Abstract
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease. However, the pathogenesis of DKD remains unclear, and no effective treatments for the disease are available. Thus, there is an urgent need to elucidate the pathogenic mechanisms of DKD and to develop more effective therapies for this disease. Human umbilical vein endothelial cells (HUVECs) were cultured using different D-glucose concentrations to determine the effect of high glucose (HG) on the cells. Alternatively, HUVECs were incubated with 100 µmol/l calcium dobesilate (CaD) to detect its effects. The authors subsequently measured HUVEC proliferation via cell counting kit-8 assays. In addition, HUVEC angiogenesis was investigated via migration assays and fluorescein isothiocyanate (FITC)-labelled bovine serum albumin (BSA) permeability assays. The content or distribution of markers of endothelial dysfunction [vascular endothelial growth factor (VEGF), VEGF receptor (R) and endocan) or inflammation [intercellular adhesion molecule (ICAM)-1, monocyte chemotactic protein (MCP)-1 and pentraxin-related protein (PTX3)] was evaluated via reverse transcription-quantitative polymerase chain reaction and western blotting. HG treatment induced increased in VEGF, VEGFR, endocan, ICAM-1, MCP-1 and PTX3 mRNA and protein expression in HUVECs. HG treatment for 24 to 48 h increased cell proliferation in a time-dependent manner, but the cell proliferation rate was decreased at 72 h of HG treatment. Conversely, CaD inhibited abnormal cell proliferation. HG treatment also significantly enhanced HVUEC migration compared to the control treatment. In contrast, CaD treatment partially inhibited HUVEC migration compared to HG exposure. HG-treated HUVECs exhibited increased FITC-BSA permeability compared to control cells cultured in medium alone; however, CaD application prevented the HG-induced increase in FITC-BSA permeability and suppressed HG-induced overexpression of endothelial markers (VEGF, VEGFR-2, endocan) and inflammation markers (ICAM-1, MCP-1, PTX3) in HUVECs. CaD has angioprotective properties and protects endothelial cells partly by ameliorating HG-induced inflammation. The current results demonstrated the potential applicability of CaD to the treatment of diabetic nephropathy, particularly during the early stages of this disease.
Keywords: diabetic nephropathy, endothelial cell, calcium dobesilate, cell proliferation, microinflammation
Introduction
Diabetes is one of the greatest global health emergencies of the 21st century. In 2015, 415 million adults had diabetes, and this number is estimated to increase to 642 million in 2040 (1). Among the microvascular complications of the diabetes mellitus, diabetic kidney disease (DKD) is a leading cause of end-stage renal disease (2). However, the pathogenesis of DKD remains unclear, and no effective treatments for this disease are available. Thus, there is an urgent need to elucidate the pathogenic mechanisms underlying DKD and to develop more effective therapies for this disease.
Endothelial cells (ECs) serve critical roles in many physiological functions, including cell proliferation and survival, vascular tone modulation, blood cell trafficking, haemostatic balance, permeability status and innate and adaptive immunity. EC injury is a major event in diabetes and results in multiple macro- and microvascular complications (3). Microvascular complications involve small vessels, such as capillaries, whereas macrovascular complications primarily involve large vessels, such as arteries and veins. Nephropathy has been recognized as a common microvascular complication of diabetes, and the underlying EC dysfunction is often underestimated in cases of DKD (4).
Given the broad function of renal ECs and the complicated endothelial pathophysiology of DKD, inflammation biomarkers [interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α] are important tools in EC research (4). The microinflammatory state plays an important role in DKD development and progression (5,6). Adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), have also been reported to be upregulated in DKD (7,8), and these factors promote inflammatory cell adhesion to the endothelium and recruit circulating immune cells to diabetic kidneys.
Calcium dobesilate (CaD, calcium 2,5-dihydroxybenzenesulfonate) is considered an angioprotective drug that can reduce blood viscosity, platelet activity and capillary permeability, as well as alleviate microcirculatory and hemorheologic abnormalities (9). CaD is widely used to treat diabetic retinopathy (10), chronic venous insufficiency (11) and various microangiopathies (12). Moreover, recent studies have demonstrated that CaD exerts protective effects against diabetic nephropathy (13) and gentamicin-induced acute kidney injury (14). Despite its broad use, very little attention has been devoted to the molecular and cellular mechanisms underlying the vasculoprotective effects of CaD.
Thus, the aim of the present study was to elucidate the molecular and cellular mechanisms underlying the protective effects of CaD against diabetes-induced endothelial dysfunction and inflammation.
Materials and methods
Human umbilical vein endothelial cell (HUVEC) culture
Primary HUVECs and EC growth medium were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Culture flasks were coated with poly-l-lysine before use, and the cells were cultured in EC supplemented with EC growth supplement, 5% FBS and penicillin/streptomycin solution at 37°C in 5% CO2. HUVECS in the 2nd to 5th passages were used in all experiments.
