Abstract
Wound healing impairment is increasingly recognized to be a consequence of hyperglycemia-induced dysfunction of endothelial precursor cells (EPCs) in type 2 diabetes mellitus (T2DM). Metformin exhibits potential for the improvement of endothelial function and the wound healing process. However, the underlying mechanisms for the observed beneficial effects of metformin application remain to be completely understood. The present study assessed whether metformin, a widely used therapeutic drug for T2DM, may accelerate wound closure in T2DM db/db mice. Genetically hyperglycemic db/db mice were used as the T2DM model. Metformin (250 mg/kg/day; intragastric) was administered for two weeks prior to EPC collection and wound model creation in db/db mice. Wound healing was evaluated by alterations in the wound area and the number of platelet endothelial cell adhesion molecule-positive cells. The function of the isolated bone marrow-derived EPCs (BM-EPCs) was assessed by a tube formation assay. The number of circulating EPCs, and the levels of intracellular nitric oxide (NO) and superoxide (O2−) were detected by flow cytometry. Thrombospondin-1 (TSP-1) expression was determined by western blot analysis. It was observed that treatment with metformin accelerated wound healing, improved angiogenesis and increased the circulating EPC number in db/db mice. In vitro, treatment with metformin reversed the impaired BM-EPC function reflected by tube formation, and significantly increased NO production while decreasing O2− levels in BM-EPCs from db/db mice. In addition, TSP-1 expression was markedly attenuated by treatment with metformin in cultured BM-EPCs. Metformin contributed to wound healing and improved angiogenesis in T2DM mice, which was, in part, associated with stimulation of NO, and inhibition of O2− and TSP-1 in EPCs from db/db mice.
Keywords: T2DM, metformin, EPCs, wound healing, TSP-1
Introduction
The prevalence of diabetes mellitus is likely to exceed 591.9 million by 2035, and is becoming a serious international health crisis (1,2). The hallmark of diabetes mellitus is chronic hyperglycemia, resulting in vascular complications, including the impairment of angiogenesis, eventually resulting in disorders of wound healing and the development of refractory low extremity ulcerations (3,4).
A previous study demonstrated that circulating endothelial precursor cells (EPCs), a multifunctional population derived from bone marrow, are important for the promotion of angiogenesis and the maintenance of vascular homeostasis (5). An impairment in circulating EPCs may contribute to the pathogenesis of diabetic vasculopathy (6–8). Also, it has been observed that patients with type 1 and type 2 diabetes mellitus (T2DM) exhibited marked dysfunction in EPCs (9,10). In addition, a marked reduction in circulating EPCs was observed in diabetic patients (11–13). Preclinical experiments have demonstrated impaired tube formation in the EPCs of diabetic mice (14). These previous data indicated that the dysfunction of EPCs may be association with a deterioration in wound healing in diabetes (15,16).
Metformin, an oral antihyperglycaemic agent, is the first-line drug in the clinic for patients with T2DM (17,18). Studies have demonstrated that metformin is able to increase the circulating EPC number in addition to improving the cellular function of EPCs in patients with T2DM (8,19). Thrombospondin-1 (TSP-1), a novel antiangiogenic adipokine, has been reported to be expressed in animal models susceptible to diabetes, including obesity and insulin resistance (20). Genetically, augmented mRNA expression of TSP-1 has been observed in diabetes mellitus (21,22). A previous study demonstrated an apparent detrimental effect of TSP-1 on EPC function, which was reported to be negatively-correlated with nitric oxide (NO) regeneration in in vitro endothelial cells (23). Although metformin has been reported to be a regulatory factor for TSP-1 in patients with polycystic ovarian syndrome, little data are currently available in diabetes (24).
Xie et al (25) reported that the function of EPCs is associated with cellular oxidative stress. The study demonstrated that a decrease in the NO level or excess generation of superoxide (O2−) may result in a detrimental effect on EPCs, as indicated by impaired angiogenesis and tube formation. Little data is available concerning the role of metformin in improving impaired wound closure in T2DM. The present study hypothesized that metformin may be able to contribute to wound healing in T2DM mice, and that this protective effect may be partly attributed to an improvement in EPC function with the involvement of TSP-1 and cellular oxidative stress.
Materials and methods
Animals
A total of 14 male C57BLKS/J db/db diabetic mice (age, 6 weeks; weight, 32–36 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and 7 male C57BL/6J non-diabetic mice (age, 6 weeks; weight, 16–18 g) were obtained from Sino-British SIPPR/BK Lab Animal Ltd. (Shanghai, China). Mice were housed in a well-ventilated holding room with a 12-h light-dark cycle at an ambient temperature of 23±2°C and 70% humidity, with free access to water and food. All studies were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Bethesda, MD, USA). The present study was approved by the Animal Care and Ethics Committee of Second Military Medical University (Shanghai, China).
