Abstract
Baicalin, an active flavone isolated from Scutellaria baicalensis Georgi, has been demonstrated to induce various beneficial biochemical effects such as anti-inflammatory, anti-viral, and antitumor effects. However, the antitumor mechanism of baicalin is not well understood. In the present study, baicalin was demonstrated to inhibit the viability and migration of a widely used ovarian cancer cell line, A2780, in a dose-dependent manner. MTT assays revealed that cell viability significantly decreased in ovarian cancer cells treated with baicalin compared with untreated cells, without effect on normal ovarian cells. Flow cytometric analysis indicated that baicalin suppressed cell proliferation by inducing apoptosis. The underlying mechanisms involved were indicated to be downregulation of the anti-apoptotic protein B-cell lymphoma 2 apoptosis regulator and activation of caspase-3 and −9. In addition, wound healing and transwell assays revealed that cell migratory potential and expression of matrix metallopeptidase (MMP)-2 and MMP-9 were significantly inhibited when cells were exposed to baicalin, compared with untreated cells. The present study therefore suggested that baicalin has the potential to be used in novel anti-cancer therapeutic formulations for treatment of ovarian cancer.
Keywords: baicalin, antitumor effect, ovarian cancer, apoptosis, migration
Introduction
Ovarian cancer is the most lethal gynecologic cancer (1): The five-year survival rate of patients is <50% post diagnosis (2). First-line clinical treatments for ovarian cancer patients are cytoreductive surgery and paclitaxel-based chemotherapy (3). However, because of multi-drug resistance to chemotherapy, systemic chemotherapy produces a disappointingly low initial response in most patients (4). Furthermore, many commonly used anti-cancer chemotherapeutics have potent cytotoxic effects in normal cells (5). Therefore, there is an urgent need to develop effective, non-cytotoxic, chemotherapeutic approaches for patients with ovarian cancer.
In recent years, many bioactive phytochemicals have been observed to exhibit anti-cancer activities (6,7). They demonstrate minimal general toxicity and adverse side effects and thus may represent potential alternative medicine to conventional cytotoxic chemotherapy (8,9). Baicalin is a flavone glycoside found in Scutellaria baicalensis Georgi, with a chemical formula of C21H18O11 (10). Baicalin has been reported to have anti-oxidation, anti-proliferation, anti-inflammation and antitumor effects (11–13). With respect to cancer, it has been reported to inhibit the proliferation of various cancer cells through induction of apoptosis and inhibition of migration (14–16). However, the effects of baicalin on ovarian cancer cells and the underlying molecular mechanisms are still not clear.
The present study aimed to evaluate whether baicalin could exert antitumor effects on ovarian cancer cells and to explore the molecular mechanism of this process. The data revealed that baicalin dose-dependently induced apoptosis and significantly reduced the migration of ovarian cancer cells. Baicalin may, therefore, be an effective active ingredient for the development of an effective drug for patients with ovarian cancer.
Materials and methods
Materials, reagents and chemicals
Antibodies against caspase-3 (cat. no. 19677-1-AP), caspase-9 (cat. no. 10,380-1-AP), B-cell lymphoma 2 apoptosis regulator (Bcl-2) (cat. no. 12789-1-AP), matrix metallopeptidase (MMP)-2 (cat. no 10373-2-AP), MMP-9 (cat. no. 10375-1-AP) and β-actin (cat. no. 20,536-1-AP) were obtained from ProteinTech Group, Inc. (Chicago, IL, USA). Secondary polyclonal anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies (cat. no. 111-035-003) were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, sodium fluoride and EDTA) was from Beyotime Institute of Biotechnology (Haimen, China). The enhanced chemiluminescence (ECL) kit was from GE Healthcare Life Sciences (Little Chalfont, UK). The Annexin V-conjugated fluorescein isothiocyanate (FITC) apoptosis detection kit with propidium iodide (PI) was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Transwells were from BD Biosciences (San Jose, American). MTT (3-(4, 5-dimethyl-2-yl)-2, 5-diphenyl tetrazolium bromide) and DAPI were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Baicalin (concentration ≥ 98%) was bought from the National Pharmaceutical Engineering Center (Jiangxi, China).
Drug preparation
Baicalin was dissolved in 100% DMSO at a concentration of 1 M as a stock solution and stored at 4°C, and diluted in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to the required concentration before each experiment. The final concentration of DMSO was <0.1% in all baicalin groups.
Cell lines and cell culture
The ovarian cancer cell line A2780 and normal ovarian cell line IOSE80 were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with 5% CO2.