Cell migration
A Transwell migration apparatus (24 wells, 6.5 mm internal diameter, 8 µm pore size; Corning Incorporated, Corning, NY, USA) was used to measure HUVEC migration. HUVECs (1×105 cells/well) were suspended in 100 µl complete medium and loaded into the upper chamber of the Transwell apparatus. The lower side of filter was coated with 0.1 mg/ml gelatin. Control or experimental medium was added to the lower chamber. Then, the chambers were incubated for 24 h at 37°C in 5% CO2. Afterwards, the non-migrated cells were removed from the top of the filter using cotton buds, whereas the migrated cells adhering to the bottom of the filter were fixed and stained with haematoxylin. The stained cells were counted in 10 randomly chosen fields at ×200 magnification. Each experiment was repeated three times (15).
Permeability assay
HUVECs were cultured on Transwell-COL membrane inserts (12 wells, 1 cm2 filter area, 0.4 µm pore size; Corning Incorporated). A total of 1×105 cells were suspended in 0.5 ml complete medium and added to the upper compartment, and 1.5 ml complete medium was added to the lower compartment. After reaching 90% confluence, the HUVECs were starved for 12 h in serum-free medium, which was subsequently replaced with control or experimental medium for 24 h. After the cells were washed three times with serum-free medium, a tracer solution of fluorescein isothiocyanate (FITC)-labelled bovine serum albumin (BSA, 250 µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in serum-free medium was added to the upper compartment of the Transwell-COL insert, and 1.5 ml medium without FITC-BSA was added to the lower compartment of the insert. Following 2 h of equilibration of the tracer solution, 100 µl medium was removed from the lower compartment. Sample fluorescence was measured using a fluorescence spectrofluorophotometer (Tecan Safire 2™; Tecan, Männedorf, Switzerland) at an emission/excitation wavelength of 495/520 nm, and the rate of albumin transfer across the monolayer was assessed by measuring the increase in FITC-BSA signal in the lower well after 2 h. Albumin flux across the monolayer was expressed as P=([A]2/txV)/(Sx([A]1-[A]2)), where V was the volume in the lower compartment, [A]2 was the albumin concentration in the lower compartment during the time interval t, [A]1 was the albumin concentration in the upper compartment, and S was the monolayer surface area. The data were expressed as the permeability ratio [P(experimental)/P(control)] (16). Each experiment was repeated in triplicate.
Cell counting kit-8 (CCK-8) assay
HUVECs were seeded in 96-well culture plates (1×104 cells/well). Following 24 h, the control or experimental medium was added. At 12, 24, 48 and 72 h, 10 µl CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well for 1 h. Absorbance values were recorded at a wavelength of 450 nm using a microplate reader (SpectraMax 250; GE Healthcare Life Sciences, Chalfont, UK).
VEGF, VEGFR, endocan, ICAM-1, MCP-1 and PTX3 mRNA expression
HUVECs were incubated in control or experimental medium for 12, 24, 48 and 72 h. Total RNA was extracted after medium removal using TRIzol reagent (Takara Bio Inc., Otsu, Japan) according to the manufacturer's instructions. Vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 2 (VEGFR), endocan, ICAM-1, monocyte chemotactic protein 1 (MCP-1) and pentraxin (PTX)3 mRNA expression was analysed via reverse transcription-quantitative polymerase chain reaction (RT-qPCR) using SYBR Green Master Mix (Takara Bio Inc.). RNA (1 µg) was reverse-transcribed to cDNA using random primers (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Moloney murine leukaemia virus (MMLV) reverse transcriptase (Takara Bio Inc.). RT-qPCR was performed using an Applied Biosystems TaqMan 7000 (Applied Biosystems; Thermo Fisher Scientific, Inc.) in 384-well plates containing 5 µl reaction mixtures consisting of 2.5 µl SYBR Green Master Mix, 0.1 µl each of forward and reverse primers, 0.1 µl Rox, 1.7 µl H2O and 0.5 µl cDNA. The amplification program consisted of 1 cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The primer sequences are presented in Table I. The relative mRNA levels in the samples were normalized to the mRNA level of GAPDH, and the data are expressed as fold increases in mRNA expression.
Table I.
Primer sequences.
| Gene | Primer sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| VEGF | Forward 5′-GGCCTTCGCTTACTCTCACC-3′ | 104 |
| Reverse 5′-CTGTCATGGGCTGCTTCTTC-3′ | ||
| VEGFR | Forward 5′-CCGTCAAGGGAAAGACTACG-3′ | 106 |
| Reverse 5′-CTCCTCCACAAATCCAGAGC-3′ | ||
| Endocan | Forward 5′-TGCTACCGCACAGTCTCA-3′ | 105 |
| Reverse 5′-GCAGATACCAAACTCTTCACCA-3′ | ||
| ICAM | Forward 5′-AAGTTGTTGGGCATAGAGACC-3′ | 126 |
| Reverse 5′-AGGGCAGTTTGAATAGCACATT-3′ | ||
| MCP-1 | Forward 5′-AAGTCTCTGCCGCCCTTCT-3′ | 170 |
| Reverse 5′-CTTGCTGCTGGTGATTCTTCTA-3′ | ||
| PTX3 | Forward 5′-CATCTCCTTGCGATTCTGTTT-3′ | 107 |
| Reverse 5′-CCATTGTCTATTTCGTTGTCCA-3′ |
VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein 1; PTX3, pentraxin 3.
