Abstract
Malignant gliomas are a group of aggressive neoplasms among human cancers. The curative effects of current treatments are finite for improving the prognosis of patients. Hyperthermia (HT) is an effective treatment for cancers; however, the effects of HT with different temperatures in treatment of MG and relevant mechanisms remain unclear. MTT assay and Annexin V-fluorescein isothiocyanate/propidium iodide staining were used for investigating the proliferation and apoptosis of C6 cells, respectively. Western blotting was applied to detect the expression of proteins. Ultrasonography was employed to evaluate the tumor formation rate, growth rate, angiogenesis rate and degree of hardness of tumors in vivo. The authors certified that HT with 42–46°C × 1 h, 1 t could inhibit proliferation, promote apoptosis, reduce tumor formation rate, growth rate, angiogenesis rate, degree of hardness of tumors, ischemic tolerance and anoxic tolerance, and have synergy with temozolomide in C6 cells. Long-term HT (43°C × 1 h, 1 t/5 d, 90 d) did not cut down the sensitivity of C6 cells to HT, and sustainably inhibited the proliferation of C6 cells. Furthermore, the authors proved HT produced these effects primarily through inhibition of the EGFR/STAT3/HIF-1A/VEGF-A pathway.
Keywords: malignant gliomas, hyperthermia, STAT3, temozolomide, apoptosis
Introduction
Malignant gliomas (MGs) are a group of heterogeneous primary central nervous system (CNS) tumors arising from glial cells. MGs account for the majority of malignant primary CNS tumors, and are associated with high morbidity and mortality (1). In spite of current approaches in their therapy, including surgical resection, radiotherapy and chemotherapy, the prognosis of these patients remains poor. The median overall survival length after first-line therapy does not exceed 15 months (2). Thus, more recently, various minimally invasive treatments are under study, including hyperthermia (HT), which is a therapeutic procedure that increases the temperature in body tissues and maintains a period of time, for inhibiting proliferation and promoting the apoptosis of cancer cells (3). In randomized control studies, HT has been reported as an effective therapy for many cancers, including gastric cancer (4), breast cancer (5) and liver cancer (6). Previous research has demonstrated that HT could inhibit proliferation or promote apoptosis in human MGs. Cha et al (7) demonstrated HT suppressed glioma cell proliferation and mobility through the induction of E2F1-mediated apoptosis. Wang et al (3) proved HT promoted apoptosis and suppressed invasion through TNF-A/P38/NF-κB pathway in C6 cells. Sun et al (8) certified MGs in the HT group exhibited growth retardation or growth termination in a clinical study with 30 pathologically diagnosed patients with grade III–IV primary or recurrent MGs. Moreover, HT has synergistic effects when combined with chemotherapeutic agents in treating cancers. However, there is no systematic research concerning the effects of HT with different doses and relevant mechanisms in MGs.
The epidermal growth factor receptor (EGFR) and signal transducers and activators of transcription (STATs) are commonly expressed and activated in many malignancies, including MGs. EGFR is one of four homologous transmembrane proteins that regulate various signaling pathways in proliferation, apoptosis and inflammation through the activation of phospholipases (9). STATs comprise a family of seven structurally and functionally related proteins. Aberrant activation of STAT3 is commonly observed in tumors and is strongly associated with tumor development and progression (10). STAT proteins participate in tumorigenesis through upregulation of genes encoding apoptosis inhibitors myeloid cell leukemia sequence 1, BCL2-like 1 and cell cycle regulators (cyclin D1/D2, MYC) (11). STAT3 is also involved in tumor progression through inducing angiogenic factors, such as vascular endothelial growth factor (VEGF) (12). Moreover, previous studies have revealed a critical role of STAT3 in maintaining EGFR-mediated cancer cell proliferation (13,14). EGFR likely activates STAT in a manner distinctive from other mechanisms of STAT activation (9). In view of the important role of EGFR/STAT3 signaling in tumor development and progression, the methods to inhibit EGFR in conjunction with oncogenic STATs may represent a novel and attractive therapeutic strategy for cancers characterized by upregulation of EGFR signaling (15). In the current study, the authors demonstrated the intense effects of HT on inducing apoptosis in C6 cells, which was associated with EGFR/STAT3 signaling.
Materials and methods
Cell culture
Rat glioblastoma cell line C6, human umbilical vein endothelial cells (HUVECs) and human renal tubular epithelial cells (HK2) were originally obtained from the American Type Culture Collection (Manassas, VA, USA). C6 and HUVECs cells were cultured in Dulbecco's modified Eagle's medium (GE Healthcare, Chicago, IL, USA) supplemented with 10% fetal bovine serum (FBS) in a standard humidified incubator at 37°C under 5% CO2 atmosphere, while HK2 was cultured in RPMI 1640 medium (GE Healthcare) with 10% FBS.