Cell viability assays
The effect of baicalin on the viability of cells was detected by MTT assay. The cells (1×104 cells/well) were seeded into 96-well plates and incubated for 24 h. Following 24 h treatment with 0 (control) 40, 80, 120, 160, 200 and 240 µM baicalin, cell viability was detected by adding 20 µl of MTT solution (5 mg/ml in PBS) to each well and incubating the mixtures for 4 h at 37°C. The MTT solution was then removed and 150 µl of dimethyl sulfoxide (DMSO) was added to the wells. The absorbance was measured using a Multiskan Ascent plate reader (Thermo Fisher Scientific, Inc.) at a wavelength of 540 nm.
DAPI staining assay
To assess the effect of baicalin on the nuclei of ovarian cancer cells, ~4×104 cells/well were treated with baicalin at 0, 80 or 160 µM for 24 h. Cells in each well were then stained with DAPI before fixation with 3.7% formaldehyde at room temperature for 15 min. The cells were then washed with PBS and detected by fluorescence microscopy. From each sample, 3 visual fields were randomly selected for evaluation.
Cell apoptosis by flow cytometry
The extent of apoptosis was evaluated by flow cytometry using an Annexin V-FITC/PI apoptosis detection kit (cat. no. CA1020, Solarbio, Beijing, China). Following treatment with either 0, 80 or 160 µM baicalin for 24 h, ovarian cancer cells (1×106 cells/well) were harvested and washed thrice with PBS, then incubated with Annexin V-FITC and PI at room temperature for 10 min in the dark. The cells were detected using a BD Accuri™ C6 flow cytometer and analyzed using BD Accuri™ C6 Software version 1.0.264.21 (BD Biosciences, Franklin Lakes, NJ, USA).
Wound healing assays
A2780 cells (1×105 cells/well) were seeded into 24-well plates and scraped with the end of 200 µl pipette tips. The plates were washed with PBS to remove detached cells and then incubated with the complete growth medium containing either 0, 20 or 40 µM baicalin solution for 24 h. Cell migration was observed under a phase-contrast microscope at 100× magnification at 0 and 24 h post-induction of injury. Migrated cells in the denuded area in each of six random fields were measured and quantified using Image J software version 1.50 (National Institutes of Health, Bethesda, MD, USA).
Transwell migration assays
Cell migration was quantified by transwell assays. Ovarian cancer cells were treated with 0, 20 or 40 µM baicalin for 24 h and harvested. A total of 2×104 cells in serum-free DMEM were added to each upper chamber and DMEM medium with 10% FBS was added to the lower chamber as a chemoattractant. After 24 h incubation at 37°C, cells remaining on the upper surface of membrane were removed and the cells that had migrated to the underside of the membrane were stained with 0.1% crystal violet for 10 min. The migrated cells on the underside of the membrane were counted under a light microscope under a 200× magnification field. A total of 6 random fields of each transwell membrane were counted and averaged.
Western blot analysis
Total protein was extracted from the cells samples using RIPA lysis buffer (Beyotime Institute of Biotechnology) with protease inhibitors (Biocolor Ltd., Beijing, China) in a proportion of 1:100. Keep on ice for 5 min, swirling the plate occasionally for uniform spreading. Centrifuge samples at 4°C, 12,000 × g for 15 min, transfer supernatant for further analysis. Equal amounts of protein (25 µg) were loaded on a 10% SDS-PAGE gel. The lysates were resolved by electrophoresis (80 V for 30 min and 120 V for 1.5 h) and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were blocked in 5% nonfat milk for 1 h at room temperature and then incubated with primary antibodies against caspase3 (1:1,000), caspase9 (1:1,000), Bcl-2 (1:1,000), MMP-2 (1:200), MMP-9 (1:200) or β-actin (1:1,000) in blocking buffer overnight at 4°C. This was followed by incubation with relevant secondary polyclonal anti-rabbit HRP-conjugated antibody (1:5,000) for 1 h at room temperature. Protein bands were visualized using a Chemiluminescent ECL assay kit (GE Healthcare Life Sciences) and the Bio-Rad ChemiDoc XRS+ image analyzer. Protein expression levels were quantitatively determined using Image J software version 1.50 (National Institutes of Health, Bethesda, MD, USA). β-actin was used as internal reference for protein expression in the treated cells.