Western blot analysis
The HUVECs were lysed with radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The total protein levels in the cell lysates was detected using the Pierce™ bicinchoninic acid protein assay kit according to the manufacturer's protocol (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples containing 50 µg protein were separated on 10% SDS-PAGE gels. Separated proteins were transferred onto nitrocellulose filter membranes (EMD Millipore, Billerica, MA, USA) blocked for 2 h at room temperature in 5% non-fat dried milk in buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl and 0.05% Tween-20, and then incubated with the primary antibody overnight at 4°C. The primary antibodies targeted to one of the following proteins (with their respective dilutions): VEGF (1:200; Abcam, Cambridge, UK; cat. no. ab1316), VEGFR (1:1,000; Abcam; cat. no. ab39256), endocan (1:1,000; Abcam; cat. no. ab103590), ICAM-1 (1:200; Abcam; cat. no. ab20), MCP-1 (1:5,000; Abcam; cat. no. ab151538) and PTX3 (1:1,000; Abcam; cat. no. ab125007). After washing the membranes three times in TBST buffer (Tris-buffered saline with 0.1% Tween-20), the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody. The signal on the nitrocellulose filter membranes were detected by the Enhanced Chemiluminescence system using chemiluminescent HRP substrates (EMD Millipore). The expression of proteins was semi-quantitatively analyzed by Image-J image analysis system (version 1.44p, National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Continuous variables were expressed as mean values (mean ± standard error of the mean). T-test was used to the comparison between two groups. One-way analysis of variance was used for the comparative analysis in multiple groups followed by the least significant difference post hoc test. P<0.05 was considered to indicate statistically significant differences. All data were analyzed by SPSS 20.0 statistical software (version, 20.0; IBM SPSS, Armonk, NY, USA).
Results
HG induced VEGF, VEGFR-2, endocan, ICAM-1, MCP-1 and PTX3 upregulation in cultured HUVECs
HUVECs were cultured with different D-glucose concentrations (15, 25 and 35 mmol/l) for 48 h to determine the effect of HG on HUVEC gene expression. Control cells were maintained in medium containing 5.5 mmol/l D-glucose to provide the cells with the basal needs for cell growth.
As illustrated in Fig. 1, VEGF, VEGFR and endocan mRNA expression levels gradually increased under HG conditions in HUVECs, but were not significantly altered in mannitol-treated cells compared with normally treated control cells, indicating that HG modulates VEGF, VEGFR and endocan mRNA expression in cultured HUVECs. Western blot analyses also revealed that HG modulates VEGF, VEGFR and endocan expression, as demonstrated in Fig. 2.
Figure 1.
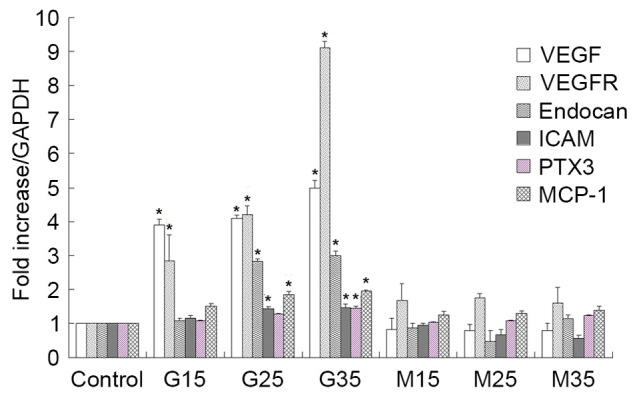
mRNA expression of VEGF, VEGFR, endocan, ICAM, MCP-1 and PTX3 mRNA in high glucose conditions in human umbilical vein endothelial cells. mRNA expression gradually increased. *P<0.05 vs. respective control. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein 1; PTX3, pentraxin 3; G15, G25, G35, D-glucose concentrations (15, 25 and 35 mmol/l, respectively); M15, M25, M35, mannitol concentrations (15, 25 and 35 mmol/l, respectively).
Figure 2.
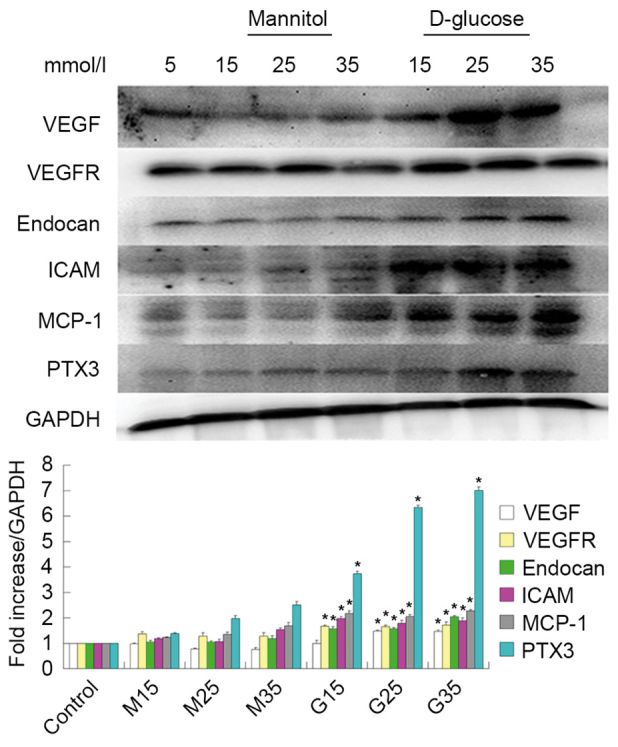
Western blot analysis of VEGF, VEGFR, endocan, ICAM, MCP-1 and PTX3 protein expression. Expression gradually increased under high glucose conditions in human umbilical vein endothelial cells. *P<0.05 vs. respective control. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein 1; PTX3, pentraxin 3.
