Abstract
Previous studies have indicated that glomerular podocyte injury serves a crucial role in proteinuria during the process of chronic kidney disease. The slit diaphragm of podocytes forms the final barrier to proteinuria. Dendrin, a constituent of the slit diaphragm protein complex, has been observed to relocate from the slit diaphragm to the nuclei in injured podocytes and promote podocyte apoptosis. However, the exact mechanism for nuclear relocation of dendrin remains unclear. The expression of WWC1 in podocyte injury induced by lipopolysaccharides (LPS) or adriamycin (ADR) was detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR), western blotting and the immunofluorescence assay. The role of WWC1 in podocyte apoptosis was detected by knockdown of WWC1 and flow cytometry. The mRNA and protein expression levels of apoptosis-associated genes Bcl-2-associated X (Bax) and Bcl-2 were measured by RT-qPCR and western blotting. The impact of WWC1 on dendrin nucleus relocation in vitro in podocytes was further evaluated by knockdown of WWC1. Expression of WWC1 significantly decreased in injured podocytes in vitro. The loss-of-function assay indicated that knockdown of WWC1 gene in vitro promoted podocyte apoptosis, accompanied with increased levels of the pro-apoptotic protein Bax and decreased levels of the anti-apoptotic protein Bcl-2. Furthermore, the relocation of dendrin protein was significantly promoted by knockdown of the WWC1 gene. In conclusion, the study indicated that loss of WWC1 may contribute to podocyte apoptosis by inducing nuclear relocation of dendrin protein, which provided novel insight into the molecular events in podocyte apoptosis.
Keywords: WWC1, dendrin, podocyte, slit diaphragm, apoptosis
Introduction
The glomerular podocyte functions as a charge barrier to prevent proteinuria. Proteinuira is the most critical clinical signature of podocyte injury predominantly by anatomical and molecular abnormalities of podocyte foot processes (FP) and the interposed slit diaphragm (SD) (1). The consistent proteinuria and podocyte apoptosis damages glomerular structure and results in progressive glomerular consecutive tubular destruction (2,3), resulting in chronic kidney disease (CKD), which is a worldwide health problem, of which the prevalence is increasing (4). Due to the fact that CKD is a worldwide chronic disease and annually millions die of cardiovascular or renal complications directly due to CKD, exploring factors intervening in the pathogenesis of podocyte injury is necessary in the development of novel strategies for CKD clinical therapy.
Previous genetic studies have highlighted the molecular makeup of SD and podocyte FP (5–6). SD is comprised by molecular proteins functioning as ‘protein gatekeepers’ in the junctional domain of the podocyte. The first observed SD protein, Nephrin, was identified by Ruotsalainen et al (7) in 1999, and more recently additional and more complex SD proteins have been identified, including podocin, CD2 associated protein (CD2AP), nephrin-like protein 1, zona occludens 1 and dendrin (8–11). Certain SD proteins have been identified to be critical in renal injury; genetic deletion of CD2AP, a scaffolding protein at the SD, leads to progressive renal failure in mice (12,13), and ectopic expression of CD2AP in podocyte prevents the development of proteinuria (14,15). Dendrin, another newly identified SD complex, interacts with CD2AP, and loss of CD2AP could drive dendrin protein from the SD to the nucleus (16). Nuclear relocation of dendrin has been identified in various podocyte injury models in vitro and in human renal diseases (17,18). In addition, nuclear dendrin could further promote apoptosis of podocytes (19). Despite of these results, the precise molecular mechanism by which dendrin regulates function of podocyte remains to be fully understood.
In yeast two hybrid screens with the human isoform of dendrin, Kremerskothen et al (20) isolated a cDNA coding for a novel protein, WWC1 (also termed KIBRA) (20). WWC1, a kidney- and brain-associated protein, was observed to be predominantly expressed in the brain and kidney, and in the kidney, WWC1 was identified to be expressed in glomerular podocytes, tubular structures and collecting ducts (21). WWC1, interaction directly with protein associated to tight junctions (PATJ), impacted the proteins associated with Lin seven-PATJ-Crumbs 3 cell polarity complex and modulated the migration of podocytes (21). However, the bio-function of WWC1 in the podocytes remains to be fully elucidated, and the functional association between WWC1 and dendrin in the podocytes was investigated in the current study.
