Abstract
Hydrogen sulfide (H2S) is a gas signaling molecule that has multiple influences on physiological and pathological processes in the mammalian body, including vasodilation, neurotransmission, inflammation, hypoxia sensing and bone remodeling. Our previous studies suggested that H2S might be involved in the periodontal tissue remodeling during the orthodontic tooth movement (OTM) via increasing periodontal ligament cell differentiation, tissue mineralization, bone formation and collagen synthesis. The aim of the present study was to investigate the effects of H2S on alveolar bone remodeling that is associated with tooth movement. Experiments were performed in an OTM mouse model. Sodium hydrosulfide (NaHS), which is a donor of H2S and DL-propargylglycine (PAG) and a cystathionine-γ-lyase (CSE) inhibitor, which could also decrease H2S expression, were administered intraperitoneally and respectively. A total of 60 male C57BL6/J mice were divided into 4 groups; Control, NaHS, PAG and combination (PAG+NaHS). The rate of OTM and the bone mineral density (BMD) of alveolar bone were scanned and measured by micro-computed tomography (micro-CT). The number of osteoclasts and expression of the tumor necrosis factor ligand superfamily member-11 (RANKL), alkaline phosphatase (ALP), osteocalcin (OCN) and osteoprotegerin (OPG) in alveolar bone were accessed to evaluate the osteoclastic activity and osteogenesis with histochemistry of tartrate-resistant acid phosphatase staining, immunohistochemistry and reverse transcription-quantitative polymerase chain reaction. In the alveolar bone, NaHS increased the OTM and decreased the BMD, respectively. PAG significantly decrease OTM and increased the BMD. NaHS combined with PAG rescued the PAG-induced changes in the OTM and the BMD. Additionally, the number of osteoclasts, the expression of RANKL, ALP, OCN and the ratio of RANKL/OPG were significantly up-regulated in the NaHS group. In contrast, PAG down-regulated the number of osteoclasts, the expression of RANKL, ALP, OCN and the ratio of RANKL/OPG. These findings suggested that H2S might facilitate the OTM by enhancing alveolar bone remodeling as a result of an increased osteoclastic activity and osteogenesis.
Keywords: hydrogen sulfide, bone remodeling, orthodontic tooth movement, mouse model
Introduction
In the process of orthodontics treatment, mechanical force is loaded on the tooth, resulting in the production of inflammatory mediators in periodontal tissues, and the surrounding alveolar bone is remodeled. According to the pressure-tension theory (1), bone resorption occurs on the side of root under pressure, while bone formation occurs on the other side of the root under tension. The bone remodeling in the dental and periodontal tissues enables the tooth to move in the alveolar bone (2,3). A previous study demonstrated alternations of the alveolar bone density due to active bone remodeling during orthodontic treatment, the reduction of alveolar bone density results from less bone mineral content as a result of resorption of pre-existing bone tissue and formation of new bone tissue during the process of bone remodeling (4). Therefore, it is likely that bone mineral density (BMD) reflects progress of bone remodeling during orthodontic treatment. Additionally, some cell factors and proteins including tumor necrosis factor ligand superfamily member-11 (RANKL), osteoprotegerin (OPG), lubricin and tumor necrosis factor-related apoptosis-inducing ligand were also proved to influence the bone remodeling in orthodontic tooth movement (OTM) (5–7).
Hydrogen sulfide (H2S) can be produced by at least three enzymes: Cystathionine β-synthetase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase in mammals (8,9). As a gasotransmitter, H2S serves a vital role in various physiological and pathophysiological processes, including vasodilation, neurotransmission, inflammation, anti-inflammation and hypoxia sensing (10–14). In rat periodontal tissues, H2S increases the osteoclast activity and upregulates RANKL expression levels (15,16). It was reported that human periodontal ligament stem cells (PDLSCs), expressed both CBS and CSE and produced H2S, which maintained the osteogenic capacity of PDLSCs (17). Our previous study indicated that human PDLSCs produced H2S via CSE, and the mechanical stimuli increased the mRNA expression levels of CSE. Another study suggested that mechanical stimuli promoted the production of H2S in human PDLSCs (18). In has also been demonstrated that H2S could promote osteogenic differentiation of human PDLSCs by activating the p38-mitogen-activated protein kinase and the extracellular signal-regulated kinase signaling pathways (19).