In addition to examining the effects of HG treatment on endothelial dysfunction marker expression, the authors examined the effects of HG treatment on inflammatory marker expression. Similar to the above results, they observed that ICAM-1, MCP-1 and PTX3 mRNA and protein expression levels increased under HG conditions in HUVECs, as demonstrated in Figs. 1 and 2.
HUVEC proliferation
Cells were incubated with HG or mannitol, which served as an osmotic control, for 3 days. HUVEC viability at 12, 24, 48 and 72 h was tested via CCK-8 assays. The results indicated that HG treatment induces increases in cell proliferation in a time-dependent manner at 24 and 48 h (P<0.05), followed by a decrease in cell proliferation at 72 h (P>0.05).
Endothelial cells were also incubated with 100 µmol/l CaD for 3 days to determine the effects of CaD on HUVEC viability. HUVEC viability was nearly identical across all time points between 12 and 72 h following 100 µmol/l CaD treatment. As demonstrated in Fig. 3, compared with HG treatment (35 mmol/l), CaD (100 µmol/l) application significantly attenuated abnormal HUVEC proliferation.
Figure 3.
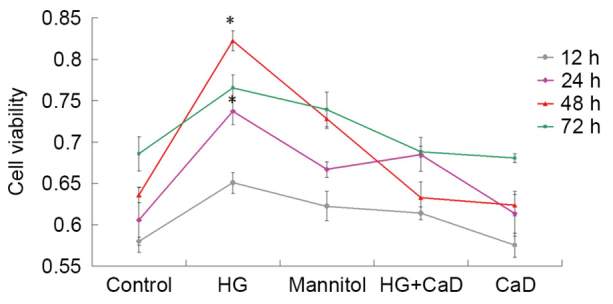
Effects of different cell treatments on HUVEC proliferation. Cell Counting kit-8 assay was performed to analysed effect on cell proliferation. HG (35 mmol/l) treatment induced increased cell proliferation at 24 and 48 h. CaD (100 µmol/l) application significantly attenuated abnormal HUVEC proliferation. *P<0.05 vs. control, mannitol, HG+CaD and CaD groups. HG, high glucose; HUVEC, human umbilical vein endothelial cell; CaD, calcium dobesilate.
CaD prevented migration of HUVECs partially induced by diabetes or high glucose
As presented in Fig. 4, HG significantly enhanced HUVEC migration compared to the control treatment (18±3 cells/HPF vs. 7±2 cells/HPF, P<0.05). CaD partially inhibited HUVEC migration compared to HG (8±2 cells/HPF vs. 18±3 cells/HPF, P<0.05) but did not completely inhibit HVUEC migration compared to the control treatment (7±1 cells/HPF vs. 7±2 cells/HPF, P>0.05).
Figure 4.
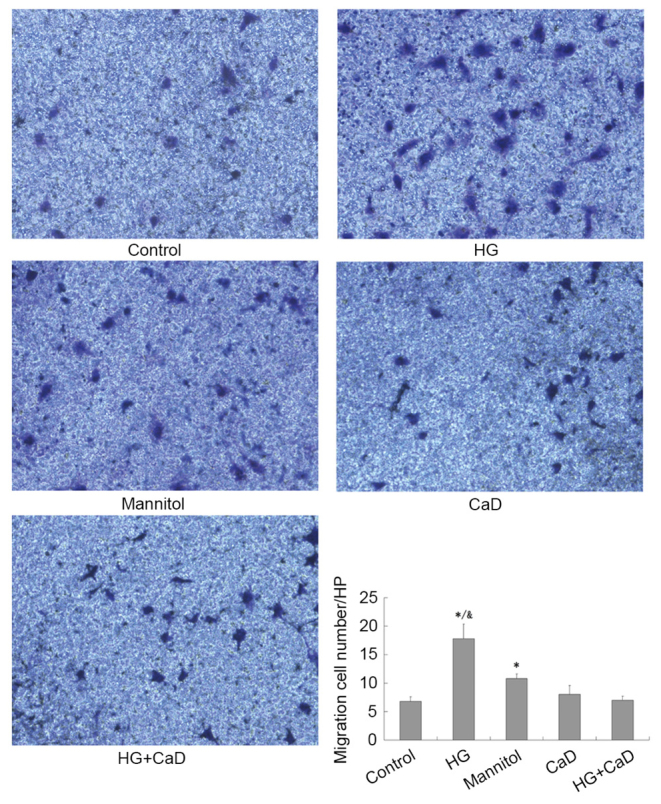
CaD prevents migration of HUVECs, which partially induced by diabetes or HG. Transwell assay analysing migration of HUVECs following cell treatment. The stained cells were counted in 10 randomly chosen fields at ×200 magnification. *P<0.05 vs. control group; &P<0.05 vs. mannitol group. CaD, calcium dobesilate; HG, high glucose; HUVECs, human umbilical vein endothelial cells.