In the present study, it was indicated that expression of WWC1 significantly was decreased in injured podocytes. Further investigation indicated that loss of WWC1 in vitro directly induced podocyte apoptosis and promoted SD protein dendrin relocation from SD into the nuclei. The results present novel insight into the molecular events in podocyte injury.
Materials and methods
Cell cultures
The conditionally immortalized mouse podocyte cell line (MPC-5) was donated by Dr Jochen Reiser (Rush University Medical Center, Chicago, IL, USA), and cultured as described previously (22–23). Cells were treated with adriamycin (ADR; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to a final concentration of 0.25 µg/ml or of lipopolysaccharides (LPS; Sigma Aldrich; Merck KGaA, Darmstadt, Germany) to 1 µg/ml for 24 h. Equal amounts of phosphate-buffered saline (PBS) were treated as the negative control.
siRNA transfection
Oligonucleotide siRNA was synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). WWC1 or scramble siRNA (Scr) was transfected into podocytes using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. WWC1 siRNA sequences used were as follows: 5′-GCACAGAGACCAGGUACUUdTdT-3′, 5′-GCACAAGAGUGAGUUGCAAdTdT-3′ and 5′-GGUGGACAGAGAGACCAAUdTdT-3′.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA samples were prepared using TRIzol RNA isolation system (Invitrogen; Thermo Fisher Scientific, Inc.) and were reverse transcribed into cDNA (50 ng) using superscript reverse transcriptase. The cDNA was used for RT-qPCR analysis using a Platinum SYBR-Green SuperMix-UDG kit (Takara Bio, Inc., Otsu, Japan) as described previously (21). The primer sequences used are listed as follows: WWC1, forward 5′-TGCTGAGGGAAACCAAAGCC-3′ and reverse 5′-CTGGACCATAGGTCGGAGTG-3′; Bcl-2-associated X (Bax), forward, 5′-CTGGACCATAGGTCGGAGTG-3′ and reverse 5′-AATTCGCCGGAGACACTCG-3′; Bcl-2, forward 5′-TGTGGCCTGATGTTGGATAAC-3′ and reverse 5′-GGTGACGAATGTGCAAATCTACT-3′; GAPDH, forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Immunofluorescence of cultured podocytes
Subsequent to being subjected to various treatments, podocytes were fixed with 4% paraformaldehyde at −20°C for 20 min, then were blocked using blocking solution of 5% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) in PBS. The primary antibodies were incubated overnight at 4°C, followed by 1 h of incubation with goat anti-rabbit Alexa Fluor 488 (4412S; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) or Texas red-donkey anti-goat IgG (ab6883; 1:250; Abcam, Cambridge, MA, USA) at room temperature. DAPI (1:1,000; Sigma-Aldrich; Merck KGaA) was stained for counterstaining. The primary antibodies used in the present study were as follows: Rabbit anti-WWC1 (sc-133374; 1:100; Santa Cruz Biotechnology, Inc.), rabbit anti-dendrin (ABT59; 1:400; EMD Millipore, Billerica, MA, USA) and goat anti-dendrin (sc-167616; 1:100; Santa Cruz Biotechnology, Inc.). All images were analyzed by two investigators blinded to the identity of the samples.
Western blotting
Protein lysates were resolved by SDS (7.5–10%)-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, and hybridized with the corresponding antibodies. The membranes were washed 3 times for 5 min with PBS following antibody incubation. The antibodies used in the present study were as follows: Rabbit anti-WWC1 (sc-133374; 1:500; Santa Cruz Biotechnology, Inc.), rabbit anti-dendrin (ABT59; 1:1,000; EMD Millipore), rabbit anti-Bax (sc-493; 1:500; Santa Cruz Biotechnology, Inc.), rabbit anti-Bcl-2 (3498; 1:1,000; Cell Signaling Technology, Inc.), rabbit anti-GAPDH (G9545; 1:5,000; Sigma-Aldrich; Merck KGaA) and rabbit anti-histone (4499; 1:2,000; Cell Signaling Technology, Inc.). The membrane was then incubated with anti-rabbit or anti-mouse IgG (211002217 and 515005071; 1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at room temperature for 1 h. Enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford, IL, USA) reagents were used for detection.