However, the effect of H2S on alveolar bone remodeling has not been extensively investigated, as most studies are based on in vitro investigation without in vivo evidence. The aim of this study was to explore the effects of H2S on alveolar bone formation and resorption in an OTM mouse model. Results of this study may provide an understanding on H2S mechanisms of action on orthodontic tooth movement and a potential approach to accelerate orthodontic treatment.
Materials and methods
Animals
A total of 60 8-week old male C57BL6/J mice were used in this study in accordance with a protocol approved by the Animal Use and Care Committee of Tongji University (Shanghai, China). This study was granted ethical approval by Medical Ethics Committee of Tongji University, Shanghai, China. Mice were housed under specific pathogen-free conditions with a controlled temperature (22±1°C), humidity (40–60%) and a 12-h light/dark cycle. The animals were given soft diet and water, ad libitum. The general condition and weight of each animal in this study were monitored during all experiments. In the present study, mice were divided into four groups, including a control (n=15), a sodium hydrosulfide (NaHS; n=15), a DL-propargylglycine (PAG; n=15) and a combination group (PAG+NaHS; n=15). Both NaHS (a H2S donor, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and PAG (a CSE inhibitor, Sigma-Aldrich; Merck KGaA) were administered intraperitoneally at a dose of 10 mg/kg/day for 14 days, and a micro-computed tomography (micro-CT) scan was performed on day 14. Following the sacrifice of mice with an overdose of anesthetic (10% chloralic hydras, 400 mg/kg, Sigma-Aldrich; Merck KGaA), tissues were collected for further histological, immunohistochemical and molecular biology analyses. Briefly, tissues were fixed in 4% paraformaldehyde at 4°C for 24 h, followed by incubation with 10% ethylenediaminetetraacetic Acid (pH 7.4) for decalcification (4°C for 4 weeks). Sections (5 µm thick) were cut by a microtome (Leica RM2235, Leica Microsystems Ltd., Wetzlar, Germany) in a mesiodistal direction parallel to the long axis of mesial root of the first molar. Sections that included mesial roots and the alveolar bone of the first molar were selected for titrate-resistant acid phosphatase (TRAP) and immunohistochemical staining.
Establishment of the OTM model
An OTM mouse model was set up as described in a previous study (20). Orthodontic force was generated by a nickel-titanium coiled spring (0.2 mm in thickness, 1 mm in diameter and 1 mm in length, Smart Technology Co, Ltd., Beijing, China) bonded between the maxillary right first molar and the incisors with flowable restorative resin (3M ESPE, St. Paul, MN, USA), producing ~35 g force (21) (Fig. 1). A dynamometer (YDM Corporation, Tokyo, Japan) was used to measure the force accurately. The distance between the first and the second molar in mice was identified as OTM.
Figure 1.
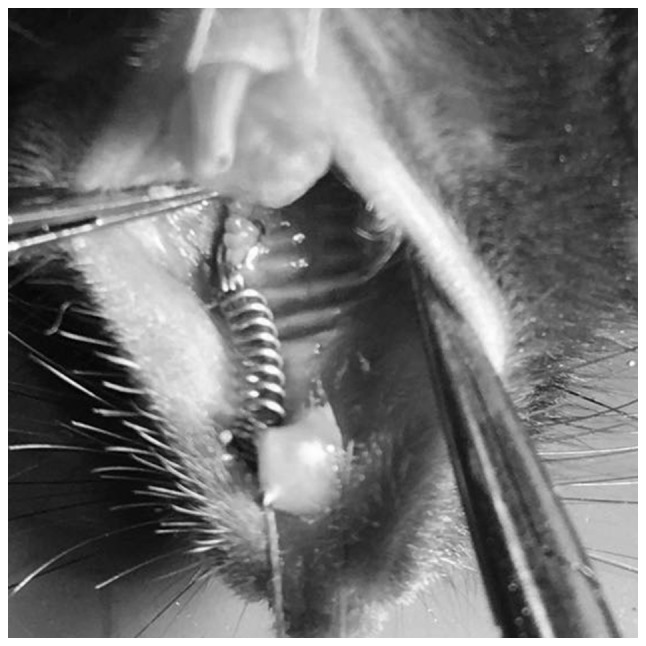
Representative image of the orthodontic appliance in an OTM mouse model.