Statistical analysis
Data are presented as the mean ± standard deviation of 3 independent experiments. For each independent experiment, the assays were performed in duplicate. Statistical differences between two groups were analyzed using a Student's t-test and multiple comparison analyses were performed by one-way analysis of variance followed by Tukey post-hoc testing. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Baicalin inhibits ovarian cancer cell viability
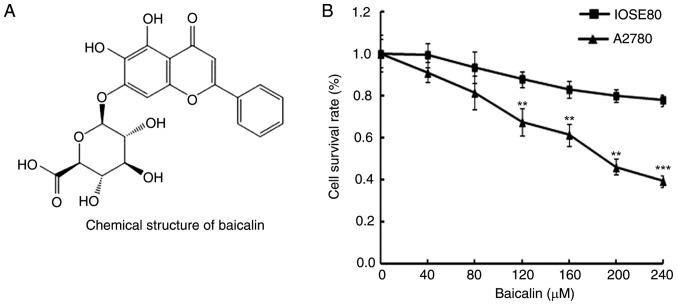
In order to determine the effects of baicalin (Fig. 1A) on the viability of A2780 ovarian cancer cells and IOSE80 normal ovarian cells, cells were treated with 0, 40, 80, 120, 160, 200 and 240 µM baicalin for 24 h, then cell viability was determined by MTT assay. Cancer cells treated with baicalin revealed significantly reduced viability compared with untreated cells, in a dose-dependent manner (Fig. 1B). However, baicalin did not affect the growth of normal ovarian cells (Fig. 1B). These data therefore indicated that baicalin inhibited the growth of ovarian cancer cells.
Figure 1.
Effects of baicalin on ovarian cancer cell and normal ovarian cell viability. (A) Chemical structure of baicalin. (B) Viability of A2780 ovarian cancer cells and IOSE80 normal ovarian cells was determined by MTT assay following treatment with 0, 40, 80, 120, 160, 200 or 240 µM baicalin for 24 h. Data are presented as the mean ± standard deviation of 3 replicates. **P<0.01 and ***P<0.001 vs. the 0 µM control group.
Baicalin induces ovarian cancer cell apoptosis
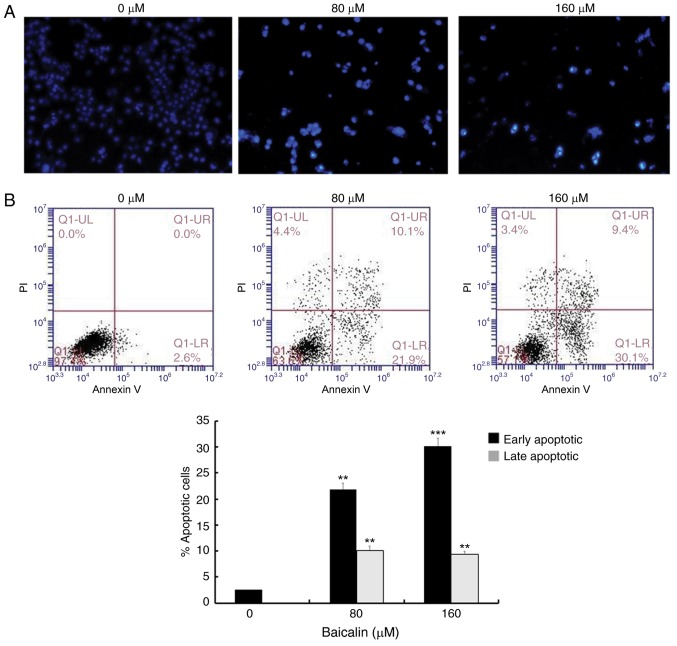
To assess whether the antitumor effects of baicalin on A2780 ovarian cancer cells were associated with apoptosis, cells were stained with DAPI and observed under a fluorescence microscope (Fig. 2A). Nuclear chromatin condensation and fragmented punctuate blue nuclear fluorescence were observed in ovarian cancer cells treated with 80 and 160 µM baicalin for 24 h, in a dose-dependent manner, while the control cells displayed normal and intact nuclei. This suggested that baicalin may induce ovarian cancer cell apoptosis. To further investigate this, apoptosis was analyzed by flow cytometry (Fig. 2B). The parentage of early- and late-stage apoptotic cells significantly increased in groups treated with baicalin compared with untreated control cells (Fig. 2B): The percentage of total apoptotic A2780 cells was 2.6% in the control cells (2.6% early-stage and 0% late-stage), 32% in the cells treated with 80 µM baicalin (21.9% early-stage and 10.9% late-stage) and 39.5% in the cells treated with 160 µM baicalin (30.1% early-stage and 9.4% late-stage). These results demonstrated that baicalin induces apoptosis in A2780 ovarian cancer cells.