Annexin V and propidium iodide (PI) staining
Apoptotic cells were determined using an Annexin V/PI apoptosis detection kit (Nanjing KeyGEN Biotech., Co., Ltd., Nanjing, China) as described previously (20).
Statistical analysis
The statistical analysis was performed using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). All experiments in vitro were performed at least in triplicate. Continuous values were expressed as mean ± standard error using one-way analysis of variance and Bonferroni non-parametric test for statistical comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of WWC1 was decreased in injured podocytes in vitro
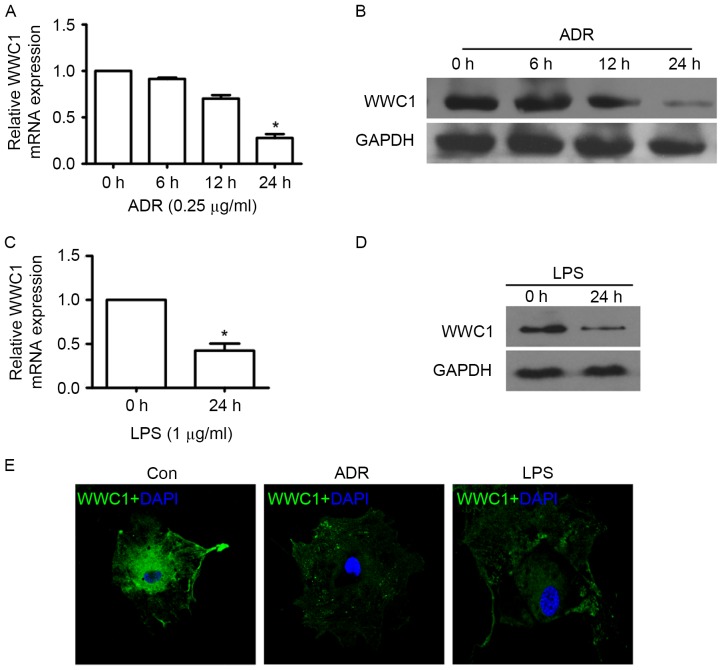
In order to evaluate whether loss of WWC1 could be induced by ADR or LPS stimulation particularly in podocytes, WWC1 expression was assessed under different exposures by RT-qPCR, immunoblotting and immunofluorescence staining. The time-response experiment indicated that ADR induced loss of WWC1 expression in a time-dependent manner. A significant decrease of WWC1 gene and protein expression was observed following 24 h exposure to 0.25 µg/ml of ADR (Fig. 1A and B). Following stimulation by LPS (1 µg/ml) for 24 h, WWC1 expression significant decreased in cultured podocyte (Fig. 1C and D). Similar results also indicated that ADR and LPS stimulation induced WWC1 expression reduction by immunofluorescence staining (Fig. 1E) (P<0.05).
Figure 1.
Expression of WWC1 is decreased in injured podocytes in vitro. (A) Podocytes were treated with ADR at a dose of 0.25 µg/ml, and RT-qPCR was performed to measure gene expression of WWC1 in different time points. (B) Representative western blots of WWC1 expression in podocyte treated as described in (A). (C and D) Podocytes were treated with LPS at a dose of 1 µg/ml or an equal amount of PBS. Gene and protein expression of WWC1 were measured by RT-qPCR and western blotting, respectively. (E) Representative immunofluorescence images of podocyte staining by anti-WWC1 antibodies under different exposure. Original magnification, ×400. Analysis of variance, *P<0.05 vs. time 0 h. ADR, adriamycin; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; LPS, lipopolysaccharides.