Micro-CT scanning
Microarchitecture of the maxillary was scanned and the distance between the maxillary first and the second molar was measured using micro-CT (SCANCO Medical AG, Bruttisellen, Switzerland). The BMD of alveolar bone in the root furcation area was analyzed quantitatively by a self-contained 3D analysis software of micro-CT (SCANCO Medical AG, Bruttisellen, Switzerland), by assessing the bone volume over total volume (BV/TV).
TRAP immunohistochemistry
TRAP staining (37°C, 1 h) was performed to identify and quantify osteoclasts using a TRAP ELISA kit (cat. no. 387A, Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocol. The osteoclasts of the alveolar bone located between the first and second molar were counted under a confocal microscope (NIKON ECLIPSE 80i, Nikon Corporation, Tokyo, Japan).
RNA extraction and reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the alveolar bone surrounding the upper region of the first molar using TRIzol reagent (Sigma-Aldrich; Merck KGaA). A RT-qPCR Kit (Takara Bio, Inc., Otsu, Japan) was used to reverse transcribe the RNA to complementary (c)DNA, according to the manufacturer's protocol, the conditions of reactions were: 30°C for 10 min, 42°C for 30 min, 99°C for 5 min and 4°C for 5 min. Relative mRNA levels of alkaline phosphatase (ALP), osteocalcin (OCN), OPG and RANKL were determined using a SYBR Green PCR Master mix and an ABI 7500 Real-Time PCR Detection System (Roche Diagnostics, Basel, Switzerland). Primer sequences are listed in Table I. The conditions of reactions were: Initial denaturation at 94°C for 3 min and followed by 35 cycles of denaturation at 95°C for 10 sec, and annealing at 57°C for 30 sec. Reactions were run in triplicate and mean-averaged the results. Relative fold changes were calculated using the method of 2−ΔΔCq (22). GAPDH was used as the housekeeping gene.
Table I.
Primer sequences of ALP, OCS, OPG, RNKL and GAPDH.
| Gene | Primer Sequence (5′-3′) | Length (bp) |
|---|---|---|
| ALP | F: ACACTCGGCCGATCGGGACT | 20 |
| R: CCGCCACCCATGATCACGTCG | 21 | |
| OCN | F: TAGTGAACAGACTCCGGCGCTA | 22 |
| R: TGTAGGCGGTCTTCAAGCCAT | 21 | |
| OPG | F: GTGGAATAGATGTCACCCTGTGT | 23 |
| R: TTTGGTCCCAGGCAAACTGT | 20 | |
| RANKL | F: CAGAAGATGGCACTCACTGCA | 21 |
| R: CACCATCGCTTTCTCTGCTCT | 21 | |
| GAPDH | F:AGCAGTCCCGTACACTGGCAAAC | 23 |
| R:TCTGTGGTGATGTAAATGTCCTCT | 24 |
F, forward; R, reverse; ALP, alkaline phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; RANKL, tumor necrosis factor ligand superfamily member-11.
Immunohistochemistry
Following routine deparaffinage with 100% xylene for 20 min at room temperature, and hydration with alcohol gradient, 3% H2O2 was used to inactivate endogenous enzymes for 30 min at room temperature. Antigen repair was performed with Trypsin (Sigma-Aldrich; Merck KGaA) for 20 min at room temperature. Sections were incubated with primary antibodies (ALP, cat. no. SAB3700030; OCN, cat. no. SAB1306277; OPG, cat. no. SAB4502041, and RANKL, cat. no. SAB4503430; all 1:200, Sigma-Aldrich; Merck KGaA) at 37°C for 1 h. Sections were also incubated with second antibody (cat. no. B3640, 1:200, Sigma-Aldrich; Merck KGaA) at 37°C for 1 h. Positive staining was visualized with the DAB (D8001, Sigma-Aldrich; Merck KGaA) and counterstained with hematoxylin.