HT
The dose of the HT refers to the temperature and duration time of treatment and therapeutic frequency. ‘h’ means hour; ‘t’ mens time; ‘d’ means day. The authors used the water bath of 42–46°C to maintain 1 h for HT (16), expressed as 42–46°C × 1 h, 1 t. Using microscope at ×200 magnification to observe before and after HT (0, 12, 24, 48, 72, 96 and 120 h), and to take pictures. For exploring the effects of long-term HT, the authors used the water bath of 43°C to maintain 1 h, once every 5 days, treatment for 90 days, expressed as 43°C × 1 h, 1 t/5 d, 90 d, named this cell line C6-90d.
MTT
Standard microplate MTT assays were used to examine the growth rate of C6 cells, which were treated at 42–46°C × 1 h. Briefly, C6 cells with/without pre-HT were plated at a density of 3×103 cells per well in 96-well plates. At the end of 24, 72, 96 and 120 h of incubation, cells were stained by 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for a further 4 h at 37°C in an incubator. The medium was removed afterwards and the intracellular formazan crystals were dissolved by adding 100 µl dimethylsulfoxide/well. Absorbance was read at 490 nm on a microplate reader (ELx808, BioTek Instruments, Inc., Winooski, VT, USA). The experiments were repeated three times.
Cell apoptosis assay
The C6 cells were plated in culture flasks and, after 24 h, cells were exposed to HT with 43–45°C × 1 h, 1 t, then the cells were maintained for 48 h. To determine the extent of spontaneous apoptosis, 1×105 cells were stained with fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide using the Annexin V-FITC Apoptosis Detection kit (4A Biotech, Beijing, China) following the manufacturer's instructions. Cell spontaneous apoptosis was determined using FACSCalibur II sorter and Cell Quest FACS system (BD Biosciences, Franklin Lakes, NJ, USA). The experiments were repeated three times.
Protein lysates and western blot analysis
Cultured cells were treated by HT with 42–45°C × 1 h, 1 t, after 48 h, then scraped into radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China) and lysed on ice for 30 min. Protein concentrations were determined using the Pierce Micro BCA protein assay system (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). From each sample, 25 µg of protein was loaded onto 12% SDS-PAGE gel for electrophoresis and transferred onto nitrocellulose membrane. The membranes were blocked with 5% non-fat milk (diluted in TBST containing 0.1% Tween 20) at room temperature for 1 h. All target proteins were immunoblotted with appropriate primary and horseradish peroxidase (HRP)-conjugated secondary antibodies. Primary antibodies were incubated at room temperature for 2 h. Following washing the membranes with PBST containing 0.1% Tween 20 three times, the bound antibodies were then detected using the secondary goat anti-rabbit or goat anti-mouse antibodies (Wuhan Sanyang Biotechnology, Wuhan, China) and protein signals were visualized by enhanced chemiluminescence using ECL Western blotting detection reagents (Beyotime Institute of Biotechnology) for 1 min and exposed to Kodak Biomax XAR film. The experiments were repeated three times. The grayscale of these data were quantified by Image J version 1.49 software (National Institutes of Health, Bethesda, Maryland, USA).
The subcutaneous tumor model of glioma
For animal experiments, 15 male Wistar rats with an average weight of ~200 g (Laboratory Animal Center of First Affiliated Hospital of Harbin Medical University, Harbin, China) were used. Under anesthetizing using pentobarbital sodium (1%; 5 ml/kg; intraperitoneal injection), the authors inoculated subcutaneous tissue of the left side of their back with C6 cells without pre-HT, and the right side of their back with C6 cells with pre-HT (44°C × 1 h, 1 time). After 15 days, the authors observed the tumors using ultrasound and hematoxylin and eosin (H&E) staining.
Ultrasound
To evaluate the tumor formation rate, growth rate, angiogenesis rate and degree of hardness of tumors in vivo, the authors used B-mode ultrasonography, color Doppler flow imaging (CDFI) and ultrasonic elastosonography (USE) of Philips iU Elite Ultrasound System (Philips Healthcare, Amsterdam, The Netherlands), respectively (17). These detections were observed by an experienced ultrasound doctor, who was blind to the study.
H&E staining for pathological examination
The tumor tissues were fixed in formalin, followed by routine embedding with paraffin and sectioning. The sections were then subjected to deparaffinization and H&E staining. An experienced pathologist who was blind to the study was asked to perform the pathological examination.