Loss of WWC1 directly induced podocyte apoptosis in vitro
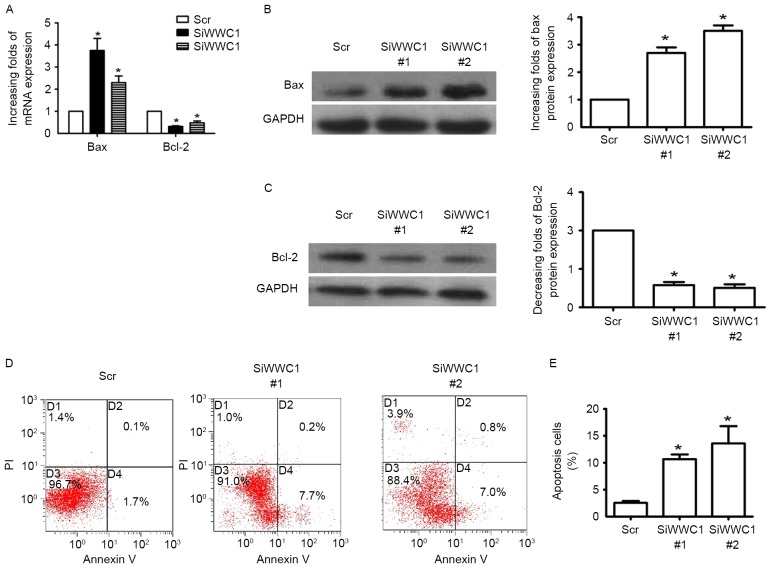
In order to investigate the direct affection of WWC1 in podocyte, WWC1 gene expression was altered in podocytes in vitro by WWC1 siRNA. Three pairs of WWC1 siRNA were transfected into podocytes and RT-qPCR was performed to detect WWC1 gene expression 48 h subsequent to transfection. Gene and protein analysis of the apoptotic factor Bax exhibited increased expression in WWC1-silenced podocytes (Fig. 2A and B), while the anti-apoptotic factor Bcl-2 was reduced (Fig. 2A and C). Annexin V and PI staining assay indicated that loss of WWC1 directly induced apoptosis process in podocytes (2.56±0.34 vs. 10.67±0.88 and 13.60±3.19, respectively; n=3) (Fig. 2D and E) (P<0.05).
Figure 2.
Loss of WWC1 directly induces podocyte apoptosis in vitro. (A) Podocytes were transfected with WWC1 siRNA or Scr. RT-qPCR was performed to analyze the expression of WWC1 in podocytes. Gene expression of apoptosis-associated genes Bax and Bcl-2 were determined by RT-qPCR. (B and C) Protein expression levels of Bax and Bcl-2 were determined by western blotting, respectively. (D) Apoptosis cells were measured by flow cytometry using Annexin V and PI staining 48 h after siRNA transfection. (E) The percentage of apoptotic cells were measured. All data are expressed as the mean ± standard error of three independent experiments. Analysis of variance, *P<0.05 vs. Scr. siRNA, small interfering RNA; Scr, scramble siRNA; Bax, Bcl-2-associated X; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; PI, propidium iodide.