Statistical analysis
Data are expressed as the mean ± standard deviation. One-way analysis of variance was performed followed by Tukey's test using the SPSS software (version 20.0, IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of H2S on OTM
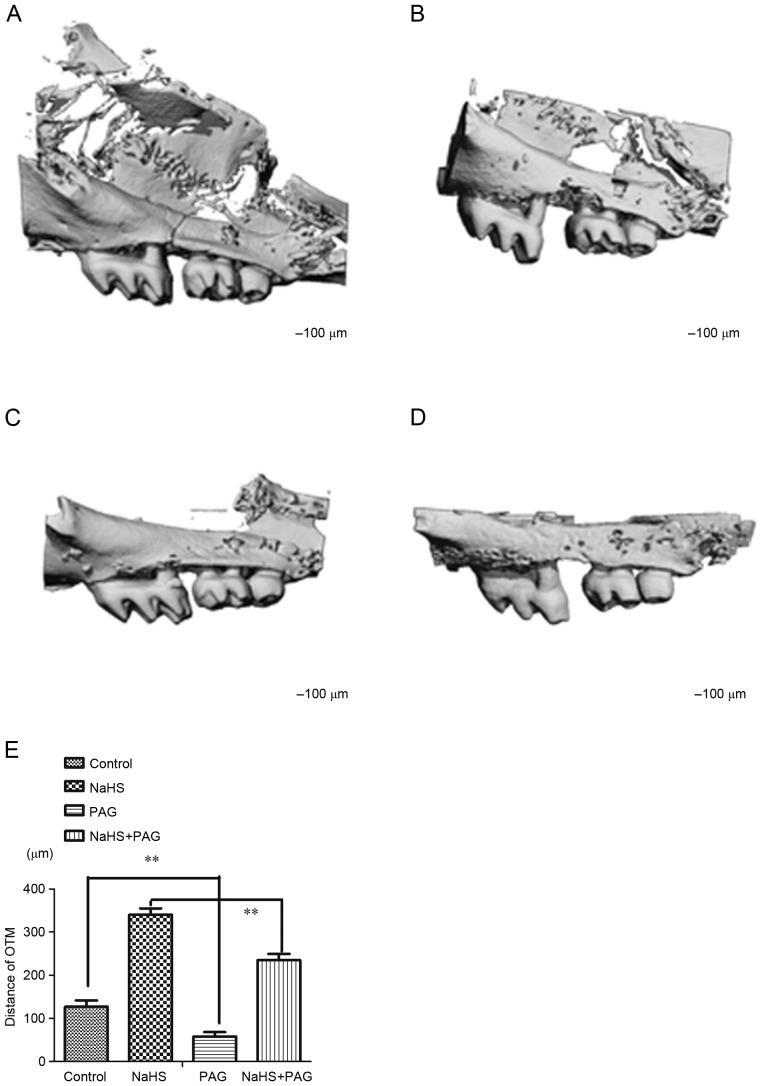
When compared with the control animals (Fig. 2A; 127±15 µm) NaHS resulted in a significant increase in the OTM (Fig. 2B; 341±14 µm, P<0.01). There was a significant decrease in the OTM in the PAG-treated group (Fig. 2C; 58±10 µm, P<0.01) compared with the control. Notably, NaHS rescued the decreased OTM induced by PAG (Fig. 2D; 235±6 µm, P<0.01 and Fig. 2E; 58±10 µm, P<0.01).
Figure 2.
Micro-computed tomography images of interdental distance between first and second molar teeth of the (A) control, (B) NaHS, (C) PAG and (D) PAG+NaHS groups, and (E) quantification of the changes in the molar tooth movement. Data are presented as the mean ± standard deviation. **P<0.01. OTM, orthodontic tooth movement; NaHS, sodium hydrosulfide; PAG, propargylglycine.
Effect of H2S on BMD
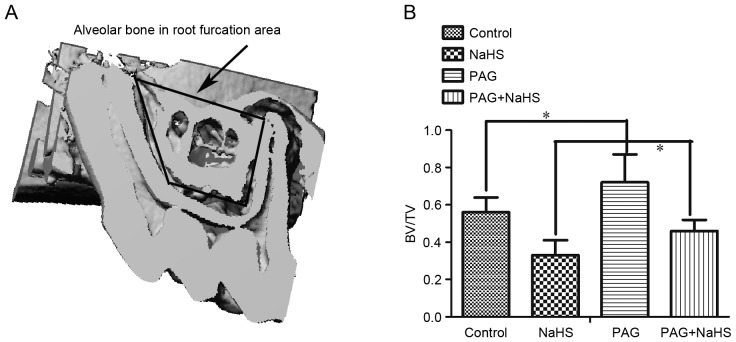
Compared with the control group (56±8%), there was a significant decrease in the BMD measured in the root furcation area in the NaSH group (33±7%, P<0.05). The PAG group (72±15%, P<0.05) demonstrated significantly higher BMD than the control group (56±8%). Finally, NaHS (46±6%, P<0.05) down-regulated the increased BMD-induced by PAG (72±15%; Fig. 3).
