Abstract
Stroke caused by atherosclerosis remains a leading cause of morbidity and mortality worldwide, associated with carotid plaque rupture and inflammation progression. However, the inflammatory biomarkers which aid in predicting the future course of plaques are less detailed. The present study investigated the association between plaque vulnerable and inflammatory biomarkers using blood and plaque specimens. Carotid plaque specimens were obtained from 80 patients following stroke, 14 patients suffering from transient ischaemic attack and 17 asymptomatic patients that underwent carotid endarterectomy. To assess changes in plaque characteristics at histological levels, plaques were categorized by the time between the latest ischemic stroke and surgical intervention within 30, 30–90, 90–180 and over 180 days following stroke. Serum levels of inflammatory biomarkers interleukin (IL)-6, IL-10 and kinin B1 receptor (B1R) were measured by ELISA. Histological assessment of plaque was used to evaluate the plaque stability, progression and the inflammatory biomarker levels. Comparisons of histological characteristics demonstrated that plaques revealed an unstable phenotype following stroke within 30, 30–90 days and then remodeled into more stable plaques following stroke at 90–180 and over 180 days. By comparing the serum levels of inflammatory biomarkers, it was observed that IL-6 and B1R levels tended to decline whereas IL-10 levels increased in stroke patients from <30 days to over 180 days. Immunohistochemical analysis of IL-6, IL-10 and B1R demonstrated similar alterations in serum levels. Correlation analyses revealed that only B1R serum level was significantly correlated with histological level in patients with carotid atherosclerosis. The findings revealed that serum B1R levels may provide prognostic information and currently act as potential indicators for progression in atherosclerosis.
Keywords: atherosclerosis, carotid atherosclerotic plaque, stroke, kinin B1 receptor, inflammation
Introduction
Stroke caused by atherosclerosis remains a leading cause of morbidity and mortality with over 15 million strokes occurring every year in the world (1). The rapture of vulnerable atherosclerotic plaque is the common reason in acute cerebrovascular event, impacting prognostic implications and eventually ending in death of patients (2). The progression of vulnerable plaques rupture has not been clearly elucidated, and it tends to be treated with routine clinical measures (3). Prognostic biomarker, which aid in predicting the future course of plaque, may be used to identify phenotype of plaques, especially the vulnerable one. Disease progression biomarker can also serve as a marker of pharmacodynamic effect, which have the potential to show the biological response occurred in a patient who received an experimental therapeutic, may help in assessing the efficacy of this treatment. Additionally, inflammation in ischemic stroke plays an important role in predicting the outcome in ischemic brain damage extent (4,5). Therefore, this study investigated changes of both serum and histological inflammatory markers in plaque of varying stages of stroke patients in order to find a prognostic biomarker in serum level which can accurately predicting the future course of plaque.
It is well documented that increased plasma levels of inflammatory markers such as interleukin (IL) which are associated with a higher cardiovascular risk (6). It has already been described that IL-6 has predictive value for future cardiovascular events and is found to be expressed in atherosclerotic tissue and have pro-inflammatory properties by activating and recruiting inflammatory cells which can contribute to plaque instability (7,8). By contrast, IL-10 is a potent anti-inflammatory cytokine with macrophage deactivating properties (9). Study has showed that serum levels of anti-inflammatory marker IL-10 were downregulated in patients with vulnerable plaques (7). However, the pattern of IL-6 and IL-10 on serum level changes in stroke patients of different stages and the best utility for assessing the progression of plaque has not been determined.
Kinins, a pro-inflammatory and vasoactive peptide, can act through the activation of two G protein-coupled receptors, denoted as B1 and B2 receptors (B1R and B2R) (10,11). B1R is weakly expressed under physiological conditions, but is significantly elevated during inflammatory conditions or after tissue damage whereas B2R is constitutively expressed (12). B1R expression is more pronounced in regions of plaques from human plaques tissue compared with regions devoid of plaques, indicating that B1R is closely related to atheromatous diseases (13). Furthermore, our preliminary study has demonstrated that B1R is upregulated in human symptomatic carotid atherosclerotic plaques (14). A variety of studies have attempted to explore inflammatory markers in plaques and its relationship with predictive value for future cerebrovascular events, but most of them failed to elaborate the dynamic changes of these inflammatory markers in different stages of human atherosclerotic plaques patients. However, an explicit study of dynamic changes of these inflammatory markers may facilitate future efforts towards early diagnosis of vulnerable plaques and monitoring of disease progression.