Loss of WWC1 induced dendrin nucleus relocation in podocytes
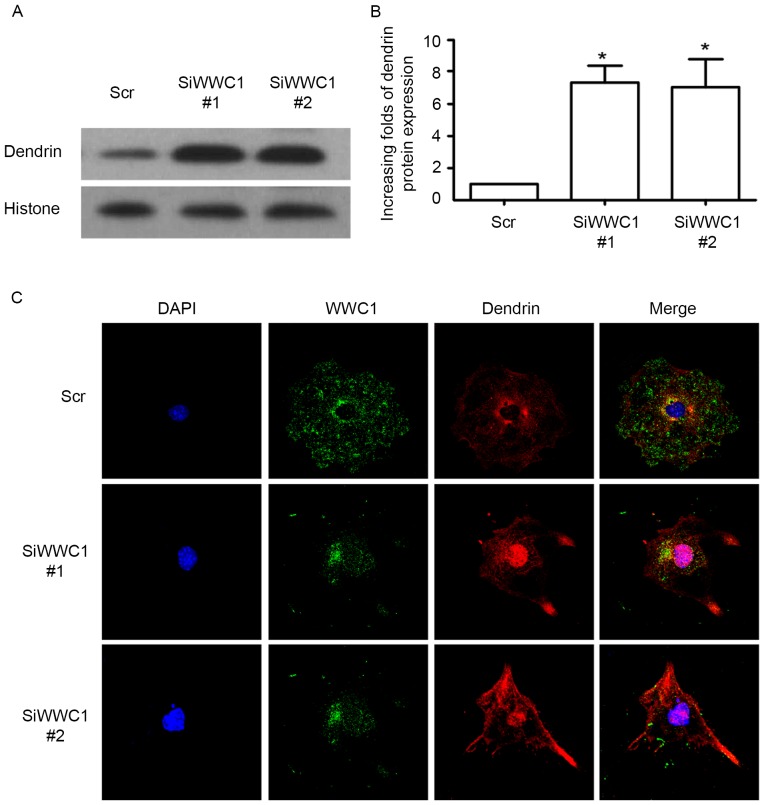
As we know, nucleus relocation of SD protein dendrin is one of the newly confirmed apoptotic mechanisms in podocytes (19). Considering the interactions between WWC1 and dendrin in normal podocytes, subcellular localization of dendrin in podocytes was assessed with silenced WWC1 gene. Notably, following silencing of the WWC1 gene in podocytes, dendrin nuclear accumulation was significantly increased (Fig. 3A and B). By immunofluorescence staining, it was identified that WWC1 and dendrin were co-located in the cytoplasm, with low levels in the nuclei of the physiological podocyte, whereas an accumulation of dendrin was detected in the podocyte nuclei in WWC1 silenced podocytes (Fig. 3C). These results indicate that loss of WWC1 could directly drive dendrin relocating into the nuclei of podocyte.
Figure 3.
WWC1 impacts dendrin nucleus relocation in podocyte. (A and B) Podocytes were transfected with WWC1 siRNA or scramble siRNA. Podocytes with either WWC1 or scramble siRNA transfection were lysated and nuclear protein was extracted using the nuclear and cytoplasmic protein extraction kit. Nucleus dendrin expression was measured by western blotting, using histone 3 as equivalent loads in the nuclei. (C) Representative photographs of triple staining of DAPI, WWC1 and dendrin showing nucleus relocation of dendrin in podocytes with silenced WWC1 gene (original magnification, ×400). Analysis of variance, *P<0.05, vs. Scr. siRNA, small interfering RNA; Scr, scramble siRNA.
Discussion
The terminally differentiated podocyte functions as a critical barrier to prevent proteinuria, and proteinuria is the clinical signature for podocyte injury, with or without loss of renal functions. Emerging experimental and clinical studies have highlighted that loss of podocyte directly causes proteinuria and glomerulosclerosis, owing to podocyte apoptosis or detachment (24–27). The present study demonstrated, to the best of our knowledge for the first time, that kidney and brain associated protein WWC1, is a critical molecular in podocyte injury. Reduced WWC1 expression was identified in injured podocytes, and loss of WWC1 directly induced podocyte apoptosis. In addition further evidence was obtained that WWC1 protected podocytes from apoptosis by preventing SD protein dendrin from relocating into nuclei.
The expression of WWC1 (KIBRA), the mammalian ortholog of Kibra, has been observed to be enriched in kidney and brain (18). In Drosophila, Kibra predominantly acts in the Merlin branch upstream of the core kinase cascade to regulate Hippo signaling, a well-recognized pathway by restricting proliferation and promoting apoptosis (28–30). In mammals, WWC1 additionally exhibits MST-independent functional regulation of Hippo signaling pathway in breast cancer (31). In a breast cancer cell line, loss of WWC1 expression displays epithelial-to-mesenchymal transition features as well as promoted migration. Directional migration regulation is additionally observed in podocytes (21). The current study focused on the observation of the response of WWC1 to podocyte apoptosis injury. A reduction of WWC1 expression was observed in ADR- and LPS-mediated podocyte injury. Due to the fact that ADR and LPS induce proteinuria in mice, these results indicated a probable endogenic protective role of WWC1 in podocytes.