Reagents and antibodies
Temozolomide (TMZ) was purchased from Aladdin Reagent Company (http://www.aladdin-e.com). Other reagents were purchased from Beyotime Institute of Biotechnology. Primary antibodies: EGFR, cat no. 18986-1-AP, 1:1,000, Proteintech Group, Inc., Chicago, IL, USA; STAT3, cat no. 9139S, 1:1,000, Cell Signaling Technology, Inc., Danvers, MA, USA; p-STAT3, cat no. 9145S, 1:1,000, Cell Signaling Technology, Inc.; HIF-1A, cat no. 14179S, 1:1,000, Cell Signaling Technology, Inc.; VEGF-A, cat no. 19003-1-AP, 1:1,000, Proteintech Group, Inc.; Bax, cat no. 50599-2-Ig, 1:1,000, Proteintech Group, Inc.; Bcl-2, cat no. 12789-1-AP, 1:1,000, Proteintech Group, Inc.; MGMT, cat no. A-1010-050, 1:1,000, Epigentek, Farmingdale, NY, USA; β-actin, cat no. TA-09, 1:1,000, OriGene Technologies, Inc., Rockville, MD, USA). HRP-conjugated secondary antibodies: HRP-conjugated Affinipure Goat anti-mouse IgG, cat no. SA00001-1, 1:2,000, Proteintech Group, Inc.; HRP-conjugated Affinipure goat anti-rabbit IgG, cat no. SA00001-2, 1:2,000, Proteintech Group, Inc.
Statistical analysis
The results are presented as the mean ± standard deviation. Data were analyzed using analysis of variance followed by LSD multiple comparison tests with SPSS software (version, 20.0; IBM SPSS, Armonk, NY, USA) to determine the level of significance between the different groups. P<0.05 was considered to indicate a statistically significant difference. P<0.01 was considered to indicate a statistically highly significant difference.
Results
The reference standard of HT in C6 cells
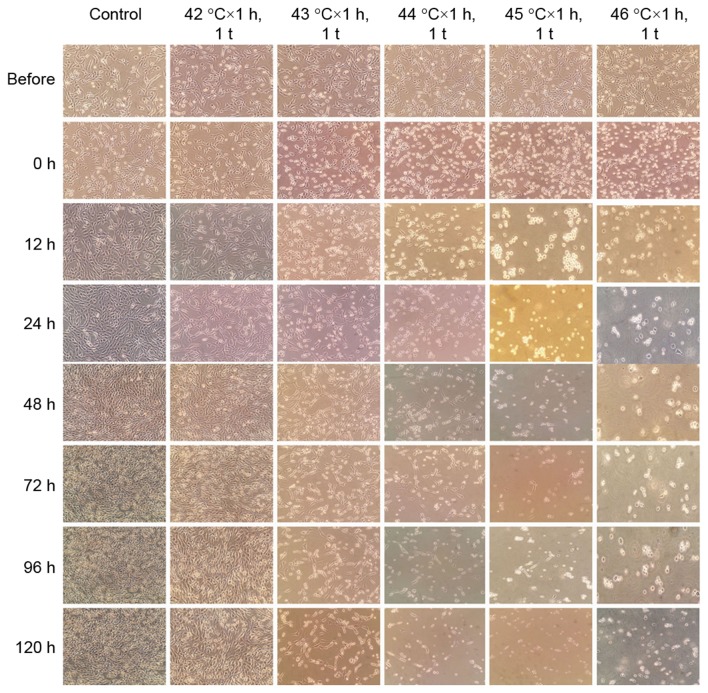
To explore the effects of HT with different doses in C6 cells, the authors adopted HT with 42–46°C × 1 h, 1 t and continuously observed the status of C6 cells before and after (0, 12, 24, 48, 72, 96 and 120 h) HT. A reference standard of HT was established with different doses in C6 cells. In addition, the results indicated that 42–46°C × 1 h, 1 time could inhibit proliferation and promote apoptosis or necrosis in a temperature dependent manner in C6 cells (Fig. 1).
Figure 1.
The reference standard of HT with different doses in C6 cells. The images of adherent C6 cells at magnification ×200 under an optical microscope before and after (0, 12, 24, 48, 72, 96 and 120 h) HT.
HT with different doses inhibited proliferation and promoted apoptosis in C6 cells
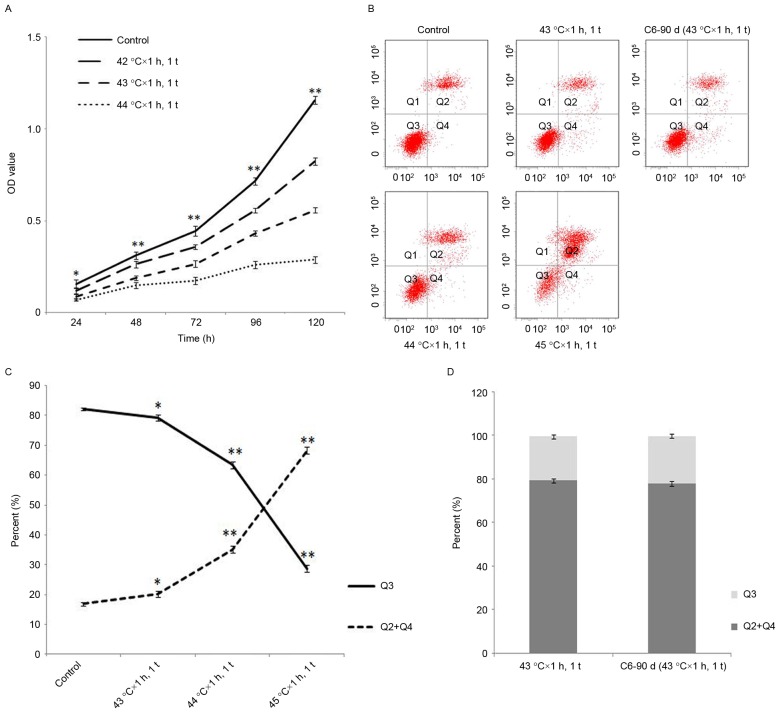
To further testify the effects of HT with different doses on inhibiting proliferation and promoting apoptosis in C6 cells, the authors applied an MTT assay and Annexin V-FITC/PI staining, respectively. The results indicated that HT with 42–44°C × 1 h, 1 t could inhibit the proliferation of glioma cells in a temperature dependent manner (Fig. 2A). When the temperature reaches 45°C, a low number of living cells adhered to the bottom of culture flasks, thus proliferation was not detected. In addition, the authors proved that HT with 43–45°C × 1 h, 1 time primarily induced apoptosis in a temperature dependent manner (Fig. 2B and C).