HG increased the permeability of HUVECs, and CaD blocked this increase
HG treatment increased FITC-BSA permeability in HUVECs compared to the control treatment (137±3% vs. 100%, P<0.05). Addition of CaD prevented the increase in permeability induced by HG (137±3% vs. 105±7%, P<0.05), although CaD alone did not alter the permeability of HUVECs to FITC-BSA compared to the control treatment (104±1% vs. 100%, P>0.05). Osmotic control treatment increased FITC-BSA permeability, although to a significantly lesser extent than HG (118±16% vs. 137±3%, P<0.05; Fig. 5).
Figure 5.
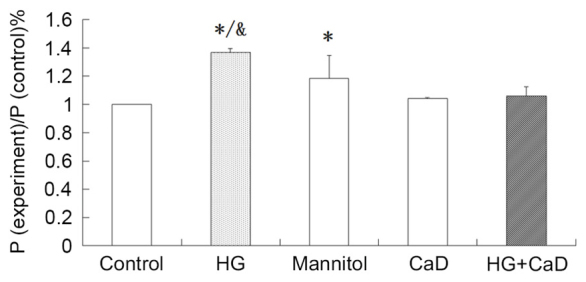
HG increased the permeability of HUVECs, which inhibited by CaD. Permeability assay analysing permeability of HUVECs following cell treatment. *P<0.05 vs. control group; &P<0.05 vs. mannitol group. HG, high glucose; HUVECs, human umbilical vein endothelial cells; CaD, calcium dobesilate.
CaD suppressed HG-induced VEGF, VEGFR-2, endocan and inflammatory marker overexpression in HUVECs
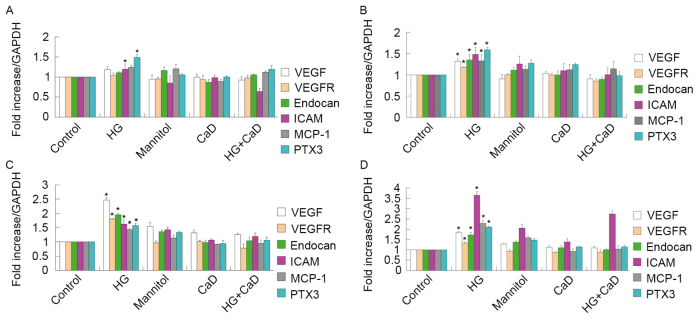
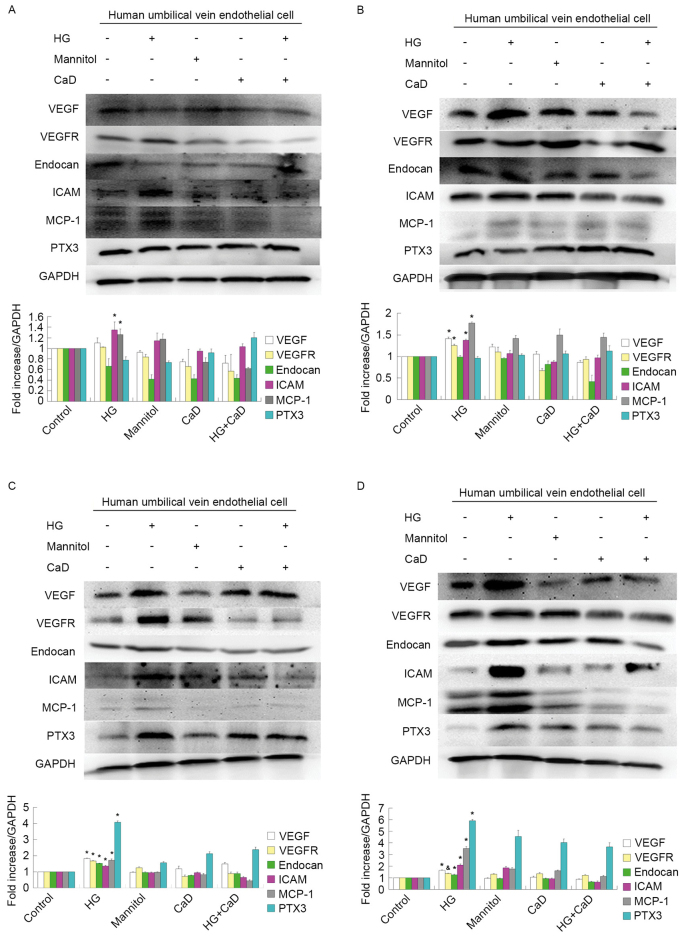
Endothelial function is mediated by interactions among VEGF, VEGFR-2 and endocan, which are expressed in HUVECs. HG treatment (35 mmol/l), but not mannitol treatment (osmotic control), increased VEGF, VEGFR-2 and endocan protein and mRNA expression levels in a time-dependent manner over 48 h in HUVECs. However, these increases diminished at 72 h of HG treatment. In addition, 100 µmol/l CaD prevented the increases in the VEGF, VEGFR-2 and endocan levels induced by HG (Figs. 6 and 7).
Figure 6.