Experimental protocols
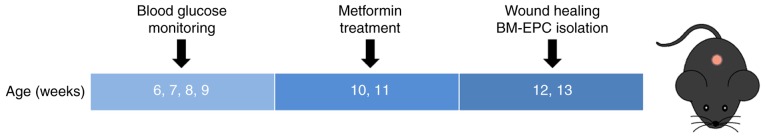
Male db/db mice with obesity and hyperglycemia were used as a model of T2DM. Male age-matched C57BL/6J mice were used as control non-diabetic mice and received treatment with a vehicle. The db/db mice were randomly divided into 2 groups, either receiving the vehicle [0.5% carboxymethyl cellulose-Na; 10 ml/kg/day; intragastric (i.g.)] or metformin (250 mg/kg/day; i.g.; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 14 consecutive days. Whole blood samples from the tail veins of the mice were used for detecting blood glucose via a monitoring system (Maochang, Taipei, China). The mice were used for wound healing experiments, or anesthetized for the harvesting of bone marrow to isolate EPCs (BM-EPCs) (Fig. 1).
Figure 1.
Illustration of experimental protocols. The blood glucose of db/db diabetic mice was monitored. Treatment with metformin (250 mg/kg/day; intragastric) was administered for 14 consecutive days. Wound healing and BM-EPC function were measured. BM-EPC, bone marrow-endothelial precursor cell.
Analysis of wound closure
A 6-mm circular wound was produced by punch biopsy, and digital images of the wound on the dorsum were captured every 2 days until the end of the experiment for all experimental mice. The wound areas were analyzed by tracing the wound margins and calculated using Image-Pro Plus software version 6.0 (Media Cybernetics, Rockville, MD, USA). The closure was expressed as a percentage area of the original wound area (26).
Wound angiogenesis
Wounds were harvested from mice on days 7 and 14 following the creation of the wound. Platelet endothelial cell adhesion molecule (CD31) staining was used to evaluate angiogenesis. Samples of skin at the wounded area and surrounding tissue (~1 cm in diameter, ~2 mm in thickness) were excised, bisected, and fixed in 10% formalin for 6 h at room temperature. The samples were subsequently embedded in paraffin. Following deparaffinization, rehydration with decreasing alcohol series, antigen retrieval (0.5 h at 90°C in 10 mM citrate buffer) and 5% serum blocking (3 h at room temperature; Chemicon International, Inc., Temecula, CA, USA), the slides were incubated with an anti-CD31 antibody (2 µg/ml; cat. no. 550274; BD Biosciences, San Jose, CA, USA) for 1 h at room temperature and subsequently incubated with a biotinylated secondary antibody (1:500; cat. no. BA-9200; Vectastain Elite ABC kit; Vector Laboratories Ltd., Peterborough, UK) for 1 h at room temperature. The samples were counterstained with hematoxylin for 2 min at room temperature (27). CD31-positive tubular structures were considered to be capillaries and the capillary density in the wound healing area was quantified. One slide from each mouse was examined and, for each slide, two high-power fields (magnification, ×200) were examined using a light microscope. The capillaries were then counted.
Quantification of circulating EPCs
Circulating EPCs were determined according to a previously-described technique (28). Peripheral blood was acquired by removing the eyeballs from anesthetized mice. The samples were dissolved in PBS (1:1), following which gradient centrifugation liquid 1083 (Sigma-Aldrich; Merck KGaA) was used for the separation of peripheral blood mononuclear cells at 400 × g for 30 min. The mononuclear fraction was extracted and the erythrocytes were lysed with red blood cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). Following washing, the samples were suspended for incubation (0.5 h at room temperature) using a buffer solution containing fluorescein isothiocyanate-ataxin-1 (Sca-1) (1:100; cat. no. 557405; BD Biosciences) and phycoerythrin-vascular endothelial growth factor receptor 2 (Flk-1) (1:100; cat. no. 555308; BD Biosciences) antibodies for flow cytometry detection and analyzed using FlowJo software version 7.6 (Tree Star Inc., Ashland, OR, USA). Sca-1/Flk-1 double-positive cells were defined as circulating EPCs.
Isolation of the BM-EPCs
The isolation and culturing of mouse BM-EPCs were in accordance with a previous technique (25). BM-EPCs were obtained from mouse tibias and femurs and seeded in 6-well plates coated with vitronectin (Sigma-Aldrich; Merck KGaA). Cells were cultured in endothelial growth medium-2 (Cambrex Corp., East Rutherford, NJ, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C, 5% CO2. A total of 4 days subsequent to cultivation, the culture medium, including nonadherent cells, was changed for fresh medium and adherent cells were subjected to further culturing for 3 days. The supernatant was collected for western blot quantification and the cells were used for in vitro studies.