Figure 2.
The proliferation and apoptosis after HT at different temperatures in C6 cells. (A) HT with 42–44°C × 1 h, 1 t inhibited the proliferation of C6 cells in a temperature dependent manner. (B and C) HT with 43–45°C 1 h, 1 t promoted the apoptosis of C6 cells in a temperature dependent manner. (B and D) The apoptosis of C6-90d cells slightly increased compared to C6 cells after HT with 43°C × 1 h, 1 t, but there was no statistical significance. Compared with the control group at the same dose of HT. *P<0.05, **P<0.01. OD, optical density; HT, hyperthermia.
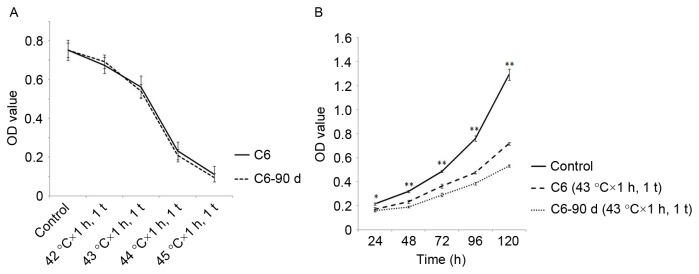
Long-term HT inhibited proliferation of C6 cells, but did not promote apoptosis
To investigate the effects of long-term HT, the authors used long-term HT with 43°C × 1 h, 1 t/5 d, 90 d, obtained a new cell line named C6-90d. The MTT assay proved that the sensitivity of C6-90d cells to HT is similar to C6 cells (Fig. 3A). Following HT (24, 48, 72, 96 and 120 h) with 43°C × 1 h, 1 t in C6-90d cells and C6 cells, it was demonstrated that the proliferation of C6-90d cells was reduced compared to C6 cells (Fig. 3B). In addition, the authors discovered the increase of apoptosis of C6-90d cells was indistinctive and without statistical significance compared to C6 cells following HT with 43°C × 1 h (Fig. 2B and D).
Figure 3.
The effects of long-term HT in C6 cells. (A) Sensitivity of C6-90d cells to HT did not change compared to C6 cells. (B) The proliferation of C6-90d cells was decreased compared to C6 cells after HT at 43°C × 1 h, 1 t. Compared with the control group at the same dose of HT, *P<0.05, **P<0.01. OD, optical density; HT, hyperthermia.
HT (44°C × 1 h, 1 t) had synergy with TMZ
Following HT (24 h) with 441°C × h, 1 t, the authors replaced the original culture solution with culture solution containing TMZ (1 mM). At 72 h later, pictures were taken, and the MTT assay was conducted for every group, proving that HT (44°C × 1 h, 1 t) had synergy with TMZ (Fig. 4).
Figure 4.
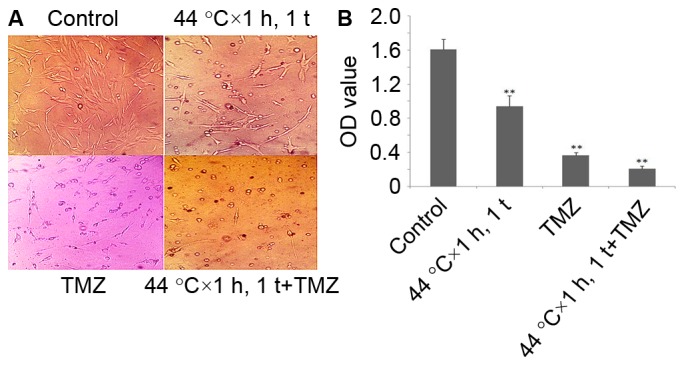
HT with 44°C × 1 h, 1 t had synergy with TMZ. (A) Photographs presented HT with 44°C × 1 h, 1 t had synergy with TMZ. Magnification, ×200. (B) MTT assay indicated that HT with 44°C × 1 h, 1 t had synergy with TMZ. **P<0.01 vs. control. HT, hyperthermia.