Figure 2.
Baicalin induces apoptosis in A2780 ovarian cancer cells. (A) Cell nucleus morphology in cells treated with 0, 80 or 160 µM baicalin was observed by DAPI staining (original magnification, ×100). (B) Apoptosis was detected by Annexin V/PI staining following treatment with 0, 80 or 160 µM baicalin. Early apoptotic cells (Annexin V+/PI−) are shown in the lower right quadrant of the chart, while the upper right quadrant indicates late apoptotic cells (Annexin V+/PI+). Quantitative data are presented as the mean ± standard deviation of 3 replicates. **P<0.01 and ***P<0.001 vs. the 0 µM control group. PI, propidium iodide.
Baicalin suppresses migration of ovarian cancer cells by antagonizing MMP-9 expression
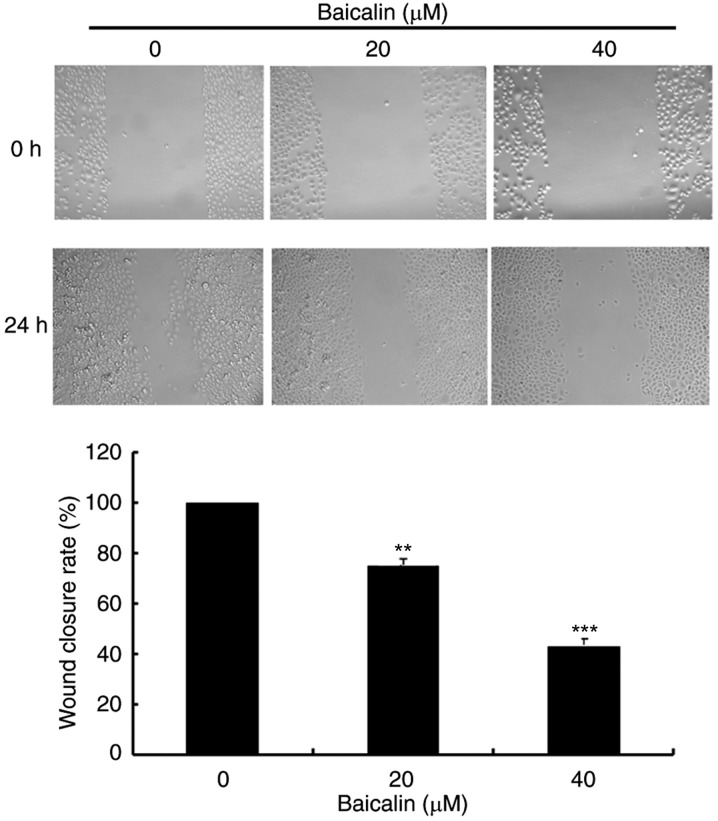
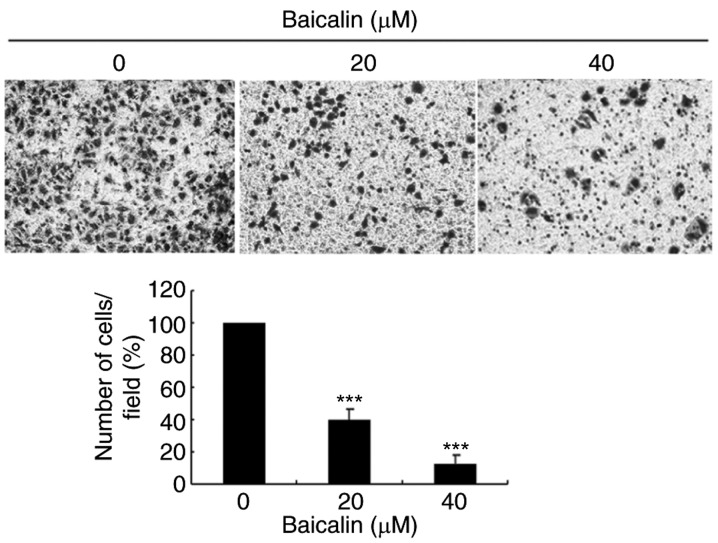
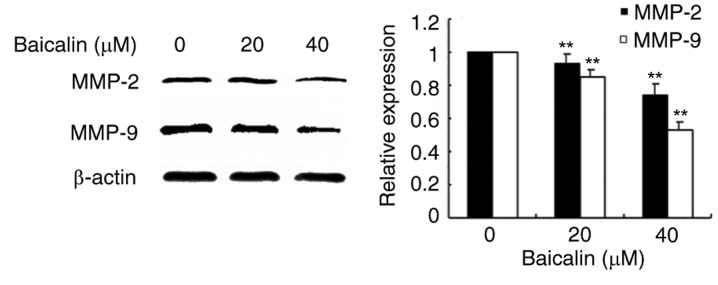
To evaluate the effects of baicalin on cell migration, wound healing assays and transwell assays were performed. In the wound healing assay, baicalin dose-dependently significantly decreased the migration of A2780 cells compared with the untreated control (Fig. 3). Likewise, baicalin significantly inhibited ovarian cancer cell migration in a 24 h transwell assay, in a dose-dependent manner, compared with untreated control cells (Fig. 4). Treatment with 20 and 40 µM baicalin inhibited A2780 cells migration by 59 and 87% respectively (P<0.001; Fig. 4). The wound healing and transwell chamber assays both suggested that baicalin suppresses the migration of ovarian cancer cells. Given the effects of baicalin on ovarian cancer cell migration, the mechanisms of this process were further investigated. Since MMPs plays an important role in cancer metastasis, MMP-2 and MMP-9 protein expression levels were detected by western blot. Baicalin dose-dependently reduced MMP-2 and MMP-9 protein expression levels in treated cells compared with untreated cells (Fig. 5). These data therefore suggested that the inhibitory effect of baicalin on the migration of ovarian cancer was at least partially associated with downregulation of MMP-2 and MMP-9 expression.
Figure 3.
