PubMed] [Google Scholar]
- 43.Muse KE, Oberley TD, Sempf JM, Oberley LW. Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochem J. 1994;26:734–753. doi: 10.1007/BF00158205. [DOI] [PubMed] [Google Scholar]
- 44.Dowling VA, Sheehan D. Proteomics as a route to identification of toxicity targets in environmental toxicology. Proteomics. 2006;6:5597–5604. doi: 10.1002/pmic.200600274. [DOI] [PubMed] [Google Scholar]
- 45.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto N, Izumi H, Miyamoto R, Bin H, Kondo H, Tawara A, Sasaguri Y, Kohno K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Invest Ophthalmol Vis Sci. 2011;52:1226–1234. doi: 10.1167/iovs.10-5775. [DOI] [PubMed] [Google Scholar]
- 49.Gonda RL, Garlena RA, Stronach B. Drosophila heat shock response requires the JNK pathway and phosphorylation of mixed lineage kinase at a conserved serine-proline motif. PLoS One. 2012;7:e42369. doi: 10.1371/journal.pone.0042369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beere HM. ‘The stress of dying’: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 51.Dorion S, Landry J. Activation of the mitogen-activated protein kinase pathways by heat shock. Cell Stress Chaperones. 2002;7:200–206. doi: 10.1379/1466-1268(2002)007<0200:AOTMAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadhwa R, Kaul SC, Sugimoto Y, Mitsui Y. Induction of cellular senescence by transfection of cytosolic mortalin cDNA in NIH 3T3 cells. J Biol Chem. 1993;268:22239–22242. [PubMed] [Google Scholar]
- 53.Kaula SC, Reddelb RR, Sugiharac T, Mitsuia Y, Wadhwac R. Inactivation of p53 and life span extension of human diploid fibroblasts by mot-2. FEBS Lett. 2000;474:159–164. doi: 10.1016/S0014-5793(00)01594-5. [DOI] [PubMed] [Google Scholar]
- 54.E Q, Liu X, Liu Y, Liu W, Zuo J. Over-expression of GRP75 inhibits liver injury induced by oxidative damage. Acta Biochim Biophys Sin (Shanghai) 2013;45:129–134. doi: 10.1093/abbs/gms098. [DOI] [PubMed] [Google Scholar]
- 55.Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 56.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 57.Dastoor Z, Dreyer J. Nuclear translocation and aggregate formation of heat shock cognate protein 70 (Hsc70) in oxidative stress and apoptosis. J Cell Sci. 2000;113:2845–2854. doi: 10.1242/jcs.113.16.2845. [DOI] [PubMed] [Google Scholar]
- 58.Lagunas L, Bradbury CM, Laszlo A, Hunt CR, Gius D. Indomethacin and ibuprofen induce Hsc70 nuclear localization and activation of the heat shock response in HeLa cells. Biochem Biophys Res Commun. 2004;313:863–870. doi: 10.1016/j.bbrc.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 59.José-Enériz ES, Román-Gómez J, Cordeu L, Ballestar E, Gárate L, Andreu EJ, Isidro I, Guruceaga E, Jiménez-Velasco A, Heiniger A, et al. BCR-ABL1-induced expression of HSPA8 promotes cell survival in chronic myeloid leukaemia. Br J Haematol. 2008;142:571–582. doi: 10.1111/j.1365-2141.2008.07221.x. [DOI] [PubMed] [Google Scholar]
- 60.Voisin PJ, Pardue S, Macouillard F, Yehia G, Labouesse J, Morrison-Bogorad M. Differential expression of heat shock 70 proteins in primary cultures from rat cerebellum. Brain Res. 1996;739:215–234. doi: 10.1016/S0006-8993(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 61.Nirdé P, Derocq D, Maynadier M, Chambon M, Basile I, Gary-Bobo M, Garcia M. Heat shock cognate 70 protein secretion as a new growth arrest signal for cancer cells. Oncogene. 2010;29:117–127. doi: 10.1038/onc.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friha I, Bradai M, Johnson D, Hilal N, Loukil S, Ben Amor F, Feki F, Han J, Isoda H, Sayadi S. Treatment of textile wastewater by submerged membrane bioreactor: In vitro bioassays for the assessment of stress response elicited by raw and reclaimed wastewater. J Environ Manage. 2015;160:184–192. doi: 10.1016/j.jenvman.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Bradai M, Han J, El Omri A, Funamizu N, Sayadi S, Isoda H. Cytotoxic effect of linear alkylbenzene sulfonate on human intestinal Caco-2 cells: Associated biomarkers for risk assessment. Environ Sci Pollut Res Int. 2014;21:10840–10851. doi: 10.1007/s11356-014-3074-6. [DOI] [PubMed] [Google Scholar]