The goal of the present study was to evaluate serum levels of inflammation markers in plaques after stroke in different stages of patients (<30, 30–90, 90–180 and >180 days) to investigate the inflammatory markers as a potential disease progression and prognostic biomarker in the process of plaque remodeling and stability and then explore their possible correlations with histological changes; the purpose was to provide experimental cues for management of patients with carotid atherosclerotic plaque using serum biomarkers.
Materials and methods
Subjects
All patients undergoing carotid endarterectomy (CEA) in two participating hospitals are asked to participate in the study. The Medical Ethics Committee of both hospitals approved the study, and participants provided written informed consent. For the current research questions we have studied the atherosclerotic plaques from 111 consecutive patients (stroke [n=80], TIA [n=14] and asymptomatic [n=17]) who were included between April 2014 and June 2017. From all patients, baseline data were obtained by extensive questionnaires including history of vascular disease, cardiovascular risk factors, and medication use (Table I). All patients were reviewed by the vascular surgeon or neurologist before CEA to assess the nature and timing of clinical symptoms. Patients who suffered from stroke were categorized into 4 different groups according to the time between ischemic event and surgery (<30, 30–90, 90–180 and >180 days). Results from asymptomatic patients served as negative values in comparison with data obtained from patients suffering from stroke, and TIA served as positive values.
Table I.
Baseline characteristics of the study population.
| Stroke | |||||||
|---|---|---|---|---|---|---|---|
| <30 days | 30–90 days | 90–180 days | >180 days | TIA | Asymptomatic | P-value | |
| N | 16 | 24 | 32 | 8 | 14 | 17 | – |
| Age, y, mean ± SD | 66.0±5.8 | 65.2±6.8 | 68.3±7.2 | 67.8±5.3 | 68.1±6.3 | 68.1±5.6 | 0.50 |
| Male, % (n) | 68.8 (11) | 70.8 (17) | 68.8 (22) | 87.5 (7) | 85.7 (12) | 70.6 (12) | 0.77 |
| Hypertension, % (n) | 68.8 (11) | 79.2 (19) | 71.9 (23) | 50.0 (4) | 71.4 (10) | 76.5 (13) | 0.73 |
| Diabetes, % (n) | 25.0 (4) | 29.1 (7) | 18.8 (6) | 12.5 (1) | 21.4 (3) | 29.4 (5) | 0.88 |
| Smoking, % (n) | 31.3 (5) | 50.0 (12) | 37.5 (12) | 25.0 (2) | 35.7 (5) | 35.3 (6) | 0.79 |
| Hyperlipidemia, % (n) | 41.7 (10) | 33.3 (8) | 62.5 (20) | 50.0 (4) | 57.1 (8) | 64.7 (11) | 0.27 |
| Statin, % (n) | 75.0 (12) | 70.8 (17) | 65.6 (21) | 62.5 (5) | 50.0 (7) | 82.4 (14) | 0.78 |
| Aspirin, % (n) | 87.5 (14) | 79.2 (19) | 59.4 (19) | 87.5 (7) | 57.1 (8) | 52.9 (9) | 0.10 |
| Anticoagulants, % (n) | 18.7 (3) | 8.3 (2) | 6.3 (2) | – | 14.3 (2) | 5.9 (1) | 0.63 |
| NSAID, % (n) | 6.3 (1) | 8.3 (2) | 12.5 (4) | 12.5 (1) | 28.6 (4) | 17.6 (3) | 0.51 |
| ACE-inhibitor, % (n) | 50.0 (8) | 37.5 (9) | 31.3 (10) | 12.5 (1) | 64.3 (9) | 41.2 (7) | 0.17 |
NSAID, nonsteroidal anti-inflammatory drug; ACE-inhibitor, angiotensin-converting enzyme-inhibitor.
Measurement of serum inflammatory markers by ELISA
Blood sampling of control patients were drawn by venipuncture one day before CEA. All serum samples was centrifuged for 10 min at 2,000 × g and then stored at −80°C until later analysis. The serum levels of the IL-6, B1R and IL-10 were measured using commercially available ELISA kits according to the manufacturer's protocol (Cusabio, Wuhan, China).