In vitro cell assays
Evaluation of the function of BM-EPCs
A tube formation assay, a previously described method, was adopted to evaluate BM-EPC function (29). A total of 40,000 BM-EPCs were seeded in 96-well plates which were precoated with 50 µl/well growth factor-induced Matrigel (BD Biosciences). Following 8 h of incubation at 37°C, images of tube morphology were captured using a computer-assisted microscope (Leica Microsystems GmbH, Wetzlar, Germany). Tube numbers were measured in five low power fields (magnification, ×50) for each sample at random.
Measurement of intracellular NO and O2−
The intracellular NO level was determined using membrane-permeable 4-amino-5-methylamino-2′, 7′-difluorofluorescein (DAF-FM) diacetate (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 7 days subsequent to BM-EPC culturing, the cells were harvested and incubated with DAF-FM diacetate (10−6 mol/l) for 30 min at 37°C and an additional 30 min at room temperature in dark. Following incubation, the DAF-FM fluorescence intensity in cells was measured by flow cytometry (25).
The intracellular O2− level was detected using the membrane-permeable dye dihydroethidium (DHE; Invitrogen; Thermo Fisher Scientific, Inc.), which is oxidized to ethidium bromide in the presence of O2−. Following 7 days of culturing, BM-EPCs were harvested and incubated with DHE (0.5×10−6 mol/l) for 30 min at room temperature in dark. Following staining, the DHE fluorescence intensity in cells was examined by flow cytometry (25).
Western blot analysis
Western blotting was performed as previously described (30). TSP-1 secreted by BM-EPCs was obtained by condensing the BM-EPC culture media using a commercial filter device. The concentration of TSP-1 was determined using a bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). For the western blotting experiments, ~30-µg samples were loaded and run on an 8% SDS-PAGE gel. The proteins were electrophoretically transferred to nitrocellulose membranes. Subsequently, the membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature, washed and incubated with the primary antibody for TSP-1 (1:500; cat. no. ab85762; Abcam, Cambridge, UK) at 4°C overnight. IRDye 800-conjugated rabbit anti-mouse IgG was used as the secondary antibody (1:5,000; cat. no. 925-32212; LI-COR Biosciences, Lincoln, NE, USA) and incubated for 0.5 h at room temperature. The bands were obtained using an Odyssey infrared imaging system (LI-COR Biosciences), and the expression levels of TSP-1 protein were quantified using Quantity One software version 4.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Statistical significance was analyzed by one-way analysis of variance followed by the Newman-Keuls multiple comparison test, using GraphPad Prism Software version 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of metformin on blood glucose and body weight in db/db mice
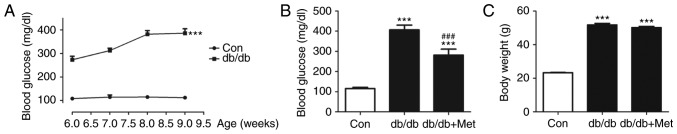
A significant increase in blood glucose was observed in db/db mice compared with the control (338.4±27.6 vs. 112.1±1.5 mg/dl; P<0.001; Fig. 2A). Pretreatment with metformin improved the blood glucose level (Fig. 2B), although it did not modify body weight in db/db mice (Fig. 2C).
Figure 2.
Alterations in blood glucose concentration and body weight of db/db mice. (A) In db/db mice, blood glucose was significantly elevated compared with the control. ***P<0.001 vs. Con. Metformin (250 mg/kg/day for 14 days; intragastric) significantly decreased (B) blood glucose, although it did not alter (C) body weight in db/db mice. ***P<0.001 vs. Con; ###P<0.001 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=7 mice/group). Con, control; Met, metformin.
Metformin accelerates wound healing and angiogenesis in db/db mice
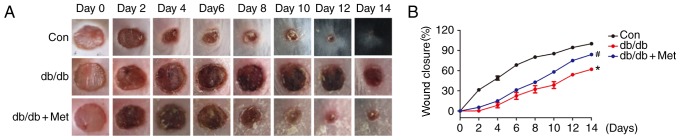
In order to examine the effects of pretreatment with metformin on wound closure in db/db mice, alterations in the wounded skin were observed on alternate days until day 14. Fig. 3A exhibits the gross appearance of the wounds during the 14 days following injury. Db/db mice exhibited a marked delay in wound closure compared with the control. By contrast, wounds in db/db mice pretreated with metformin underwent gradual and progressive healing until reaching complete closure (Fig. 3A). Statistically, treatment with metformin significantly accelerated wound closure in db/db mice when compared with the untreated db/db mice (P<0.05; Fig. 3B).
Figure 3.