Apoptosis has been reported to cause podocyte loss and glomerulosclerosis, and according to the biogenetic pleiotropy of WWC1 (31–34), it was hypothesized that reduction of WWC1 may be associated with podocyte apoptosis as a response to injury. Subsequently, it was investigated whether loss of WWC1 directly induces podocyte apoptosis. Increased apoptotic cells were observed following knockdown of endogenic WWC1 gene in podocyte by Annexin V and PI staining. In addition, the apoptosis-associated gene Bax and Bcl-2 were observed to be activated and inhibited respectively with WWC1 gene silencing.
These results suggested that endogenic WWC1 could serve a critical role in protecting podocytes from apoptotic injury. Total podocyte number is a balance between proliferation and loss, owing to several critical processes including DNA synthesis, DNA damage, hypertrophy, detachment and apoptosis. The proteins or molecules those are stably present in podocyte are essential for normal function, such as survival. For example, several SD proteins, such as CD2AP and nephrin, had been demonstrated to serve a critical role in proteinuria protection (35,36). The results provide new evidence supporting the role of WWC1 in podocyte protection.
The mechanisms of WWC1 in the protection of podocyte from apoptosis require further investigation. WWC1 was originally isolated by dendrin in a yeast two hybrid screen (20), and accumulating evidence has indicated that dendrin is additionally one of the SD complex proteins present in podocytes (37,38). Nuclear relocation of dendrin has been identified both in human glomerular diseases and various podocyte injury models, and notably, it was observed that nuclear dendrin induced podocyte apoptosis (17,18). It was investigated whether WWC1 could contribute to normal location of dendrin in the SD domain; it was identified that loss of WWC1 in podocytes directly promoted movement of dendrin proteins from SD into the nuclei, indicating that WWC1 is a crucial molecular component in regulating the stabilization of dendrin in podocytes.
The present study systematically evaluated the functions of WWC1 in the podocyte. The results demonstrated that expression of WWC1 was significantly decreased in injured podocytes. In addition, loss of WWC1 in podocytes directly induced apoptosis of podocytes. Nuclear relocation of the SD complex protein dendrin was promoted following WWC1 gene silencing in podocytes. These results indicate that endogenic WWC1 could serve a critical role in podocyte apoptosis. Taken together, the results demonstrate that WWC1 may serve as a promising molecular target to tackle proteinuric kidney diseases.
Acknowledgements
The present study was supported by the National Nature and Science Grants (grant nos. 81270784, 181470930 and 81500560); Nature and Science Grant of Guangdong Province (grant nos. 2014A030310106 and 2014A030310107) and National Clinical Key Specialty Construction Projects.
References
- 1.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MM. The role of podocyte injury in the pathogenesis of focal segmental glomerulosclerosis. Ren Fail. 2000;22:663–684. doi: 10.1081/JDI-100101955. [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, Lemley KV. The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens. 1999;8:489–497. doi: 10.1097/00041552-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: Epidemiology, social, and economic implications. Kidney Int Suppl (Suppl) 2005:S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawachi H, Han GD, Miyauchi N, Hashimoto T, Suzuki K, Shimizu F. Therapeutic targets in the podocyte: Findings in anti-slit diaphragm antibody-induced nephropathy. J Nephrol. 2009;22:450–456. [PubMed] [Google Scholar]
- 6.Kriz W, Lemley KV. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 2017;91:1283–1286. doi: 10.1016/j.kint.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes; Proc Natl Acad Sci USA; 1999; pp. 7962–7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attié T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI200112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahola H, Heikkilä E, Aström E, Inagaki M, Izawa I, Pavenstädt H, Kerjaschki D, Holthöfer H. A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol. 2003;14:1731–1737. doi: 10.1097/01.ASN.0000075553.33781.9F. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 13.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 14.Huber TB, Kwoh C, Wu H, Asanuma K, Gödel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest. 2006;116:1337–1345. doi: 10.1172/JCI27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunkemeyer JA, Kwoh C, Huber TB, Shaw AS. CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem. 2005;280:29677–29681. doi: 10.1074/jbc.M504004200. [DOI] [PubMed] [Google Scholar]