Effects of baicalin on A2780 ovarian cancer cell migration, assessed by wound healing assay. Cells were scraped with a pipette tip and treated with 0, 20 or 40 µM baicalin for 24 h. Cells were imaged under a light microscope (original magnification, ×100) before and after injury. Cell migration was quantified by measuring wound closure areas before and after injury. Quantitative data are presented as the mean ± standard deviation of 3 replicates. **P<0.01 and ***P<0.001 vs. the 0 µM control group.
Figure 4.
Effects of baicalin on A2780 ovarian cancer cell migration, assessed by transwell assay. Cells were treated. Images represent cell migration to the underside of transwell membranes following treatment with 0, 20 or 40 µM baicalin for 24 h (original magnification, ×200). Quantitative data are presented as the mean ± standard deviation of 3 replicates, relative to the 0 µM control group. **P<0.01 and ***P<0.001 vs. the 0 µM control group.
Figure 5.
Effects of baicalin on protein expression levels of MMP-2 and −9. Protein expression levels of MMP-9 and MMP-2 were assessed by western blotting analysis following treatment with 0, 20 or 40 µM baicalin for 24 h. β-actin served as a loading control. Quantitative data are presented as the mean ± standard deviation of 3 replicates. **P<0.01 vs. the 0 µM control group. MMP, matrix metallopeptidase.
The effects of baicalin on apoptosis-related proteins
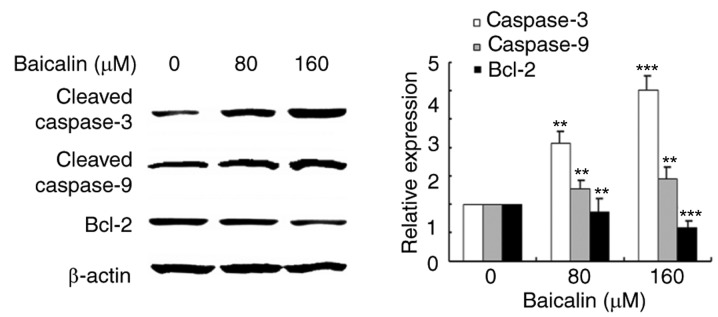
Since baicalin was demonstrated to induce apoptosis in ovarian cancer cells, the expression and activation of apoptosis related proteins was investigated by western blotting analysis (Fig. 6). Protein expression levels of Bcl-2, an anti-apoptotic protein, decreased in ovarian cancer cells treated with baicalin in a dose-dependent manner, compared with untreated control cells. Cleavage of caspase-3 and caspase-9 was also measured in the study: The results revealed that cleaved-caspase-3 and cleaved-caspase-9 levels increased in baicalin-treated ovarian cancer cells compared with untreated control cells. These results suggested that baicalin may activate the caspase-dependent apoptosis pathway in A2780 cells.
Figure 6.