Metformin therapy accelerates wound closure in db/db mice. A dorsal skin wound was created via a 6-mm circular punch biopsy and digital images of the wound were captured every 2 days until day 14. (A) Representative images of wound healing. (B) Metformin therapy accelerated wound closure compared with control in db/db mice. *P<0.05 vs. Con; #P<0.05 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=5 mice/group). Con, control; Met, metformin.
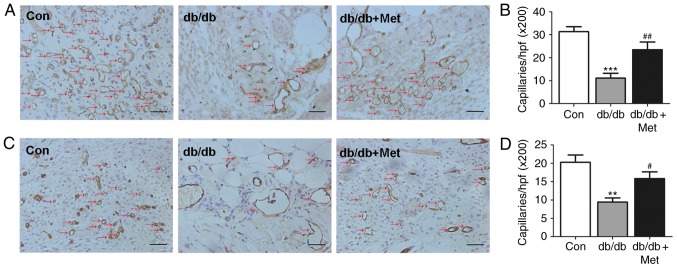
In order to further examine the role of metformin treatment in skin neovascularization, the number of tubular structures, indicated by CD31 staining of the skin tissue harvested from the wound area, were calculated (Fig. 4). A significant decrease in capillary formation was observed in db/db mice on days 7 (P<0.001; Fig. 4B) and 14 (P<0.01; Fig. 4D) compared with the control. Capillary formation in db/db mice was significantly improved on days 7 (P<0.01; Fig. 4B) and 14 (P<0.05; Fig. 4D) following pretreatment with metformin.
Figure 4.
Metformin therapy enhances wound angiogenesis in db/db mice. A dorsal skin wound was created via a 6-mm circular punch biopsy and wound angiogenesis was measured on days 7 and 14. (A) Representative images of CD31 staining on day 7 and (B) quantitative analysis. (C) Representative images of CD31 staining on day 14 and (D) quantitative analysis. Red arrows indicate CD31-positive capillaries (magnification, ×200; scale bar, 50 µm). Quantitative analysis of capillaries in each field demonstrated that wound capillaries in metformin-treated db/db mice were increased on days 7 and 14 when compared with the untreated db/db mice. ***P<0.001, **P<0.01 vs. Con; ##P<0.01, #P<0.05 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=5 mice/group). Con, control; Met, metformin; hpf, high-power field.
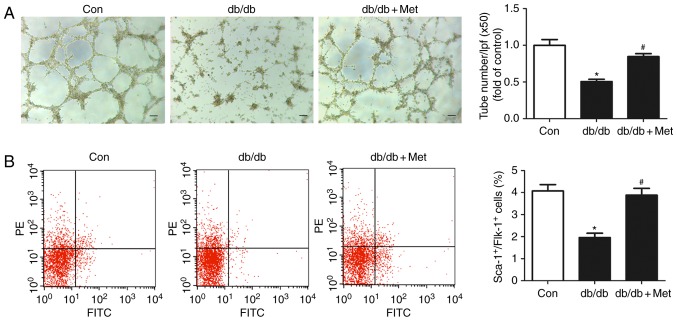
Metformin improves BM-EPC function in db/db mice
In order to further examine the mechanism underlying the role of metformin in accelerating wound closure, the tube formation capacity of EPCs from db/db mice was determined. Impaired EPCs were observed in db/db mice compared with the control. Pretreatment with metformin significantly improved tube formation capacity in db/db mice (0.70±0.04 vs. 0.54±0.04; P<0.05; Fig. 5A). A decrease in the circulating EPC number was observed in db/db mice compared with the control, which was partially reversed by pretreatment with metformin (2.18±0.32% vs. 1.11±0.18%; P<0.05; Fig. 5B).
Figure 5.
Metformin therapy ameliorates BM-EPC function in db/db mice. (A) Representative images of the tube formation assay of BM-EPCs. The number of tubes in each sample was calculated from 5 fields (magnification, ×50; scale bar, 100 µm) at random. Treatment with metformin ameliorated the tube formation of BM-EPCs. (B) EPC numbers were detected by flow cytometry and the percentage of Sca-1+/Flk-1+ cells was calculated. Metformin elevated the circulating EPC number in db/db mice. *P<0.05 vs. Con; #P<0.05 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=7 mice/group). Con, control; Met, metformin; BM-EPC, bone marrow-endothelial precursor cell; PE, phycoerythrin; FITC, fluorescein isothiocyanate; Sca-1, ataxin-1; Flk-1, vascular endothelial growth factor receptor 2; lpf, low-power field.