Histological analysis
The atherosclerotic lesions were dissected into 5 mm segments. Segment was fixed in formaldehyde 4% and then embedded in paraffin. The segment having the greatest plaque area was used to assess the plaque phenotype for histological analyses. For measuring changes in plaque composition in histology, 4 µm thick sections were cut from each paraffin block and stained with hematoxylin and eosin (H&E) for histology and Picro-sirius red for collagen. For immunohistochemical analyses, deparaffinized sections were incubated with 1% H2O2 in methanol for 3 min to eliminate endogenous peroxidase activity. After blocking with 10% normal serum, the sections were incubated for 1 h at room temperature with the primary antibodies with CD68 antibody for macrophages (1:300, GB13067-M-1, Servicebio, WuHan, China); IL-6 (1:500, GB1117, Servicebio) and IL-10 (1:100, sc-32815, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and B1R antibody (1:100, Bs-8675R, Bioss, WuHan, China). After washes in phosphate-buffered saline for three times, the sections were incubated with biotinylated secondary antibodies for 1 h and then with the avidin-biotinylated horseradish peroxidase complex (ABC Elite kit; Vector Laboratories Inc., Burlingame, CA, USA) for 30 min. Counterstaining was performed on representative sections with hematoxylin, and then the sections were subject to image analysis (Leica TCS SP2; Leica, Wetzlar, Germany) and analyzed using the Image Pro Plus 6.0 software package (Media Cybernetics Inc., Rockville, MD, USA) to calculate the percentage of positive area in each microscopic view at 200× magnification in plaques.
Statistical analysis
All results were expressed as means ± SD. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). For statistical analyses of baseline characteristics of patients, the one-factor ANOVA test was used for continuous variables and the χ2 test for categorical variables. For analysis of serum and histological data, ANOVA followed by Tukey multiple comparison tests (α=0.05) was used to calculate the difference between any two groups of patients. Spearman's correlation analysis was used to predict the association between serum and histological parameters in patients. Receiver operating curve was constructed to identify the cutoff point. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical and histological characteristics
The study population includes 17 asymptomatic, 14 TIA and 80 patients with stroke of different stages (<30, 30–90, 90–180 and >180 days). Baseline characteristics for all patients are documented in Table I. There were no statistically significant differences among different groups with respect to clinical characteristics, risk factors or drug use.
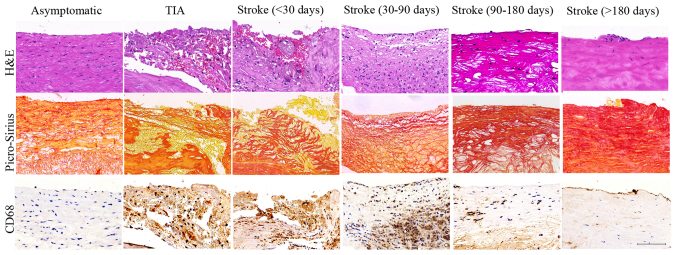
H&E, Picro-sirius red and CD68 staining were used to reveal the plaque histological characteristics in stroke patients of <30, 30–90, 90–180 and >180 days; typical images are showed in Fig. 1. Compared with asymptomatic group, plaques from stroke patients showed intense macrophage infiltration, intraplaque hemorrhage and large lipid core size at stroke of <30 days; and then declined at stroke of 30–90 days; this decline was notably at stroke of 90–180 days and to basal levels at >180 days (ANOVA, all P<0.05). By contrast, smooth muscle cells (SMC) content increased progressively in plaques of stroke from <30 to >180 days (ANOVA, P<0.05). However, no significant difference was found in stroke of <30 days and TIA groups; stroke of >180 days and asymptomatic groups (all P>0.05). These results suggest that stroke patients of <30 and 30–90 days presented the early stage of vulnerable plaque, whereas stroke patients of 90–180 and >180 days exhibited the process of vulnerable plaques remodeling to stable plaques.
Figure 1.
Histological characteristics of plaques. Representative images of plaques in asymptomatic, TIA, stroke patients of <30, 30–90, 90–180 and >180 days. H&E staining showed inflammatory cell infiltrations (TIA, Stroke <30 d and Stroke 30–90 d groups) and intraplaque hemorrhage (TIA, Stroke <30 d groups) in the vulnerable plaques and intact cap and predominantly fibrous tissue in the stable plaques (Asymptomatic, Stroke 90–180 d and Stroke >180 d groups). Picro-Sirius Red staining showed the large cholesterol crystals in the lipid core in vulnerable plaques (TIA, Stroke <30 d and Stroke 30–90 d groups) and collagen fibrous tissue in stable plaques (Asymptomatic, Stroke 90–180 d and Stroke >180 d groups). CD 68 staining showed histological appearance of a plaque with substantial macrophage infiltration in the vulnerable plaques (TIA, Stroke <30 d and Stroke 30–90 d groups) and minor macrophage infiltration in stable plaques (Asymptomatic, Stroke 90–180 d and Stroke >180 d groups). Scale bar: 100 µm.