Effects of baicalin on protein expression levels of apoptosis-related proteins. Protein expression levels of cleaved caspase-3, cleaved caspase-9 and Bcl-2, were assessed by western blotting analysis following treatment with 0, 80 or 160 µM baicalin for 24 h. β-actin served as a loading control. Quantitative data are presented as the mean ± standard deviation of 3 replicates. **P<0.01 and ***P<0.001 vs. the 0 µM control group. Bcl-2, B-cell lymphoma 2 apoptosis regulator.
Discussion
Apoptosis is a fundamental life phenomenon through the whole process of life (17). It has been reported that in many human tumor cells, the proliferation of cells is unrestricted if cells apoptosis is definitely hindered (18). Therefore, the antitumor effects of baicalin on ovarian cancer cells was investigated as a strategy to identify new and effective drugs for patients with ovarian cancer. Baicalin has previously been demonstrated to inhibit platelet-derived growth factor-BB-stimulated vascular smooth muscle cell proliferation through suppressing β-type platelet-derived growth factor receptor/extracellular signal-regulated kinase signaling (19). Baicalin, a phytochemical component of Scutellaria baicalensis Georgi has widespread applications as anti-inflammatory, anti-hepatitis and anti-oxidation agent (11,20,21). Furthermore, the anti-cancer effect of baicalin has also been previously documented (22). The present study aimed to explore the effects of baicalin on ovarian cancer cells and analyze the mechanisms underlining the observed effects.
Monomer compounds extracted from plants have previously been reported to induce apoptosis (23–25). Apoptosis is programmed cell death and plays a vital role in eliminating mutated or hyper-growing cancer cells. Various natural compounds have been shown to suppress the growth of tumor cells by inducing apoptosis (26–28). Therefore, induction of apoptosis has become the major target of most anti-cancer agents. It has been reported that baicalin inhibits the proliferation of HeLa cells via the induction of apoptosis through the intracellular mitochondrial pathway (29). The present study indicated that baicalin significantly reduces the viability of ovarian cancer cells, with no significant effect observed in normal ovarian cells, and that ovarian cancer cells treated with baicalin displayed specific apoptotic morphological changes. In addition, the percentage of early and late apoptotic ovarian cancer cells significantly increased following treatment with baicalin. Thus, baicalin may specifically and significantly induce apoptosis of ovarian cancer cells without affecting normal ovarian cells. Mitochondrial proteins directly activate cellular apoptotic programs (30,31). Bcl-2 is involved in the mitochondria-associated apoptotic pathway (32). Downregulation of Bcl-2 expression could lead to loss of mitochondrial membrane potential and trigger a series of apoptotic events such as activation of caspase-9 and caspase-3, as observed in this study. Peng et al (29) indicated that baicalin-induces apoptosis in HeLa cells through activation of caspase-3 through the intracellular mitochondrial pathway and the surface death receptor pathway, however, it did not show that baicalin could suppress migration of ovarian cancer cells by antagonizing MMP2/9 expression. The present study demonstrated that baicalin not only induced ovarian cancer cell apoptosis via the intracellular mitochondrial pathway but also suppressed they migratory ability of ovarian cancer cells by antagonizing MMP2/9 expression. These data suggested that baicalin could induce cell death through the mitochondria-associated apoptotic pathway in A2780 epithelial ovarian cancer cells, but requires further confirmation in other cell models of ovarian cancer.
Additionally, the present study demonstrated that baicalin effectively suppressed ovarian cancer cell migration. MMP-9 and MMP-2 belong to the gelatin enzyme class of proteases (33,34). A recent study has suggested that the expression of MMP-9 is associated with metastasis in ovarian cancer (35). Also, the inhibition of MMP-2 expression suppresses the metastatic potential of prostate cancer cells (36). The present study demonstrated that baicalin significantly inhibited ovarian cancer cell migration and that MMP-9 and MMP-2 protein expression was reduced by baicalin treatment in a dose-dependent manner. These findings indicate that baicalin may suppress ovarian cancer cell migration through downregulation of MMP-9 and MMP-2 expression.
In conclusion, to the best of our knowledge, the present study is the first to demonstrate that baicalin may function as a selective antitumor agent for ovarian cancer by inhibiting cell viability, inducing apoptosis and suppressing ovarian cancer cell migration. These data thus suggested that baicalin may potentially be used in the formulation of a novel and effective antitumor treatment for ovarian cancer patients.
Acknowledgements
We are grateful to Professor Nazir Ahmad from the University of Agriculture, Faisalabad, Pakistan for detailed correction of the manuscript. The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 31572590, 31772815 and 31502138), Shandong Province (grant no. BS2015NY001), and Higher Educational Science and Technology Program of Shandong Province (grant no. J15LF03).