HT (44°C × 1 h, 1 t) in vivo
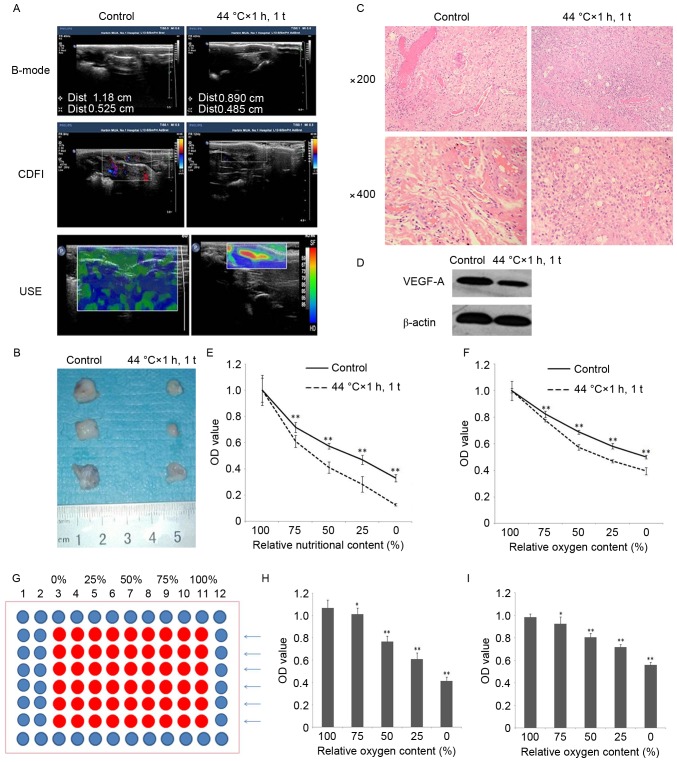
For animal experiments, 15 rat models were established and, after 15 d, the authors used B-mode ultrasonography, CDFI and USE to evaluate the tumor formation rate, growth rate, angiogenesis rate and degree of hardness of tumor in vivo, respectively, and found that the aforementioned indexes of 44°C × 1 h, 1 time group were reduced compared with the control group (Table I; Fig. 5A). The pathological results were consistent with the ultrasonography results (Fig. 5B and C). Additionally, the expression of vital factor of angiogenesis VEGF-A was reduced after HT (Fig. 5D).
Table I.
Tumor formation rate of Wistar rats.
| Control | |||
|---|---|---|---|
| 44°C × 1 h, 1 time | + | − | Total (n) |
| + | 5 | 0 | 5 |
| − | 8 | 2 | 10 |
| Total (n) | 13 | 2 | 15 |
Tumorigenesis (+), non-tumorigenesis (−). The tumor formation rate of 44°C × 1 h, 1 t group was reduced compared to control group, 33% vs. 87%, P<0.01.
Figure 5.
HT in vivo. (A) B-mode ultrasonography discovered that the tumor growth rate was reduced. CDFI determined that tumor angiogenesis rate was reduced. USE found the degree of tumor hardness was reduced after HT with 44°C × 1 h, 1 t. (B) After removing tumors from tumor-burdened rats, the authors found that the tumor growth rate was reduced after HT with 44°C × 1 h, 1 t. (C) Hematoxylin and eosin staining indicated that the tumor angiogenesis rate was reduced after HT with 44°C ×1 h, 1 time (magnification ×200, ×400). (D) Western blotting indicated that the expression of VEGF-A in the tumor tissue was reduced after HT with 44°C × 1 h, 1 t. (E) Ischemic tolerance of C6 cells were decreased after HT with 44°C × 1 h, 1 t. **P<0.01 vs. Control. (F) Hypoxia tolerance of C6 cells were decreased after HT with 44°C × 1 h, 1 t. **P<0.01 vs. Control. (G) 96-well plate gradient hypoxia model. (H) Using the HUVECs proved that the 96-well plate gradient hypoxia model was effective. *P<0.05, **P<0.01 vs. 100% relative oxygen content. (I) Using the HK2 proved that the 96-well plate gradient hypoxia model was effective. **P<0.01 vs. 100% relative oxygen content. CDFI, color Doppler flow imaging; USE, ultrasonic elastosonography; HT, hyperthermia.
Moreover, in the center of the tumor of the 44°C × 1 h, 1 t group, the authors discovered necrosis, even the tumors were small. A hypothesis was established suggesting that HT augmented the sensibility of C6 to ischemia and hypoxia, hence the authors designed an ischemia model and a 96-well plate gradient hypoxia model (Fig. 5G) to certify the hypothesis, and this produced positive results (Fig. 5E and F). Following this, human umbilical vein endothelial cells (HUVECs) and human renal tubular epithelial cells (HK2) were used to examine the 96-well plate gradient hypoxia model, and proved it was effective (Fig. 5H and I).