Serum levels of inflammatory markers
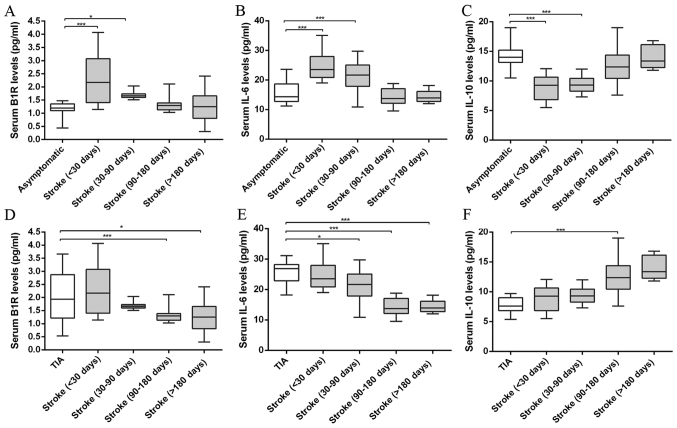
The ELISA was used to measure the inflammatory marker levels of stroke patients of <30, 30–90, 90–180 and >180 days, and the results are showed in Fig. 2. After analysis and comparison the serum inflammatory marker levels, we found the B1R levels were significantly higher in the vulnerable plaques of the TIA and stroke patients of <30, 30–90 days than that of asymptomatic and stroke patients of 90–180 and >180 days (ANOVA, P<0.001). In addition, compared with asymptomatic group, stroke patients showed B1R level increase at <30 days and this increased B1R level tended to decline after stroke from 30–90 to >180 days (ANOVA, P<0.001). The B1R level decreased approximately two-fold in stroke patients of >180 days compared to that of <30 days. Likewise, the IL-6 levels showed a similar pattern in stroke patients from <30 to >180 days (ANOVA, P<0.001). IL-6 levels were significantly higher in the vulnerable plaques of the TIA and stroke patients of <30, 30–90 days than that of asymptomatic and stroke patients of 90–180 and >180 days (ANOVA, P<0.001). By contrast, the IL-10 was significantly reduced in the vulnerable plaques of the TIA and stroke patients of <30, 30–90 days compared with that of asymptomatic and stroke patients of 90–180 and >180 days (ANOVA, P<0.001). The IL-10 levels were gradually upregulated in stroke patients in spatiotemporal course (ANOVA, P<0.001). However, no significant differences were found in serum B1R, IL-6 and IL-10 levels between TIA and stroke groups of <30 days; asymptomatic and stroke groups of >180 days (all P>0.05).
Figure 2.
Serum levels of proinflammatory and anti-inflammatory markers in asymptomatic, TIA and stroke patients of <30, 30–90, 90–180 and >180 days measured by ELISA. (A and D) B1R levels of plaques in stroke patients of <30, 30–90, 90–180 and >180 days compared with asymptomatic or TIA patients. (B and E) IL-6 levels of plaques in stroke patients compared with asymptomatic or TIA patients. (C and F) IL-10 levels of plaques in stroke patients compared with asymptomatic or TIA patients. Compared with asymptomatic group, stroke patients showed increased B1R and IL-6 levels at <30 days and this increased serum B1R and IL-6 levels tended to decline after stroke from 30–90 to >180 days. By contrast, the anti- inflammatory marker IL-10 levels were gradually upregulated in stroke patients in spatiotemporal course from<30 days to >180 days. (*P<0.05, ***P<0.001 vs. asymptomatic or TIA group).
All results indicate that stroke patients at <30, 30–90 days showed unstable plaque phenotype with increased pro-inflammatory marker IL-6 and B1R levels and decreased anti-inflammatory marker IL-10 level; and then further remodeled to more stable plaques with decreased IL-6, B1R levels and increased IL-10 level at 90–180 and >180 days.