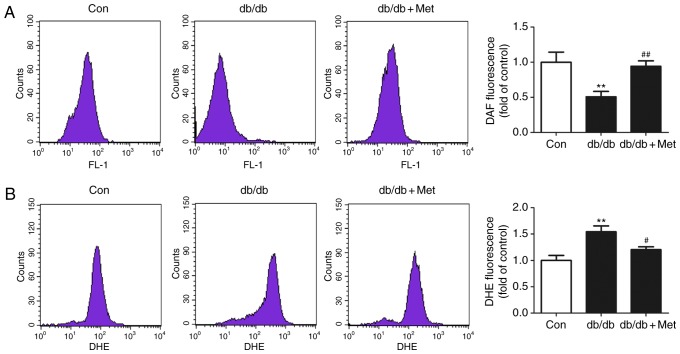
Metformin increases intracellular NO production and decreases the intracellular O2− level in BM-EPCs from db/db mice
There was significant decrease in intracellular NO in BM-EPCs from db/db mice compared with the control (P<0.01; Fig. 6A), and pretreatment with metformin significantly increased the intracellular NO level of BM-EPCs in db/db mice (P<0.01; Fig. 6A). By contrast, the intracellular O2− level in BM-EPCs from db/db mice was significantly elevated compared with the control (P<0.01; Fig. 6B). However, pretreatment with metformin significantly decreased the levels of intracellular O2− in BM-EPCs from db/db mice (P<0.05; Fig. 6B).
Figure 6.
Metformin therapy enhances intracellular NO and suppresses intracellular O2− levels in BM-EPCs from db/db mice. (A) The intracellular NO level was determined by DAF-FM-staining and flow cytometry. Metformin increased the NO level in BM-EPCs from db/db mice. (B) DHE fluorescence intensity was determined by flow cytometry. Metformin decreased the intracellular O2− level in BM-EPCs from db/db mice. **P<0.01 vs. Con; ##P<0.01, #P<0.05 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=6–7 mice/group). Con, control; Met, metformin; BM-EPC, bone marrow-endothelial precursor cell; DAF-FM, 4-amino-5-methylamino-2′, 7′-difluorofluorescein; DHE, dihydroethidium; O2−, superoxide; NO, nitric oxide.
Metformin inhibits TSP-1 secretion in BM-EPCs from db/db mice
Elevated TSP-1 secretion was observed in BM-EPCs from db/db mice, as indicated by western blot analysis of the supernatant from the cell culture medium, when compared with the control (P<0.05; Fig. 7). By contrast, a significant decrease in TSP-1 was detected in the culture medium of BM-EPCs from metformin-pretreated db/db mice (P<0.05; Fig. 7).
Figure 7.
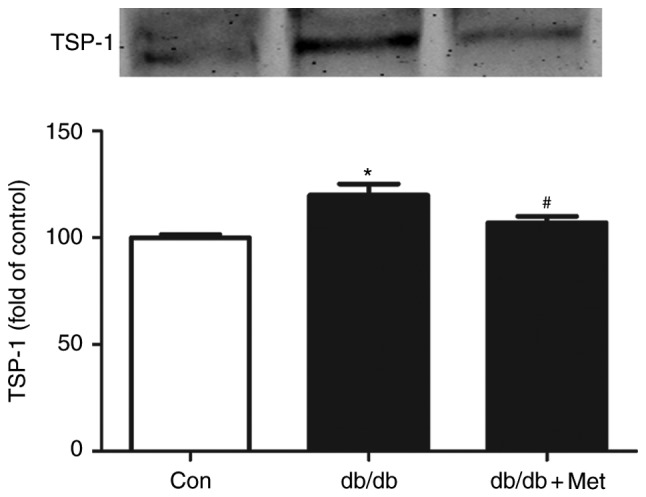
Metformin inhibits the secretion of TSP-1 in BM-EPC culture media from db/db mice. BM-EPCs were isolated and cultured from anesthetized mice. TSP-1 in BM-EPC culture media was subjected to western blotting, and metformin significantly decreased the expression of TSP-1 in BM-EPCs from db/db mice. *P<0.05 vs. Con; #P<0.05 vs. db/db. Values are expressed as the mean ± standard error of the mean (n=3 mice/group). Con, control; Met, metformin; TSP-1, thrombospondin-1.
Discussion
The principal findings of the present study were as follows: i) Metformin accelerated wound closure and improved angiogenesis in db/db mice; ii) metformin improved BM-EPC function in db/db mice; iii) metformin augmented the decreased NO level and reduced the increased O2− production in the BM-EPCs of db/db mice; and iv) metformin decreased the serum TSP-1 level secreted from the BM-EPCs of db/db mice.