HG (35 mmol/l) increased the mRNA levels of VEGF, VEGFR-2, endocan in a time-dependent manner from 12 to 48 h. However, the growth trend began to slow down in 72 h. 100 µmol/l CaD prevented the increase of VEGF, VEGFR-2 and endocan levels induced by HG. HG also increased the mRNA levels of ICAM, MCP-1 and PTX3 in a time-dependent manner from 12 to 72 h. 100 µmol/l CaD prevented the increase the expression of these inflammatory factors induced by HG. (A) HUVECs were incubated with control or experimental medium for 12 h. (B) HUVECs were incubated with control or experimental medium for 24 h. (C) HUVECs were incubated with control or experimental medium for 48 h. (D) HUVECs were incubated with control or experimental medium for 72 h. *P<0.05 vs. respective control or respective mannitol, HG+CaD, CaD group. HG, high glucose; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein 1; PTX3, pentraxin 3; CaD, calcium dobesilate; HUVECs, human umbilical vein endothelial cells.
Figure 7.
HG (35 mmol/l) increased the protein levels of VEGF, VEGFR-2, endocan in a time-dependent manner from 12 to 48 h. However, the growth trend began to slow down in 72 h. 100 µmol/l CaD prevented the increase of VEGF, VEGFR-2 and endocan levels induced by HG. HG also increased the protein levels of ICAM, MCP-1 and PTX3 in a time-dependent manner from 12 to 72 h. 100 µmol/l CaD prevented the increase the expression of these inflammatory factors induced by HG. (A) HUVECs were incubated with control or experimental medium for 12 h. (B) HUVECs were incubated with control or experimental medium for 24 h. (C) HUVECs were incubated with control or experimental medium for 48 h. (D) HUVECs were incubated with control or experimental medium for 72 h. *P<0.05 vs. control, mannitol, HG+CaD and CaD; &P<0.05 vs. control group. HG, high glucose; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein 1; PTX3, pentraxin 3; CaD, calcium dobesilate; HUVECs, human umbilical vein endothelial cells.
Furthermore, HG treatment increased ICAM-1, MCP-1 and PTX3 mRNA and protein expression in a time-dependent manner from 12 to 72 h. However, 100 µmol/l CaD treatment prevented these HG-induced increases in inflammatory marker expression (Figs. 6 and 7).
Discussion
To the best of the authors' knowledge, the current study is the first to demonstrate that CaD inhibits changes in endothelial and inflammatory protein levels. Specifically, CaD significantly inhibited the expression of VEGF and VEGFR, which are known to mediate abnormal HUVEC proliferation (17). CaD also prevented the HG-induced increases in HUVEC migration and permeability, most likely by suppressing ICAM-1, PTX3 and MCP-1 expression.
CaD, an antioxidant and angioprotective agent, blocks the increase in capillary permeability induced by reactive oxygen species, enhances the activity of nitric oxide synthase in endothelial cells, and inhibits apoptosis and inflammatory cytokine expression in blood vessels and other tissues (18). CaD effectively treats diabetic retinopathy at the systemic and local ocular levels and is therefore widely used to treat this disorder (18). DKD and retinopathy often coexist, develop in parallel, and have similar pathogeneses (19). Zhang et al (13) observed that CaD exerted therapeutic effects on type 2 diabetes patients with microalbuminuria; however, the mechanism underlying the effects of CaD on diabetic nephropathy is unclear.
It is generally known that diabetic nephropathy is characterized by an early increase followed by a gradual decrease in the glomerular filtration rate (GFR). The increase in GFR may be caused by capillary angiogenesis. A streptozotocin-induced diabetes model demonstrated increases in glomerular capillary number, capillary length and capillary cross-sectional area (20–22). Correspondingly, neoangiogenesis was observed in a human study (23). Immature neovessels exhibit thin basement membranes and endothelial swelling, which may limit permselectivity and lead to microalbuminuria (23–27). New blood vessel formation involves EC proliferation and migration (28). The present study demonstrated that HG treatment induces concentration- and time-dependent increases in cell proliferation at 12 and 48 h of treatment followed by decreases in cell proliferation at 72 h of treatment. In addition, the authors indicated that CaD ameliorates these abnormal increases in cell proliferation. CaD also prevents the migration of HUVECs partially induced by HG. The abovementioned early increased and subsequent gradual decrease in the number of glomerular capillaries may be attributed to corresponding changes in VEGF expression (26). The present study also demonstrated that HG induced early increases in VEGF, VEGFR-2 and endocan, although the rate of these expression increases was reduced at 72 h, which conformed to the pattern of cell proliferation. The important role of VEGF in diabetic microangiopathy has been studied extensively in the setting of proliferative diabetic retinopathy, which is characterized by the formation of new vessels inside the retina along with abnormal vascular architecture and permeability. In the kidney, VEGF is produced primarily by podocytes and is upregulated in early diabetic nephropathy, promoting new blood vessel formation (26,29). In contrast, VEGF expression and activity are downregulated in advanced diabetic nephropathy, leading to glomerular capillary rarefaction and GFR decreases, possibly due to podocyte loss (30–33). CaD suppressed HG-induced VEGF, VEGFR-2 and endocan overexpression in the current study. These results demonstrated that CaD has angioprotective properties.