Histological analysis of inflammatory markers
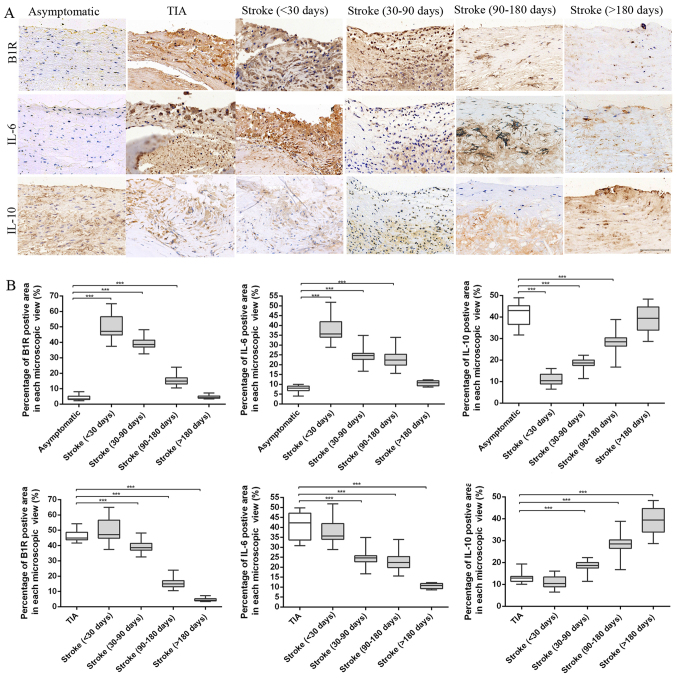
Immuno-histochemical analysis was used to stain these inflammation markers to confirm histological changes in plaques; typical images are shown in Fig. 3. The B1R and IL-6 staining appeared in plaques of different groups, and percentages of positive area in each microscopic view were markedly increased in plaques obtained from TIA and stroke patients of <30 and 30–90 days compared with asymptomatic and stroke patients of 90–180 and >180 days (ANOVA, P<0.001). Stroke plaques showed the initial increases of B1R and IL-6 contents at <30 days, and subsequent reduction from 30–90 to >180 days (both P<0.001). By contrast, the IL-10 content was significantly reduced in the vulnerable plaques of the TIA and stroke patients of <30, 30–90 days compared with that of asymptomatic and stroke patients of 90–180 and >180 days (ANOVA, P<0.001). In stroke plaques, we showed a noticeable increase in IL-10 contents from <30 to >180 days (ANOVA, P<0.001). However, no significant differences were found in the contents of B1R, IL-6 and IL-10 in plaques from stroke patients of <30 days and TIA groups; stroke patients of >180 days compared to asymptomatic group.
Figure 3.
Immunohistochemical results. (A) Representative immunohistochemical images of B1R, IL-6 and IL-10 in plaques of asymptomatic, TIA and stroke patients of <30, 30–90, 90–180 and >180 days. (B) Quantification of immunohistochemistry analysis of B1R, IL-6 and IL-10 staining of section from asymptomatic, TIA and stroke patients of <30, 30–90, 90–180 and >180 days. Percentage of positive staining area in each microscopic view (at 200x) showed that stroke plaques experienced initial increases of B1R and IL-6 contents at <30 days, and subsequent reduction from 30–90 to >180 days. However, a noticeable increase in IL-10 contents was found in stroke plaques from <30 to >180 days. (***P<0.001 vs. asymptomatic or TIA group). Scale bar: 100 µm.
Correlation analysis between serum and histological inflammatory marker levels
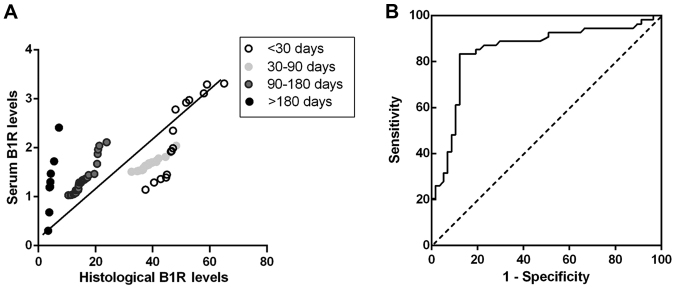
Correlation analysis between serum inflammation marker and histological levels were performed in the plaques from stroke, TIA and asymptomatic patients to test whether B1R, IL-6 and IL-10 in serum level can indicate plaque instability. Spearman correlation test showed that there was a close and highly significant relationship between serum level of B1R with histological staining of B1R in stroke patients (r=0.54, P<0.001) (Fig. 4A), TIA (r=0.81, P<0.001) and asymptomatic patients (r=0.61, P<0.001), suggesting of a notable correlation between changes in serum B1R level and changes in plaques in the progression of atherosclerotic plaques. However, serum IL-6, IL-10 levels have non-significant correlations with histological levels in plaques of stroke (r=0.15, P>0.05; r=0.13, P>0.05), TIA (r= −0.29, P>0.05; r=0.02, P>0.05) and asymptomatic patients (r=0.08, P>0.05; r=0.01, P>0.05). These results suggest that patients with carotid atherosclerotic plaques share uniform correlations between serum and histological levels of B1R in the remodeling progression of unstable plaques.
Figure 4.