In the present study, db/db mice, with genetic hyperglycemia akin to adult-onset T2DM as a consequence of an inactive gene mutation affecting leptin receptors, were specifically selected as the T2DM model (31–33); this model has been widely recognized to be well-established animal model for investigating the association between hyperglycemia, EPC function and wound closure (34). Wound healing following tissue damage is a sophisticated pathophysiological process which requires comprehensive interactions between cells and a variety of signaling molecules (35). However, diabetes may cause disorders in the tissue microenvironment, in addition to impairments in cellular function and wound healing. Angiogenesis is important for promoting vessel formation which, in turn, provides regenerating tissue with the required oxygen and nutrients (35,36). CD31 is a typical marker for tracing vascular and endothelial cells (37). It was observed in the present study that healing capacity was decreased in genetically diabetic db/db mice, as indicated by staining of CD31, when compared with non-diabetic mice. These data were consistent with a previous report (38).
Currently, regarding the role of metformin on EPC function and wound healing, the published literature is subjected to a degree of controversy. For example, previous studies have indicated that metformin contributed to accelerated wound healing and an enhanced quantity of circulating EPCs and BM-EPC function in diabetes (39,40). Another study held the opposite opinion, that metformin treatment did not result in any alterations in the circulating EPC number, and even caused delayed wound healing in diabetes (41). The results of the present study demonstrated that treatment with metformin accelerated wound healing and improved angiogenesis.
EPCs, as precursors of endothelial cells, can mobilize from the bone marrow into the circulation and have been implicated in neovascularization following tissue injury (5,23,42). Reduced EPC numbers and impaired EPC function have been observed in diabetic patients (43). Metformin, a biguanide family member, is a commonly applied therapeutic drug for T2DM (44,45). Studies have demonstrated that metformin contributed substantially to increasing the circulating EPC number and maintaining endothelial cell function in patients with T2DM (19), while the associated mechanisms remain largely unclear. In the present study, db/db mice pretreated with metformin (250 mg/kg/day) for 2 weeks exhibited an increased EPC number and improved EPC function.
Gao et al (46) demonstrated that NO serves an important role in regulating tube formation in EPCs. Gallagher et al (16) demonstrated that induced expression of NO in bone marrow was highly correlated with increased mobilization of EPCs to the circulation. In the present study, metformin contributed to the elevation of NO levels in EPCs from db/db mice, in addition to a significantly increased circulating EPC number and improved EPC function. It has been demonstrated that a decreased intracellular O2− level may be accompanied by an improvement in EPC dysfunction and an enhanced NO level in EPCs (25,26). In the present study, it was observed that the generation of O2− was increased, and treatment with metformin was able to inhibit the production of O2− in EPCs from db/db mice.
TSP-1, an endogenous anti-angiogenic mediator, has been demonstrated to be involved in vascular complications in diabetes (47). In a T2DM animal model, TSP-1 in the vessel walls was elevated at the mRNA and protein levels (22). In addition, preclinical studies suggested that the proangiogenic factor NO was a biological antagonist of TSP-1 (48,49). Ridnour et al (49) observed that the protective effect of NO on endothelial cells was accompanied by TSP-1 downregulation. Xu et al (50) reported that the TSP-1 level was upregulated in impaired retinal capillaries provoked by nitrative stress in diabetic rats. The results of the present study identified that TSP-1 was increased and NO was decreased in db/db mice, accompanied by wound healing delay, angiogenesis impairment and EPC dysfunction. Treatment with metformin was able to significantly alleviate disorders in wound healing, stimulate angiogenesis and improve EPC function, with TSP-1 levels inhibited and NO levels upregulated in BM-EPCs from db/db mice.
In conclusion, the results of the present study demonstrated that wound healing, angiogenesis and EPC function were impaired in db/db mice. Treatment with metformin was able to accelerate wound healing, which was possibly associated with an improvement in EPC function via a TSP-1/NO pathway.
Acknowledgements
The present study was supported by grants from the Natural Science Foundation of Hangzhou (grant nos. 20131813A20, 20130733Q41 and 20150633B58) and the Natural Science Foundation of Zhejiang (grant no. 2013RCB014). The abstract was presented at the American Association of Pharmaceutical Scientists Annual Meeting and Exposition, 13th-17th November 2016, in Denver, CO, USA.
Glossary
Abbreviations
- T2DM
type 2 diabetes mellitus
- BM-EPCs
bone marrow-endothelial precursor cells
- NO
nitric oxide
- O2-
superoxide
- TSP-1
thrombospondin-1
- CD31
platelet endothelial cell adhesion molecule
- DAF-FM
4-amino-5-methylamino-2′, 7′-difluorofluorescein
- DHE
dihydroethidium
- i.g.