- 16.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Möller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asanuma K, Akiba-Takagi M, Kodama F, Asao R, Nagai Y, Lydia A, Fukuda H, Tanaka E, Shibata T, Takahara H, et al. Dendrin location in podocytes is associated with disease progression in animal and human glomerulopathy. Am J Nephrol. 2011;33:537–549. doi: 10.1159/000327995. [DOI] [PubMed] [Google Scholar]
- 18.Kodama F, Asanuma K, Takagi M, Hidaka T, Asanuma E, Fukuda H, Seki T, Takeda Y, Hosoe-Nagai Y, Asao R, et al. Translocation of dendrin to the podocyte nucleus in acute glomerular injury in patients with IgA nephropathy. Nephrol Dial Transplant. 2013;28:1762–1772. doi: 10.1093/ndt/gfs500. [DOI] [PubMed] [Google Scholar]
- 19.Asanuma K, Campbell KN, Kim K, Faul C, Mundel P. Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes; Proc Natl Acad Sci USA; 2007; pp. 10134–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremerskothen J, Plaas C, Büther K, Finger I, Veltel S, Matanis T, Liedtke T, Barnekow A. Characterization of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res Commun. 2003;300:862–867. doi: 10.1016/S0006-291X(02)02945-5. [DOI] [PubMed] [Google Scholar]
- 21.Duning K, Schurek EM, Schluter M, Bayer M, Reinhardt HC, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem MA, et al. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol. 2008;19:1891–1903. doi: 10.1681/ASN.2007080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Zhang L, Shi W, Zhang B, Liang X, Liu S, Wang W. NFAT2 mediates high glucose-induced glomerular podocyte apoptosis through increased Bax expression. Exp Cell Res. 2013;319:992–1000. doi: 10.1016/j.yexcr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Zhang B, Liu S, Xie S, Yang Y, Ma J, Deng Y, Wang W, Xu L, Li R, et al. 1,25-dihydroxyvitamin D(3) inhibits podocyte uPAR expression and reduces proteinuria. PLoS One. 2013;8:e64912. doi: 10.1371/journal.pone.0064912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L, Lauria F, Miji M, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 25.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Benya RV. Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2011;108:807–816. doi: 10.1172/JCI200112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moleirinho S, Chang N, Sims AH, Tilston-Lünel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy C, Faratian D, et al. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene. 2013;32:1821–1830. doi: 10.1038/onc.2012.196. [DOI] [PubMed] [Google Scholar]
- 32.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. doi: 10.1038/ncomms7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papenberg G, Salami A, Persson J, Lindenberger U, Bäckman L. Genetics and functional imaging: Effects of APOE BDNF, COMT, and KIBRA in aging. Neuropsychol Rev. 2015;25:47–62. doi: 10.1007/s11065-015-9279-8. [DOI] [PubMed] [Google Scholar]
- 34.Yang S, Ji M, Zhang L, Chen Y, Wennmann DO, Kremerskothen J, Dong J. Phosphorylation of KIBRA by the extracellular signal-regulated kinase (ERK)-ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration. Cell Signal. 2014;26:343–351. doi: 10.1016/j.cellsig.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffer M, Mundel P, Shaw AS, Böttinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 36.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremerskothen J, Kindler S, Finger I, Veltel S, Barnekow A. Postsynaptic recruitment of Dendrin depends on both dendritic mRNA transport and synaptic anchoring. J Neurochem. 2006;96:1659–1666. doi: 10.1111/j.1471-4159.2006.03679.x. [DOI] [PubMed] [Google Scholar]
- 38.Patrakka J, Xiao Z, Nukui M, Takemoto M, He L, Oddsson A, Perisic L, Kaukinen A, Szigyarto CA, Uhlén M, et al. Expression and subcellular distribution of novel glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and 2310066E14Rik. J Am Soc Nephrol. 2007;18:689–697. doi: 10.1681/ASN.2006060675. [DOI] [PubMed] [Google Scholar]