Serum B1R levels for predicting vulnerable plaque in patients of stroke groups. (A) Histological levels of B1R in individual patients were correlated with the serum values of B1R in Stroke groups of <30, 30–90, 90–180 and >180 days (r=0.54, P<0.001). The regression curve line and significance values are shown in the plots. (B) Receiver-operating curve of B1R level for predicting vulnerable plaque in patients. The area under the curve of B1R levels were obtained from stroke patients.
Diagnostic efficiency of serum B1R level on carotid plaque
From our correlation analysis, it seems that serum B1R level had significantly positive and uniform correlation with histological level in plaque which is closely associated with the progression and instability of plaque. Receiver-operating curve of serum B1R level was used for predicting vulnerable plaques of patients. Because stroke patients of different stages represented dynamic changes of vulnerable plaques, only the data from stroke patients were included in the analysis. After stroke, the area under the curve was 0.85 (95% confidence interval: 0.77 to 0.93; P<0.001). A B1R cutoff level of 1.49 pg/ml distinguished vulnerable plaques from stable plaques with 83.3% sensitivity and 87.7% specificity (Fig. 4B).
Discussion
To provide basic evidence regarding the serum markers in patients with carotid atherosclerotic plaque, we studied the serum inflammation biomarkers and atherosclerotic plaque composition in stroke patients of varying stages. We found the remodeling progression of vulnerable atherosclerotic plaques over time after stroke with a significant decrease in macrophage content and increase in smooth muscle cells. By comparing inflammation markers in serum levels, vulnerable plaques were distinguished from stable ones before histological changes. The serum biomarkers of IL-6 and B1R tended to decline while IL-10 tended to increase over time after stroke. And, the histological levels of BIR, IL-6 and IL-10 showed similar dynamic patterns as serum level changes. In addition, serum B1R levels were significantly associated with histological levels in all patients with carotid atherosclerotic plaque. These results suggest that serum B1R changes can reveal unstable plaques and indicate progression of plaques after ischemic cerebrovascular events. To the best of our knowledge, this is the first study that details the dynamic changes of inflammation biomarkers in both serum and histological levels after stroke and explores their utility as prognostic biomarker in development of carotid atherosclerotic plaques.
Growing evidence has shown that atherosclerotic carotid plaques from symptomatic patients reveal a vulnerable phenotype early after acute ischemic event (15) and then remodel into stable ones over time (8). This period often involves several pathophysiological variables and complex interactions, including local and systematic inflammation alterations (16). Elucidation of the inflammatory alterations can facilitate early vulnerable plaques diagnosis. However, most patients already have late-stage plaque rupture at diagnosis and then developed an acute ischemic event because of the prior absence of featured clinical signs; thus, it is impractical to explore vulnerable plaque-related pathophysiological changes in patients. Plaques obtained from stroke patients of different stages facilitate the study of a serial of pathological changes in the progression of vulnerable plaques. In this study, plaques from stroke patients showed a significant reduction of macrophage content and a clear trend of increasing numbers of smooth muscle cells from <30 to >180 days. However, plaque from stroke of <30 and 30–90 days showed unstable phenotype and then tended to stable ones at stroke of 90–180 and >180 days. Thus, based on these findings, plaques from stroke patients of four stages used in this study can mimic the progression of vulnerable plaques in clinical.
Using ELISA, we showed noticeable increases in serum inflammatory markers of IL-6 and B1R in stroke patients from <30 to 180 days. Conversely, IL-10 reduced in stroke patients from <30 to >180 days in serum levels. There are several causes explaining the inflammatory changes of plaques observed after stroke. Because circulating biomarkers reliably predict the vascular events, the serum inflammatory biomarkers as surrogates that could reflect processes associated with atherosclerotic disease progression (17). IL-6 has pro-inflammatory properties by activating and recruiting inflammatory cells and has been reported to be higher in local plaques from patients (18). IL-6 are also reported elevated in patients with acute coronary syndromes and may contribute to the exacerbation of atherosclerosis (19,20). By contrast, IL-10, an anti-inflammatory marker can control inflammation by inhibiting the synthesis of several cytokines produced by macrophages (21). Kamaly et al also showed controlled-release polymeric nanoparticles incorporating the anti-inflammatory cytokine IL-10 can be an effective treatment for atherosclerotic plaques in advanced lesions of high fat-fed low-density lipoprotein receptor-/-mice (22). However, it must be noted that the dynamic changes of inflammatory biomarkers on both serum and histological levels from stroke patients with different stages of plaques compared with asymptomatic controls were found in the present study. Thus, it is required to learn the dynamic changes in inflammatory biomarkers when using serum biomarkers to monitor progression in vulnerable plaques.