References
- 1.Temkin SM, Tanner EJ, Dewdney SB, Minasian LM. Reducing overtreatment in gynecologic oncology: The case for less in endometrial and ovarian cancer. Front Oncol. 2016;6:118. doi: 10.3389/fonc.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: A systematic review. Gynecol Oncol. 2007;105:801–812. doi: 10.1016/j.ygyno.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Markman M. Current status and future directions of platinum/paclitaxel-based chemotherapy of ovarian cancer. Semin Oncol. 1997;24(4 Suppl 11):S11–S27. [PubMed] [Google Scholar]
- 4.Fujiwara Y, Takaishi K, Nakao J, Ikeda T, Katabuchi H, Takeya M, Komohara Y. Corosolic acid enhances the antitumor effects of chemotherapy on epithelial ovarian cancer by inhibiting signal transducer and activator of transcription 3 signaling. Oncol Lett. 2013;6:1619–1623. doi: 10.3892/ol.2013.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy J, Maltais R, Jegham H, Poirier D. Libraries of 2β-(N-substituted piperazino)-5α-androstane-3α, 17β-diols: Chemical synthesis and cytotoxic effects on human leukemia HL-60 cells and on normal lymphocytes. Mol Divers. 2011;15:317–339. doi: 10.1007/s11030-010-9273-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Xu T, Zhang Y, Zhu M, Zhu W, Wang Z, Gu H, Wang H, Li P, Ying J, et al. Transcriptional network in ovarian cancer cell line SKOV3 treated with Pinellia pedatisecta Schott extract. Oncol Rep. 2016;36:462–470. doi: 10.3892/or.2016.4779. [DOI] [PubMed] [Google Scholar]
- 7.Yung MM, Ross FA, Hardie DG, Leung TH1, Zhan J, Ngan HY, Chan DW. Bitter Melon (Momordica charantia) extract inhibits tumorigenicity and overcomes cisplatin-resistance in ovarian cancer cells through targeting AMPK signaling cascade. Integr Cancer Ther. 2016;15:376–389. doi: 10.1177/1534735415611747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Ahmed KM. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent J. 2013;25:141–147. doi: 10.1016/j.sdentj.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HB, Lu P, Cao WB, Zhang ZH, Meng XL. The effect-enhancing and toxicity-reducing activity of Hypericum japonicum Thunb. Extract in murine liver cancer chemotherapy. Mol Clin Oncol. 2013;1:395–399. doi: 10.3892/mco.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47:1749–1758. doi: 10.3892/ijo.2015.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Chi S, Cong X, Li H, Jiang Z, Cao R, Tian W. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod Toxicol. 2015;57:196–203. doi: 10.1016/j.reprotox.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Sun SJ, Wu XP, Song HL, Li GQ. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. Int J Clin Exp Med. 2015;8:22063–22072. [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Pei M, Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med. 2015;8:8958–8967. [PMC free article] [PubMed] [Google Scholar]
- 14.Cortés-Castell E, Veciana-Galindo C, Torró-Montell L, Palazón-Bru A, Sirvent-Segura E, Gil-Guillén V, Rizo-Baeza M. Protection by polyphenol extract from olive stones against apoptosis produced by oxidative stress in human neuroblastoma cells. Nutr Hosp. 2016;33:118–122. doi: 10.20960/nh.39. [DOI] [PubMed] [Google Scholar]
- 15.Kim GT, Lee SH, Kim YM. Torilis japonica extract-generated intracellular ROS induces apoptosis by reducing the mitochondrial membrane potential via regulation of the AMPK-p38 MAPK signaling pathway in HCT116 colon cancer. Int J Oncol. 2016;49:1088–1098. doi: 10.3892/ijo.2016.3578. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S, Cho GS, Ryu S, Kim HJ, Song HY, Yune TY, Ju C, Kim WK. Post-ischemic treatment of WIB801C, standardized Cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J Ethnopharmacol. 2016;186:169–180. doi: 10.1016/j.jep.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Afford S, Randhawa S. Apoptosis. Mol Pathol. 2000;53:55–63. doi: 10.1136/mp.53.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin SJ, Lennon SV, Bonham AM, Cotter TG. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 19.Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ, Sun RH, Wu Y, Han M. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRbeta-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010;20:1252–1262. doi: 10.1038/cr.2010.111. [DOI] [PubMed] [Google Scholar]