Mechanisms of HT with different doses
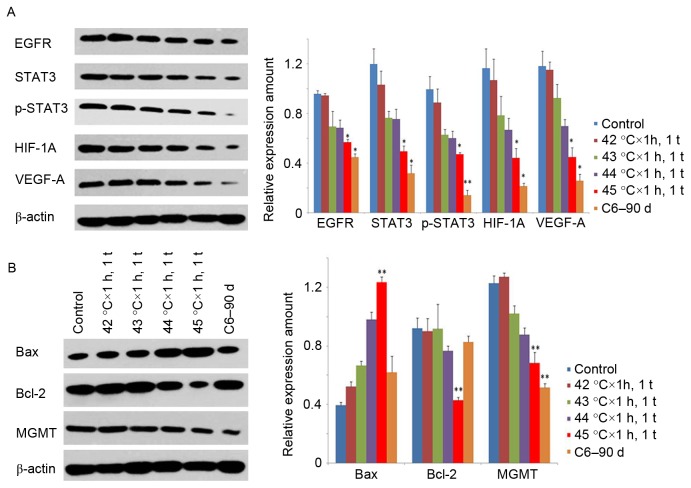
To determine whether it was C6 cells rather than other cells in the tumor microenvironment that cut down the expression of VEGF-A, the authors tested the expression of VEGF-A following HT at different temperatures in C6 cells, and found that they were reduced in a temperature dependent manner. Following this the EGFR/STAT3/HIF-1A pathway which is upstream of VEGF-A, was investigated (18–20), and found that the expression and activation of these genes were reduced in the same manner (Fig. 6A).
Figure 6.
Mechanisms of HT with different temperatures. (A) The expression of EGFR, STAT3, p-STAT3, HIF-1A and VEGF-A gradually reduced with the increasing of temperature, the C6-90d group was the minimum. (B) The expression of Bax increased after HT, Bcl-2 and MGMT decreased. *P<0.05, **P<0.01 vs. Control. EGFR, epidermal growth factor receptor; STAT3, signal transducers and activators of transcription; HIF-1A, hypoxia inducible factor-1A; VEGF-A, vascular endothelial growth factor-A; HT, hyperthermia.
Apoptosis related genes Bax and Bcl-2, both downstream of STAT3, can lead to mitochondrion mediated apoptosis (21), and the present study detected an increase in Bax and decrease in Bcl-2 after HT. In addition, the expression of MGMT, which is related to the drug resistance of MGs to TMZ, was in accordance with results of STAT3 (Fig. 6B).
Discussion
HT is one of the newest therapies for tumors in recent years, however there has been no systematic research conducted concerning using HT at different time periods. Therefore, we conducted this study. The authors carried out HT at conditions of 42–46°C × 1 h, 1 t, and continuously observed the status of C6 cells before and after (0,12, 24, 48, 72, 96 and 120 h) HT, and found that the scope of temperatures of HT in C6 cells were narrow, just 42–46°C. HT could inhibit proliferation and promote apoptosis in this scope of temperatures in a temperature dependent manner in C6 cells. To explore the effects of long-term HT, long-term HT was adopted with 43°C × 1 h, 1 t/5 d, 90 d, and obtained a new cell line named C6-90d. Sensitivity of C6-90d to HT was similar to C6 cells. HT with 43°C × 1 h, 1 t could inhibit the proliferation of C6-90d cells, but could not significantly promote apoptosis compared to C6 cells. In order to facilitate communication, the authors recommend the above methods to represent the dose of HT. Wang et al (3) have demonstrated that HT could inhibit the migration and invasion of C6 cells, so similar studies were not conducted. TMZ is one of the clinical standard chemotherapy drugs for glioma, but drug resistance has been demonstrated to always interfere with the efficacy of TMZ (22). The present study testified that HT had synergy with TMZ.
In subcutaneous tumor models of glioma, the authors found the tumor formation rate, growth rate, angiogenesis rate and degree of hardness of tumor were reduced following HT at 44°C × 1 h, 1 t. The ultrasonography results were consistent with the pathological results. Then it was detected that the vital factor of angiogenesis VEGF-A was reduced. To determine whether it was C6 cells rather than other cells in the tumor microenvironment that cut down the expression of VEGF-A, the authors tested the expression of VEGF-A after HT with different dose in C6 cells, and found that they were reduced in a temperature dependent manner. Then the authors detected the EGFR/STAT3/HIF-1A pathway, upstream of VEGF-A, and discovered that the expression and activation of these genes are reduced in the same manner. According to the results of the present study, the C6-90d group is the most obvious group. Because STAT3 plays an important role in the occurrence and progress of tumor (23), it can promote the proliferation, migration, invasion and resistance to TMZ of glioma (24). Therefore, the authors believe that HT can suppress the proliferation, migration, invasion, tumorigenic rate, growth rate, angiogenesis rate and degree of hardness of tumors through inhibiting the EGFR/STAT3/HIF-1A/VEGF-A pathway.