The most striking and important aspect of our findings is that serum B1R change in stroke patients over time, increasing as disease progresses. Kinins are pro-inflammatory peptides that are released during tissue injury (23). The effects of kinins are mediated by B1 and B2 receptor (B1R and B2R) and comprise induction of edema formation and release of pro-inflammatory mediators (24). Austinat et al reported that B1R knockout mice developed significantly smaller brain infarctions and less neurological deficits compared to wild-type controls (25). In our study, it is true that serum and histological B1R levels were highest in stroke patients of <30 days compared with other groups. Besides detailing dynamic changes in serum and histological inflammatory markers, we also explored the association of serum changes with histological in patients with carotid atherosclerotic plaques. From our correlation analysis, it seems that the serum levels of B1R remains closely related with histological levels even in patients with asymptomatic plaques. In this respect, B1R is currently the potential biochemical biomarker in serum of atherosclerosis disease progression. Since patients comfort and compliance are important considerations, obtaining serum for B1R quantification may be more practical than the more invasive option of surgery for plaques. Thus, B1R may have utility as a potential pharmacodynamic biomarker insofar as showing that an experimental therapeutic that the changes of B1R in serum would provide evidence of underlying biological effect of treatment.
We acknowledge that our study has several limitations. First, drug use for patients associated with the different time intervals may determine possible confounding effects. However, no differences between the groups have been observed, indicating that this confounder could not explain the results. Second, our findings are based on a small number of different samples and our data need confirmation in larger studies in other populations at risk. However, because nowadays patients are being operated much faster after the event, it is hard to recruit larger number of samples to confirm in this study. Third, although HE staining was used as a gold standard for the diagnosis of vulnerable plaques, other techniques such as MRI or 18F-FDG-PET/CT can be used to further monitor the unstable plaque in these patients (26,27).
In summary, carotid atherosclerosis is a progression with the upregulation of pro-inflammatory factors and the downregulation of anti-inflammatory factors leading to plaque destabilization, which may eventually result in acute cerebrovascular events. This study demonstrates that the remodeling and progression of vulnerable plaques after stroke is significantly associated with the decrease of serum inflammatory factors IL-6 and B1R and an increase of anti-inflammatory marker-IL-10 levels. In particular, serum B1R represent the most promising potential pharmacodynamic biomarkers available today that are suitable for further investigation in the context of future clinical trials.
Acknowledgements
The present study work was supported by funding from the National Natural Science Foundation of China (81371435; 81671299), the Hunan Natural Science Foundation (2016JC2057) and the Xiangya Hospital foundation (xywm2015I32).
References
- 1.Rothstein L, Jickling GC. Ischemic stroke biomarkers in blood. Biomark Med. 2013;7:37–47. doi: 10.2217/bmm.12.104. [DOI] [PubMed] [Google Scholar]
- 2.Mauriello A, Servadei F, Sangiorgi G, Anemona L, Giacobbi E, Liotti D, Spagnoli LG. Asymptomatic carotid plaque rupture with unexpected thrombosis over a non-canonical vulnerable lesion. Atherosclerosis. 2011;218:356–362. doi: 10.1016/j.atherosclerosis.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Stefanadis C, Antoniou CK, Tsiachris D, Pietri P. Coronary atherosclerotic vulnerable plaque: Current perspectives. J Am Heart Assoc. 2017;6:pii: e005543. doi: 10.1161/JAHA.117.005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttolomondo A, Pecoraro R, Casuccio A, Di Raimondo D, Buttà C, Clemente G, Corte V Della, Guggino G, Arnao V, Maida C, et al. Peripheral frequency of CD4+ CD28- cells in acute ischemic stroke: Relationship with stroke subtype and severity markers. Medicine (Baltimore) 2015;94:e813. doi: 10.1097/MD.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttolomondo A, Pedone C, Pinto A, Di Raimondo D, Fernandez P, Di Sciacca R, Licata G. Gruppo Italiano di Farmacoepidemiologia dell'Anziano (GIFA) researchers: Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int J Cardiol. 