Figure 3.
NaHS decreases BMD of alveolar bone in root furcation area. (A) Representative image depicting the region of root furcation and (B) quantification of BMD of alveolar bone in all groups. Data are presented as the mean ± standard deviation. *P<0.05. OTM, orthodontic tooth movement; NaHS, sodium hydrosulfide; PAG, propargylglycine; BMD, bone mass density; BV/TV, bone volume over total volume.
Effect of H2S on osteoclast numbers
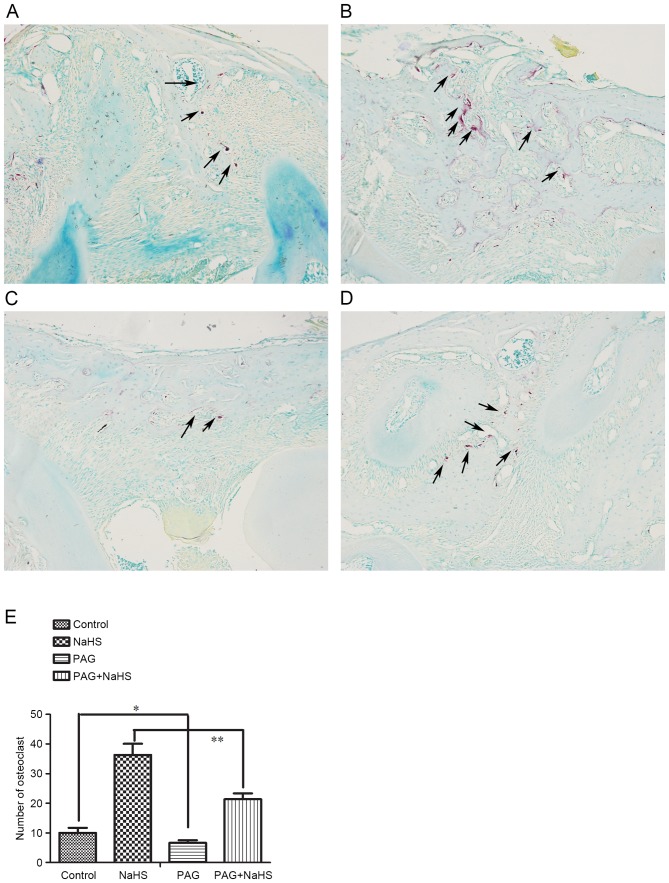
To explore the effect of H2S on bone resorption, the osteoclasts in the alveolar bone between the first and the second molar were identified and quantified with TRAP staining (Fig. 4A-D). Treatment of NaHS (Fig. 3A and B; 36±4, P<0.05) increased the number of osteoclasts whereas PAG (7±1, P<0.05) decreased their number, compared with the control group (Fig. 4C and E; 10±2). In addition, the PAG+NaHS group (21±2, P<0.01) indicated a significant increase in the number of osteoclasts compared with the PAG group (7±1; Fig. 4D and E).
Figure 4.
Tartrate-resistant acid phosphatase+ osteoclasts in alveolar bone between first and second molar in the (A) control, (B) NaHS, (C) PAG and (D) PAG+NaHS groups. Magnification, ×100. (E) Quantification of the number of osteoclasts among the groups following each treatment. Arrows indicate the osteoclasts. Data are presented as the mean ± standard deviation. *P<0.05; **P<0.01. OTM, orthodontic tooth movement; NaHS, sodium hydrosulfide; PAG, propargylglycine.