intragastric
- Sca-1
ataxin-1
- Flk-1
vascular endothelial growth factor receptor 2
References
- 1.Chan JC, Cho NH, Tajima N, Shaw J. Diabetes in the Western Pacific Region-past, present and future. Diabetes Res Clin Pract. 2014;103:244–255. doi: 10.1016/j.diabres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Thandavarayan RA, Garikipati VN, Joladarashi D, Babu S Suresh, Jeyabal P, Verma SK, Mackie AR, Khan M, Arumugam S, Watanabe K, et al. Sirtuin-6 deficiency exacerbates diabetes-induced impairment of wound healing. Exp Dermatol. 2015;24:773–778. doi: 10.1111/exd.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanas N, Demetzos C, Pippa N, Maltezos E, Tentolouris N. Efficacy of a new heparan sulfate mimetic dressing in the healing of foot and lower extremity ulcerations in type 2 diabetes: A case series. Int J Low Extrem Wounds. 2016;15:63–67. doi: 10.1177/1534734616629302. [DOI] [PubMed] [Google Scholar]
- 4.Zgheib C, Liechty KW. Shedding light on miR-26a: Another key regulator of angiogenesis in diabetic wound healing. J Mol Cell Cardiol. 2016;92:203–205. doi: 10.1016/j.yjmcc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Li DW, Liu ZQ, Wei J, Liu Y, Hu LS. Contribution of endothelial progenitor cells to neovascularization (Review) Int J Mol Med. 2012;30:1000–1006. doi: 10.3892/ijmm.2012.1108. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, de Kreutzenberg S Vigili, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 7.Kovacic JC, Moore J, Herbert A, Ma D, Boehm M, Graham RM. Endothelial progenitor cells, angioblasts, and angiogenesis-old terms reconsidered from a current perspective. Trends Cardiovasc Med. 2008;18:45–51. doi: 10.1016/j.tcm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Liao YF, Chen LL, Zeng TS, Li YM, Fan Yu, Hu LJ, Ling Yue. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med. 2010;15:279–285. doi: 10.1177/1358863X10367537. [DOI] [PubMed] [Google Scholar]
- 9.Tepper OM. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.CIR.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 10.Wang CH, Ting MK, Verma S, Kuo LT, Yang NI, Hsieh IC, Wang SY, Hung A, Cherng WJ. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006;152:1051.e1–8. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi: 10.1186/s12933-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue WS, Lau KK, Siu CW, Wang M, Yan GH, Yiu KH, Tse HF. Impact of glycemic control on circulating endothelial progenitor cells and arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2011;10:113. doi: 10.1186/1475-2840-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badr G, Hozzein WN, Badr BM, Al Ghamdi A, Eldien HM Saad, Garraud O. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. J Cell Physiol. 2016;231:2159–2171. doi: 10.1002/jcp.25328. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher KA, Goldstein LJ, Thom SR, Velazquez OC. Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular. 2006;14:328–337. doi: 10.2310/6670.2006.00057. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori Y, Hattori K, Hayashi T. Pleiotropic benefits of metformin: Macrophage targeting its anti-inflammatory mechanisms. Diabetes. 2015;64:1907–1909. doi: 10.2337/db15-0090. [DOI] [PubMed] [Google Scholar]
- 18.Li DJ, Huang F, Lu WJ, Jiang GJ, Deng YP, Shen FM. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol (Oxf) 2015;213:711–721. doi: 10.1111/apha.12421. [DOI] [PubMed] [Google Scholar]
- 19.Chen LL, Liao YF, Zeng TS, Yu F, Li HQ, Feng Y. Effects of metformin plus gliclazide compared with metformin alone on circulating endothelial progenitor cell in type 2 diabetic patients. Endocrine. 2010;38:266–275. doi: 10.1007/s12020-010-9383-8. [DOI] [PubMed] [Google Scholar]
- 20.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, III, Lee MJ, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 23.Tie L, Chen LY, Chen DD, Xie HH, Channon KM, Chen AF. GTP cyclohydrolase I prevents diabetic-impaired endothelial progenitor cells and wound healing by suppressing oxidative stress/thrombospondin-1. Am J Physiol Endocrinol Metab. 2014;306:E1120–E1131. doi: 10.1152/ajpendo.00696.2013. [DOI] [PubMed] [Google Scholar]
- 24.Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, Lehnert H, Randeva HS. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res. 2009;83:566–574. doi: 10.1093/cvr/cvp131. [DOI] [PubMed] [Google Scholar]