Reduced tumor angiogenesis leaded to ischemia and hypoxia after HT, plus HT augmented the sensitivity of C6 cells to ischemia and hypoxia, which might be attributed to the vast energy and organic ingredients for cell repair following HT or the reduction of glioma stem cells caused by HT (25). Forming a benign positive feedback mechanism that reduced tumor angiogenesis leads to ischemia and hypoxia after HT. The C6 cells that could not tolerate ischemia and hypoxia underwent apoptosis, further reducing the total expression of VEGF-A. For detecting the tolerance of C6 cells to hypoxia after HT, the authors designed a 96-well plate gradient hypoxia model, this model creates graded hypoxia through four mechanisms: First, the concentration of O2 is gradually reduced from 12 side orientation to 1 side, because O2 is gradually consumed; second, the space for accommodating O2 gradually shrink from 12 side orientation to 1 side. third, water vapor pressure is higher on 1 side; fourth, CO2 pressure is higher on 1 side because CO2 discharged by cell respiration cannot be eliminated. All of them reduce the amount and speed of entering of O2. It can be used to roughly determine the hypoxia tolerance of cells, because it can simulate the gradient hypoxia and change of CO2 content of perivascular tissue. The authors proved its effect using HUVECs and HK2.
Hou et al (26) indicated that HT induced apoptosis of osteosarcoma cells was mediated by mitochondrial apoptosis pathway. STAT3, as a multifunctional gene, plays crucial regulatory roles in many aspects. Wang et al (27) certified that the JAK2/STAT3 pathway could increase the expression of Bax and decrease the expression of Bcl-2, then induced mitochondria-mediated apoptosis. Therefore, the authors tested the expression of Bax and Bcl-2, and found that HT increased expression of Bax and decreased expression of Bcl-2. The EGFR/STAT3/HIF-1A/VEGF-A pathway of the C6-90d group was significantly inhibited compared to 45°C × 1 h, 1 t group, but the apoptosis of the C6-90d group did not increased correspondingly. Hence, the authors assumed that there were other mechanisms involved in HT with 45°C 1 h, 1 t induced apoptosis. The high expression of MGMT is the primary reason of drug resistance of MGs to TMZ. Kohsaka et al (28) proved inhibition of STAT3 could reduce the expression of MGMT. The expression of MGMT was reduced after HT. The synergy of HT and TMZ is attributed to the inhibition of the EGFR/STAT3/MGMT pathway.
In conclusion, these experiments explored the curative effects of HT at different time periods, certified HT could inhibit proliferation, promote apoptosis, reduce tumor formation rate, growth rate, angiogenesis rate, degree of hardness of tumors, ischemic tolerance and anoxic tolerance, and have synergy with TMZ in C6 cells. Long-term HT (43°C × 1 h, 1 t/5 d, 90 d) did not impair the sensitivity of C6 cells to HT, and sustainably inhibited the proliferation of C6 cells. The authors further proved HT produced these effects mainly through inhibition of the EGFR/STAT3/HIF-1A/VEGF-A pathway. However, the mechanisms underlying how HT at 45°C × 1 h, 1 t led to increased apoptosis are not completely clear, and will be focused on in future research of the authors.
However, due to the current technical conditions, HT cannot be maintained accurately at higher temperatures (45°C), as it would damage normal brain tissue (29). However, with the development of nanotechnology-dependent precise HT (30), improvement of treatment strategies, and adoption of auxiliary measures, HT will become an important member of combined treatments using MGs in the near future.
Acknowledgements
The present study was funded by the Natural Science Foundation of China (grant no. 81171346).