Effect of H2S on mRNA and protein expression levels of OCN, ALP, RANKL, OPG and RANKL/OPG
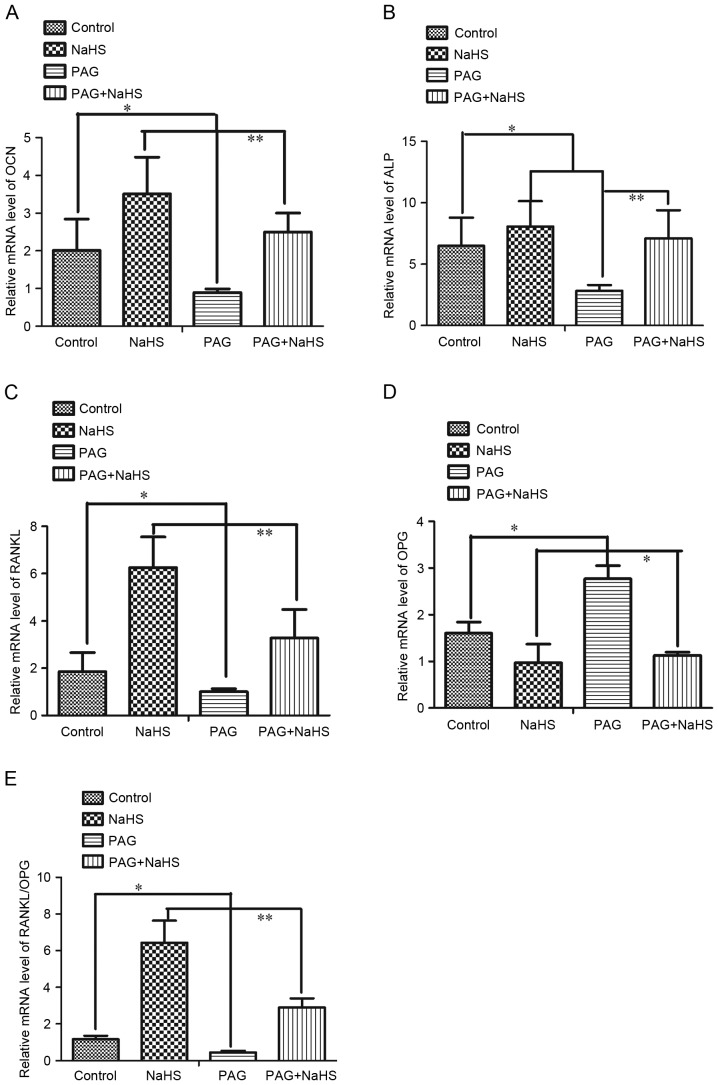
To further understand the effect of H2S on bone resorption and bone formation, mRNA and protein expression levels of OCN, ALP, RANKL and OPG in the alveolar bone were investigated. Results demonstrated that both the treatment of NaHS alone and NaHS combined with PAG significantly up-regulated the mRNA expression levels of OCN, ALP and RANKL, whereas it down-regulated the mRNA expression levels of OPG. However, PAG administration decreased significantly the mRNA expression levels of OCN, ALP, and RANKL and increased the mRNA expression levels of OPG (Fig. 5A-D). The mRNA expression ratio of RANKL/OPG was up-regulated by NaHS and down-regulated by PAG (Fig. 5E). Similarly, both the treatment of NaHS alone and NaHS combined with PAG significantly up-regulated the protein expression levels of OCN, ALP and RANKL whereas down-regulated OPG protein expression levels. Additionally, PAG administration decreased significantly the protein expression levels of OCN, ALP and RANKL, and increased OPG protein expression levels (Fig. 6).
Figure 5.
mRNA expression levels of (A) OCN, (B) ALP, (C) RANKL, (D) OPG and (E) ratio of RANKL/OPG in the alveolar bone surrounding the upper first molar region. Data are presented as the mean ± standard deviation. *P<0.05; **P<0.01. OTM, orthodontic tooth movement; NaHS, sodium hydrosulfide; PAG, propargylglycine; ALP, alkaline phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; RANKL, tumor necrosis factor ligand superfamily member-11.