- 25.Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56:1137–1144. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li ZP, Xin RJ, Yang H, Jiang GJ, Deng YP, Li DJ, Shen FM. Diazoxide accelerates wound healing by improving EPC function. Front Biosci (Landmark Ed) 2016;21:1039–1051. doi: 10.2741/4439. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Lu S, Yao Z, Deng YP, Zhang LD, Yu JW, Ren GF, Shen FM, Jiang GJ. Glibenclamide attenuates myocardial injury by lipopolysaccharides in streptozotocin-induced diabetic mice. Cardiovasc Diabetol. 2014;13:106. doi: 10.1186/s12933-014-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120:4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JK, Deng YP, Jiang GJ, Liu YZ, Zhao T, Shen FM. Establishment of tube formation assay of bone marrow-derived endothelial progenitor cells. CNS Neurosci Ther. 2013;19:533–535. doi: 10.1111/cns.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MH, Choi EN, Jeon YJ, Jung SC. Possible role of transforming growth factor-β1 and vascular endothelial growth factor in Fabry disease nephropathy. Int J Mol Med. 2012;30:1275–1280. doi: 10.3892/ijmm.2012.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyama H, Daitoku H, Fukamizu A. Nutrient control of phosphorylation and translocation of Foxo1 in C57BL/6 and db/db mice. Int J Mol Med. 2006;18:433–439. [PubMed] [Google Scholar]
- 32.Bao Q, Shen X, Qian L, Gong C, Nie M, Dong Y. Anti-diabetic activities of catalpol in db/db mice. Korean J Physiol Pharmacol. 2016;20:153–160. doi: 10.4196/kjpp.2016.20.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura Y, Murayama T, Minami M, Yokode M, Arai H. Differential effect of statins on diabetic nephropathy in db/db mice. Int J Mol Med. 2011;28:683–687. doi: 10.3892/ijmm.2011.769. [DOI] [PubMed] [Google Scholar]
- 34.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galeano M, Altavilla D, Cucinotta D, Russo GT, Calò M, Bitto A, Marini H, Marini R, Adamo EB, Seminara P, et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes. 2004;53:2509–2517. doi: 10.2337/diabetes.53.9.2509. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XN, Ma ZJ, Wang Y, Li YZ, Sun B, Guo X, Pan CQ, Chen LM. The four-herb Chinese medicine formula Tuo-Li-Xiao-Du-San accelerates cutaneous wound healing in streptozotocin-induced diabetic rats through reducing inflammation and increasing angiogenesis. J Diabetes Res. 2016;2016:5639129. doi: 10.1155/2016/5639129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caligiuri G, Groyer E, Khallou-Laschet J, Al Haj Zen A, Sainz J, Urbain D, Gaston AT, Lemitre M, Nicoletti A, Lafont A. Reduced immunoregulatory CD31+ T cells in the blood of atherosclerotic mice with plaque thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1659–1664. doi: 10.1161/01.ATV.0000172660.24580.b4. [DOI] [PubMed] [Google Scholar]
- 38.Tellechea A, Kafanas A, Leal EC, Tecilazich F, Kuchibhotla S, Auster ME, Kontoes I, Paolino J, Carvalho E, Nabzdyk LP, Veves A. Increased skin inflammation and blood vessel density in human and experimental diabetes. Int J Low Extrem Wounds. 2013;12:4–11. doi: 10.1177/1534734612474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complications. 2013;27:519–525. doi: 10.1016/j.jdiacomp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Lin JT, Chen HM, Chiu CH, Liang YJ. AMP-activated protein kinase activators in diabetic ulcers: From animal studies to Phase II drugs under investigation. Expert Opin Investig Drugs. 2014;23:1253–1265. doi: 10.1517/13543784.2014.922951. [DOI] [PubMed] [Google Scholar]
- 41.Ochoa-Gonzalez F, Cervantes-Villagrana AR, Fernandez-Ruiz JC, Nava-Ramirez HS, Hernandez-Correa AC, Enciso-Moreno JA, Castañeda-Delgado JE. Metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS One. 2016;11:e0150900. doi: 10.1371/journal.pone.0159468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao L, Huang M, Chen SC, Li YN, Xia YP, He QW, Wang MD, Huang Y, Zheng L, Hu B. Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neurosci Ther. 2014;20:460–468. doi: 10.1111/cns.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KA, Shin YJ, Kim JH, Lee H, Noh SY, Jang SH, Bae ON. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res. 2012;35:223–234. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Lakshmanan AP, Hwang MJ, Kubba H, Mushannen A, Triggle CR, Ding H. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem Pharmacol. 2015;98:412–421. doi: 10.1016/j.bcp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, He Z, Jia Z, Xu C. Inhibitory effect of metformin combined with gemcitabine on pancreatic cancer cells in vitro and in vivo. Mol Med Rep. 2016;14:2921–2928. doi: 10.3892/mmr.2016.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, Chang E, Chao L, Chao J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3:e001194. doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem. 2008;283:5699–5707. doi: 10.1074/jbc.M706435200. [DOI] [PubMed] [Google Scholar]
- 48.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1; Proc Natl Acad Sci USA; 2005; pp. 13147–13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Xun G, Yao Z, Liu Y, Qiu Y, Liu K, Zhu D, Gu Q, Xu X, Ho PC. Effects of generated trans-arachidonic acids on retinal capillary during nitrative stress in diabetic rats. Ophthalmologica. 2008;222:37–41. doi: 10.1159/000109277. [DOI] [PubMed] [Google Scholar]