Diabetic nephropathy is caused by not only uncontrolled hemodynamics and hyperglycaemia but also low-grade inflammation and chronically activated innate immunity (34,35). Studies indicate that the expression levels of inflammatory cytokines, such as TNF-α, IL-1, IL-6 and IL-18, are elevated in the kidneys of diabetes models (36,37). In diabetic rat models, the elevated expression levels of TNF-α and IL-6 are associated with increases in kidney weight and urinary albumin excretion (35). Serum IL-18 and TNF-α levels were higher in patients with DKD than in controls and positively correlated with the degree of albuminuria in patients with diabetes (38,39). These cytokines promote vascular endothelial cell permeability in vitro, leading to glomerular basement membrane thickening, contributing to EC apoptosis, and even exerting a direct toxic effect on renal cells (40–48). In addition, VCAM-1 was identified to be upregulated in DKD patients (8) and may promote inflammatory cell adhesion to the endothelium and recruit circulating immune cells to diabetic kidneys. The results of the present study are consistent with the above research findings. HG induced increases in HUVEC permeability, as well as concentration- and time-dependent increases in ICAM-1, MCP-1 and PTX3 protein and mRNA expression from 12 to 72 h of treatment. Furthermore, CaD prevented the HG-induced increases in the expression of these inflammatory factors and ameliorated the HG-mediated increase in HUVEC permeability. These results indicated that CaD protects ECs partly by ameliorating HG-induced inflammation.
In conclusion, CaD suppressed HG-induced VEGF, VEGFR-2 and endocan overexpression, indicating that CaD has angioprotective properties. CaD also protected ECs partly by ameliorating HG-induced inflammation. The current findings demonstrated the potential application of CaD to the treatment of diabetic nephropathy, particularly during the early stages of this disease.
Acknowledgements
The present study was supported in part by the National Natural Science Foundation of China (grant nos. 81370794 and 81570604) as well as by a grant (grant no. ZHYY-ZXYJHZX-1-02) from the Shanghai Municipal Commission of Health and Family Planning. The authors would like to thank Shanghai Zhaohui pharmaceutical Co., Ltd, who provided the original powder of CaD.
Glossary
Abbreviations
- DKD
diabetic kidney disease
- HUVECs
human umbilical vein endothelial cells
- HG
high glucose
- CaD
calcium dobesilate (calcium 2,5-dihydroxybenzenesulfonate)
- CCK-8
Cell Counting Kit-8
- FITC
fluorescein isothiocyanate
- BSA
bovine serum albumin
- ECs
endothelial cells
- ICAM-1
intercellular cell adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- ECM
endothelial cells growth medium
- GFR
glomerular filtration rate
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor 2
- MCP-1
monocyte chemotactic protein 1
- PTX3
pentraxin 3
References
- 1.International Diabetes Federation, corp-author. www.diabetesatlas.org Diabetes Atlas. (7th edition) 2015 [Google Scholar]
- 2.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308:F287–F297. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Haematol Disord Drug Targets. 2014;14:22–33. doi: 10.2174/1871529X14666140401110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-González JF, Mora-Fernández C, de Fuentes M Muros, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 6.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Shikata K, Hirata K, Akiyama K, Matsuda M, Kushiro M, Shikata Y, Miyatake N, Miyasaka M, Makino H. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: Glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997;46:2075–2081. doi: 10.2337/diab.46.12.2075. [DOI] [PubMed] [Google Scholar]
- 8.Seron D, Cameron JS, Haskard DO. Expression of VCAM-1 in the normal and diseased kidney. Nephrol Dial Transplant. 1991;6:917–922. doi: 10.1093/ndt/6.12.917. [DOI] [PubMed] [Google Scholar]
- 9.Tejerina T, Ruiz E. Calcium dobesilate: Pharmacology and future approaches. Gen Pharmacol. 1998;31:357–360. doi: 10.1016/S0306-3623(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 10.Javadzadeh A, Ghorbanihaghjo A, Adl FH, Andalib D, Khojasteh-Jafari H, Ghabili K. Calcium dobesilate reduces endothelin-1 and high-sensitivity C-reactive protein serum levels in patients with diabetic retinopathy. Mol Vis. 2013;19:62–68. [PMC free article] [PubMed] [Google Scholar]
- 11.Rabe E, Jaeger KA, Bulitta M, Pannier F. Calcium dobesilate in patients suffering from chronic venous insufficiency: A double-blind, placebo-controlled, clinical trial. Phlebology. 2011;26:162–168. doi: 10.1258/phleb.2010.010051. [DOI] [PubMed] [Google Scholar]
- 12.Hall JF. Modern management of hemorrhoidal disease. Gastroenterol Clin North Am. 2013;42:759–772. doi: 10.1016/j.gtc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X. Therapeutic effects of calcium dobesilate on diabetic nephropathy mediated through reduction of expression of PAI-1. Exp Ther Med. 2013;5:295–299. doi: 10.3892/etm.2012.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafarey M, Ashtiyani S Changizi, Najafi H. Calcium dobesilate for prevention of gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2014;8:46–52. [PubMed] [Google Scholar]
- 15.Pan SL, Guh JH, Huang YW, Chern JW, Chou JY, Teng CM. Identification of apoptotic and antiangiogenic activities of terazosin in human prostate cancer and endothelial cells. J Urol. 2003;169:724–729. doi: 10.1097/00005392-200302000-00074. [DOI] [PubMed] [Google Scholar]