- 20.Ye C, Li S, Yao W, Xu L, Qiu Y, Liu Y, Wu Z, Hou Y. The anti-inflammatory effects of baicalin through suppression of NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes. Innate Immun. 2016;22:196–204. doi: 10.1177/1753425916631032. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Liu L, Wang Y, Mao T, Li J. Effects of a combination of puerarin, baicalin and berberine on the expression of proliferator-activated receptor-γ and insulin receptor in a rat model of nonalcoholic fatty liver disease. Exp Ther Med. 2016;11:183–190. doi: 10.3892/etm.2015.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange I, Moschny J, Tamanyan K, Khutsishvili M, Atha D, Borris RP, Koomoa DL. Scrophularia orientalis extract induces calcium signaling and apoptosis in neuroblastoma cells. Int J Oncol. 2016;48:1608–1616. doi: 10.3892/ijo.2016.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Hong S, Seong GJ, Kim CY. Cigarette smoke extract causes injury in primary retinal ganglion cells via apoptosis and autophagy. Curr Eye Res. 2016;41:1367–1372. doi: 10.3109/02713683.2015.1119856. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimzadeh-Bideskan AR, Hami J, Alipour F, Haghir H, Fazel AR, Sadeghi A. Protective effects of ascorbic acid and garlic extract against lead-induced apoptosis in developing rat hippocampus. Metab Brain Dis. 2016;31:1123–1132. doi: 10.1007/s11011-016-9837-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim MK, Choi HS, Cho SG, Shin YC, Ko SG. Rubus coreanus Miquel extract causes apoptosis of doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK phosphorylation. Mol Med Rep. 2016;13:4065–4072. doi: 10.3892/mmr.2016.4996. [DOI] [PubMed] [Google Scholar]
- 26.Lin R, Li Z, Lin J, Ye J, Cai Q, Chen L, Peng J. Ethanolic extract of Tulipa edulis Bak induces apoptosis in SGC-7901 human gastric carcinoma cells via the mitochondrial signaling pathway. Oncol Lett. 2015;10:2371–2377. doi: 10.3892/ol.2015.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Listyawati S, Sismindari, Mubarika S, Murti YB, Ikawati M. Anti-proliferative activity and apoptosis induction of an ethanolic extract of boesenbergia pandurata (Roxb.) schlecht. Against HeLa and vero cell lines. Asian Pac J Cancer Prev. 2016;17:183–187. doi: 10.7314/APJCP.2016.17.1.183. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Wang B. Ginkgo biloba extract attenuates oxidative stress and apoptosis in mouse cochlear neural stem cells. Phytother Res. 2016;30:774–780. doi: 10.1002/ptr.5572. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y, Fu ZZ, Guo CS, Zhang YX, Di Y, Jiang B, Li QW. Effects and mechanism of baicalin on apoptosis of cervical cancer HeLa cells in-vitro. Iran J Pharm Res. 2015;14:251–261. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M, Li SJ, Xin YN, Ji SS, Xie RJ, Xuan SY. Ferric nitrilotriacetate (Fe-NTA)-induced reactive oxidative species protects human hepatic stellate cells from apoptosis by regulating Bcl-2 family proteins and mitochondrial membrane potential. Int J Clin Exp Med. 2015;8:18074–18081. [PMC free article] [PubMed] [Google Scholar]
- 31.Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006;1762:191–201. doi: 10.1016/j.bbadis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Soriano ME, Scorrano L. The interplay between BCL-2 family proteins and mitochondrial morphology in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:97–114. doi: 10.1007/978-1-4419-6706-0_6. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Zeng YP, Zhou Q, Guan JX, Lu ZN. The effect of captopril on the expression of MMP-9 and the prognosis of neurological function in herpes simplex encephalitis mice. Neurol Res. 2016;38:733–739. doi: 10.1080/01616412.2016.1202462. [DOI] [PubMed] [Google Scholar]
- 34.Li NA, Wang H, Zhang J, Zhao E. Knockdown of hypoxia inducible factor-2α inhibits cell invasion via the downregulation of MMP-2 expression in breast cancer cells. Oncol Lett. 2016;11:3743–3748. doi: 10.3892/ol.2016.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li XM, Dai JM, He H, Wang J, Peng HJ, et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett. 2015;359:241–249. doi: 10.1016/j.canlet.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Chen A, Guo F, Yuan L. A short-hairpin RNA targeting osteopontin downregulates MMP-2 and MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett. 2010;295:27–37. doi: 10.1016/j.canlet.2010.02.012. [DOI] [PubMed] [Google Scholar]