2008;125:391–396. doi: 10.1016/j.ijcard.2007.03.109. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati E, Moroni F, Norata GD, Magnoni M, Camici PG. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015;2015:718329. doi: 10.1155/2015/718329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shindo A, Tanemura H, Yata K, Hamada K, Shibata M, Umeda Y, Asakura F, Toma N, Sakaida H, Fujisawa T, et al. Inflammatory biomarkers in atherosclerosis: Pentraxin 3 can become a novel marker of plaque vulnerability. PLoS One. 2014;9:e100045. doi: 10.1371/journal.pone.0100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters W, Hellings WE, de Kleijn DP, de Vries JP, Moll FL, Vink A, Pasterkamp G. Carotid atherosclerotic plaques stabilize after stroke: Insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol. 2009;29:128–133. doi: 10.1161/ATVBAHA.108.173658. [DOI] [PubMed] [Google Scholar]
- 9.Roquilly A, Lejus C, Asehnoune K. Brain injury, immunity and infections. Ann Fr Anesth Reanim. 2012;31:e97–e100. doi: 10.1016/j.annfar.2012.04.012. (In French) [DOI] [PubMed] [Google Scholar]
- 10.Golias C, Charalabopoulos A, Stagikas D, Charalabopoulos K, Batistatou A. The kinin system-bradykinin: Biological effects and clinical implications. Multiple role of the kinin system-bradykinin. Hippokratia. 2007;11:124–128. [PMC free article] [PubMed] [Google Scholar]
- 11.Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros R, Cabrini DA, Ferreira J, Fernandes ES, Mori MA, Pesquero JB, Bader M, Avellar MC, Campos MM, Calixto JB. Bradykinin B1 receptor expression induced by tissue damage in the rat portal vein: A critical role for mitogen-activated protein kinase and nuclear factor-kappaB signaling pathways. Circ Res. 2004;94:1375–1382. doi: 10.1161/01.RES.0000128404.65887.08. [DOI] [PubMed] [Google Scholar]
- 13.Duchene J, Cayla C, Vessillier S, Scotland R, Yamashiro K, Lecomte F, Syed I, Vo P, Marrelli A, Pitzalis C, et al. Laminar shear stress regulates endothelial kinin B1 receptor expression and function: Potential implication in atherogenesis. Arterioscler Thromb Vasc Biol. 2009;29:1757–1763. doi: 10.1161/ATVBAHA.109.191775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Liu T, Li X, Zhang M, Shi L, Liu H. Expression of the genes encoding kinin receptors are increased in human carotid atherosclerotic plaques. Biomed Rep. 2015;3:398–402. doi: 10.3892/br.2015.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatsukami TS, Ferguson MS, Beach KW, Gordon D, Detmer P, Burns D, Alpers C, Strandness DE., Jr Carotid plaque morphology and clinical events. Stroke. 1997;28:95–100. doi: 10.1161/01.STR.28.1.95. [DOI] [PubMed] [Google Scholar]
- 16.Marnane M, Prendeville S, McDonnell C, Noone I, Barry M, Crowe M, Mulligan N, Kelly PJ. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke. 2014;45:801–806. doi: 10.1161/STROKEAHA.113.003657. [DOI] [PubMed] [Google Scholar]
- 17.Chen YC, Huang AL, Kyaw TS, Bobik A, Peter K. Atherosclerotic plaque rupture: Identifying the straw that breaks the camel's back. Arterioscler Thromb Vasc Biol. 2016;36:e63–e72. doi: 10.1161/ATVBAHA.116.307993. [DOI] [PubMed] [Google Scholar]
- 18.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 19.Poredos P, Spirkoska A, Lezaic L, Mijovski MB, Jezovnik MK. Patients with an inflamed atherosclerotic plaque have increased levels of circulating inflammatory markers. J Atheroscler Thromb. 2017;24:39–46. doi: 10.5551/jat.34884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan V, Akram HR, Ahmed SS. Genetic predisposition of IL-10 promoter polymorphisms with risk of multiple sclerosis: A meta-analysis. J Neuroimmunol. 2017;306:11–18. doi: 10.1016/j.jneuroim.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn R, Mossberg M, Ståhl AL, Johansson K, Lindman I Lopatko, Heijl C, Segelmark M, Mörgelin M, Leeb-Lundberg LM, Karpman D. Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int. 2017;91:96–105. doi: 10.1016/j.kint.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renné T, Kleinschnitz C. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–293. doi: 10.1161/STROKEAHA.108.526673. [DOI] [PubMed] [Google Scholar]
- 26.Chan JM, Monaco C, Wylezinska-Arridge M, Tremoleda JL, Gibbs RG. Imaging of the vulnerable carotid plaque: Biological targeting of inflammation in atherosclerosis using iron oxide particles and MRI. Eur J Vasc Endovasc Surg. 2014;47:462–469. doi: 10.1016/j.ejvs.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA, Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: Ready for prime time? J Am Coll Cardiol. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]