- 16.Irwin DC, van Patot MC Tissot, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol. 2005;288:L849–L859. doi: 10.1152/ajplung.00294.2004. [DOI] [PubMed] [Google Scholar]
- 17.Demirtas S, Caliskan A, Guclu O, Yazici S, Karahan O, Yavuz C, Mavitas B. Can calcium dobesilate be used safely for peripheral microvasculopathies that require neoangiogenesis? Med Sci Monit Basic Res. 2013;19:253–257. doi: 10.12659/MSMBR.889427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Liu W, Wu S, Jin J, Li W, Wang N. Calcium dobesilate for diabetic retinopathy: A systematic review and meta-analysis. Sci China Life Sci. 2015;58:101–107. doi: 10.1007/s11427-014-4792-1. [DOI] [PubMed] [Google Scholar]
- 19.Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
- 20.Seyer-Hansen K. Renal hypertrophy in experimental diabetes mellitus. Kidney Int. 1983;23:643–646. doi: 10.1038/ki.1983.71. [DOI] [PubMed] [Google Scholar]
- 21.Seyer-Hansen K. Renal hypertrophy in streptozotocin-diabetic rats. Clin Sci Mol Med. 1976;51:551–555. doi: 10.1042/cs0510551. [DOI] [PubMed] [Google Scholar]
- 22.Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993;36:189–194. doi: 10.1007/BF00399948. [DOI] [PubMed] [Google Scholar]
- 23.Osterby R, Nyberg G. New vessel formation in the renal corpuscles in advanced diabetic glomerulopathy. J Diabet Complications. 1987;1:122–127. doi: 10.1016/S0891-6632(87)80069-7. [DOI] [PubMed] [Google Scholar]
- 24.Wehner H, Nelischer G. Morphometric investigations on intrarenal vessels of streptozotocin-diabetic rats. Virchows Arch A Pathol Anat Histopathol. 1991;419:231–235. doi: 10.1007/BF01626353. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanesaki Y, Suzuki D, Uehara G, Toyoda M, Katoh T, Sakai H, Watanabe T. Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am J Kidney Dis. 2005;45:288–294. doi: 10.1053/j.ajkd.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Min W, Yamanaka N. Three-dimensional analysis of increased vasculature around the glomerular vascular pole in diabetic nephropathy. Virchows Arch A Pathol Anat Histopathol. 1993;423:201–207. doi: 10.1007/BF01614771. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Fang W, Lin A, Ni Z, Qian J. Angiopoietin-2/Tie2 signaling involved in TNF-α induced peritoneal angiogenesis. Int J Artif Organs. 2012;35:655–662. doi: 10.5301/ijao.5000123. [DOI] [PubMed] [Google Scholar]
- 29.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 30.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69:1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 31.Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis. 2004;43:636–650. doi: 10.1053/j.ajkd.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Baelde HJ, Eikmans M, Lappin DW, Doran PP, Hohenadel D, Brinkkoetter PT, Van der Woude FJ, Waldherr R, Rabelink TJ, de Heer E, Bruijn JA. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71:637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 33.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 34.García-García PM, Getino-Melián MA, Domínguez-Pimentel V, Navarro-González JF. Inflammation in diabetic kidney disease. World J Diabetes. 2014;5:431–443. doi: 10.4239/wjd.v5.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 36.Navarro JF, Milena FJ, Mora C, León C, García J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 37.Sekizuka K, Tomino Y, Sei C, Kurusu A, Tashiro K, Yamaguchi Y, Kodera S, Hishiki T, Shirato I, Koide H. Detection of serum IL-6 in patients with diabetic nephropathy. Nephron. 1994;68:284–285. doi: 10.1159/000188281. [DOI] [PubMed] [Google Scholar]
- 38.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, Okamura H, Koga M, Fukuchi M, Hada T. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Metabolism. 2003;52:605–608. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 39.Navarro JF, Mora C, Muros M, García J. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant. 2006;21:3428–3434. doi: 10.1093/ndt/gfl469. [DOI] [PubMed] [Google Scholar]
- 40.Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am J Physiol. 1989;257:L399–L410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 42.Vestra M Dalla, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–S82. doi: 10.1681/ASN.2004110961. [DOI] [PubMed] [Google Scholar]
- 43.Ruef C, Budde K, Lacy J, Northemann W, Baumann M, Sterzel RB, Coleman DL. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int. 1990;38:249–257. doi: 10.1038/ki.1990.193. [DOI] [PubMed] [Google Scholar]
- 44.Mariño E, Cardier JE. Differential effect of IL-18 on endothelial cell apoptosis mediated by TNF-alpha and Fas (CD95) Cytokine. 2003;22:142–148. doi: 10.1016/S1043-4666(03)00150-9. [DOI] [PubMed] [Google Scholar]
- 45.Stuyt RJ, Netea MG, Geijtenbeek TB, Kullberg BJ, Dinarello CA, van der Meer JW. Selective regulation of intercellular adhesion molecule-1 expression by interleukin-18 and interleukin-12 on human monocytes. Immunology. 2003;110:329–334. doi: 10.1046/j.1365-2567.2003.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134:419–430. [PMC free article] [PubMed] [Google Scholar]
- 47.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113–F121. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- 48.Dipetrillo K, Gesek FA. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am J Nephrol. 2004;24:352–359. doi: 10.1159/000079121. [DOI] [PubMed] [Google Scholar]