References
- 1.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Wang DC, Zhang Y, Chen HY, Li XL, Qin LJ, Li YJ, Zhang HY, Wang S. Hyperthermia promotes apoptosis and suppresses invasion in C6 rat glioma cells. Asian Pac J Cancer Prev. 2012;13:3239–3245. doi: 10.7314/APJCP.2012.13.7.3239. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ruan J, Yang M, Pan F, Gao G, Qu S, Shen YL, Dang YJ, Wang K, Jin WL, Cui DX. Human induced pluripotent stem cells labeled with fluorescent magnetic nanoparticles for targeted imaging and hyperthermia therapy for gastric cancer. Cancer Biol Med. 2015;12:163–174. doi: 10.7497/j.issn.2095-3941.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Sun J, Yang X. Molecular imaging-guided interventional hyperthermia in treatment of breast cancer. Biomed Res Int. 2015;2015:505269. doi: 10.1155/2015/505269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra SK, Ghoshal G, Gartia MR, Wu Z, De AK, Ye M, Bromfield CR, Williams EM, Singh K, Tangella KV, et al. Trimodal therapy: Combining hyperthermia with repurposed bexarotene and ultrasound for treating liver cancer. ACS Nano. 2015;9:10695–10718. doi: 10.1021/acsnano.5b05974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha J, Jeon TW, Lee CG, Oh ST, Yang HB, Choi KJ, Seo D, Yun I, Baik IH, Park KR, et al. Electro-hyperthermia inhibits glioma tumorigenicity through the induction of E2F1-mediated apoptosis. Int J Hyperthermia. 2015;31:784–792. doi: 10.3109/02656736.2015.1069411. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Guo M, Pang H, Qi J, Zhang J, Ge Y. Treatment of malignant glioma using hyperthermia. Neural Regen Res. 2013;8:2775–2782. doi: 10.3969/j.issn.1673-5374.2013.29.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 10.Cheong JH, Hong SY, Zheng Y, Noh SH. Eupatilin inhibits gastric cancer cell growth by blocking STAT3-mediated VEGF expression. J Gastric Cancer. 2011;11:16–22. doi: 10.5230/jgc.2011.11.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: Role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 12.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulatesVEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 13.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 14.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol. 2001;19:1155–1160. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, Cao X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in Sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs. 2012;10:2055–2068. doi: 10.3390/md10092055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagoubi N, Jomni Y, Sakly M. Hyperthermia-induced febrile seizures have moderate and transient effects on spatial learning in immature rats. Behav Neurol. 2015;2015:924303. doi: 10.1155/2015/924303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan CX, Luo ZY, Liu XM, Huang PT, Mo GQ, Hong YR, Wen Q, Pan MQ, Weng HF. Ultrasonic scores of conventional ultrasound and ultrasound elastography in the diagnosis of thyroid nodular lesions. Zhonghua Yi Xue Za Zhi. 2013;93:1630–1633. (In Chinese) [PubMed] [Google Scholar]
- 18.Li D, Li XP, Wang HX, Shen QY, Li XP, Wen L, Qin XJ, Jia QL, Kung HF, Peng Y. VEGF induces angiogenesis in a zebrafish embryo glioma model established by transplantation of human glioma cells. Oncol Rep. 2012;28:937–942. doi: 10.3892/or.2012.1861. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Yuan J, Zhang J, Tian R, Ji W, Zhou Y, Yang Y, Song W, Zhang F, Niu R. Anxa2 binds to STAT3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget. 2015;6:30975–30992. doi: 10.18632/oncotarget.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XF, Lin GS, Lin ZX, Chen YP, Chen Y, Zhang JD, Tan WL. Association of pSTAT3-VEGF signaling pathway with peritumoral edema in newly diagnosed glioblastoma: An immunohistochemical study. Int J Clin Exp Pathol. 2014;7:6133–6140. [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 22.Perazzoli G, Prados J, Ortiz R, Caba O, Cabeza L, Berdasco M, Gónzalez B, Melguizo C. Temozolomide resistance in glioblastoma cell lines: Implication of MGMT, MMR, p-glycoprotein and CD133 expression. PLoS One. 2015;10:e0140131. doi: 10.1371/journal.pone.0140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Li Z, Zhang C, Zhang S, Ji Y, Chen F. Knockdown of PKCε expression inhibits growth, induces apoptosis and decreases invasiveness of human glioma cells partially through Stat3. J Mol Neurosci. 2015;55:21–31. doi: 10.1007/s12031-014-0341-4. [DOI] [PubMed] [Google Scholar]
- 24.Nie XH, Ou-yang J, Xing Y, Li DY, Dong XY, Liu RE, Xu RX. Paeoniflorin inhibits human glioma cells via STAT3 degradation by the ubiquitin-proteasome pathway. Drug Des Devel Ther. 2015;9:5611–5622. doi: 10.2147/DDDT.S93912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Man J, Shoemake JD, Ma T, Rizzo AE, Godley AR, Wu Q, Mohammadi AM, Bao S, Rich JN, Yu JS. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75:1760–1769. doi: 10.1158/0008-5472.CAN-14-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int J Mol Sci. 2014;15:17380–17395. doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Wang JJ, Chen XL, Du SM, Li DS, Pei ZJ, Lan H, Wu LB. The JAK2/STAT3 and mitochondrial pathways are essential for quercetin nanoliposome-induced C6 glioma cell death. Cell Death Dis. 2013;4:e746. doi: 10.1038/cddis.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, Tanino M, Kimura T, Nishihara H, Tanaka S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11:1289–1299. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 29.Yi GQ, Gu B, Chen LK. The safety and efficacy of magnetic nano-iron hyperthermia therapy on rat brain glioma. Tumour Biol. 2014;35:2445–2449. doi: 10.1007/s13277-013-1324-8. [DOI] [PubMed] [Google Scholar]
- 30.Mamani JB, Pavon LF, Miyaki LA, Sibov TT, Rossan F, Silveira PH, Cárdenas WH, Junior E Amaro, Gamarra LF. Intracellular labeling and quantification process by magnetic resonance imaging using iron oxide magnetic nanoparticles in rat C6 glioma cell line. Einstein (Sao Paulo) 2012;10:216–221. doi: 10.1590/S1679-45082012000200009. (In English, Portuguese) [DOI] [PubMed] [Google Scholar]