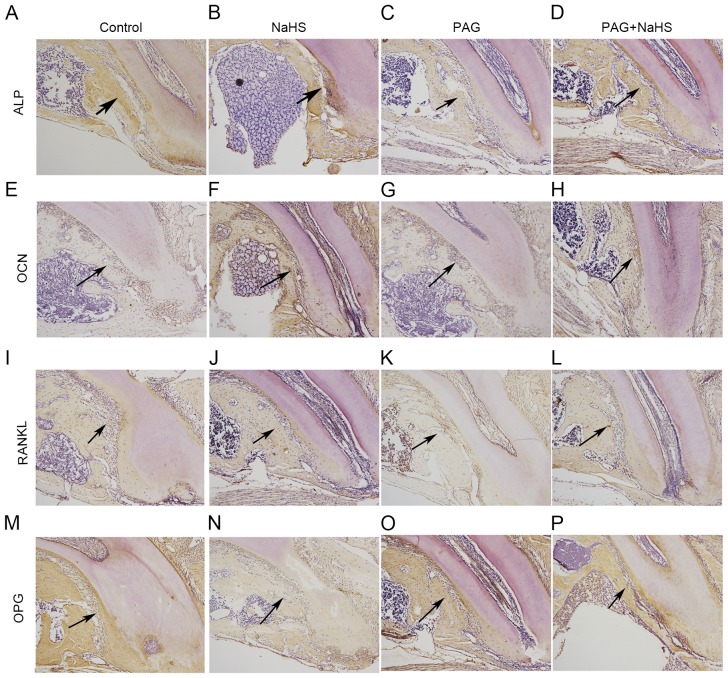
Figure 6.
Protein expression levels of ALP in the (A) control, (B) NaHS, (C) PAG and (D) PAG+NaHS groups; of OCN in the (E) control, (F) NaHS, (G) PAG and (H) PAG+NaHS groups; of RANKL in the (I) control, (J) NaHS, (K) PAG and (L) PAG+NaHS groups; of OPG in the (M) control, (N) NaHS, (O) PAG and (P) PAG+NaHS groups. Original magnification, ×100. Arrows indicate positive staining. OTM, orthodontic tooth movement; NaHS, sodium hydrosulfide; PAG, propargylglycine; ALP, alkaline phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; RANKL, tumor necrosis factor ligand superfamily member-11.
Discussion
Orthodontic treatment usually lasts 24 to 30 months (23). The long treatment duration could increase the risk of caries development, root resorption and periodontal problems (24–26). Accelerating OTM and shortening the time of treatment has become a crucial research area. OTM occurs in the presence of a mechanical stimulus sequenced by remodeling of the alveolar bone and periodontal ligament (1). It was reported that cyclical forces of 60Hz could increase the rate of OTM (27). Periodontally accelerated osteogenic orthodontics has also been proved to be a safe technique to accelerate tooth movement by reducing the treatment time (28). It is well known that the OTM can be controlled by the size of the applied force and the biological responses from the periodontal ligament, such as the release of inflammatory mediators including cytokines, growth factors, neurotransmitters and arachidonic acid metabolites (2). One of the cytokines, RANKL is considered to be involved in the acceleration of tooth movement by binding to the RANK on osteoclasts stimulating osteoclastogenesis (29). OPG, a circulating glycoprotein, binds to RANKL and inhibits osteoclast activities by competitively inhibiting the RANKL-RANK interaction (30). The balance between RANKL and OPG levels determines osteoclast activation, skeletal calcium release and bone remodeling (31).
H2S, a gasotransmitter, serves many physiological and pathophysiological roles in maintaining the normal activity of various organs in the neuronal, cardiovascular, gastrointestinal and respiratory systems (10,11,32,33). A previous study indicated that bone marrow mesenchymal stem cells could produce H2S, regulating osteogenic differentiation and cell self-renewal, and that H2S deficiency could lead to defects in their differentiation (34). Our previous in vitro study also demonstrated that H2S could promote osteogenic differentiation by up-regulating the expression ratio of OPG/RANKL expression in human PDLSCs (18). It was also demonstrated that H2S significantly increased the expression of ALP, OCN, runt-related transcription factor 2 and collagen 1 in the human PDLSCs with tension force stimulation, and promoted osteogenic differentiation of human PDLSCs by activating the p38-MAPK and ERK signaling pathways (19,35). In the present study, exogenous H2S could up-regulate the expression of ALP and OCN, the ratio of RANKL/OPG and the number of osteoclasts in the alveolar bone. ALP has been commonly studied as an early marker of osteogenic differentiation in tension force experiments and OCN is a key transcription factor in the modulation of osteogenic differentiation and bone formation (19). The results of the present study revealed that H2S could up-regulate the process of osteogenesis, osteoclastic activities and bone remodeling. These findings are consistent with our previous study, where mechanical stimulation and H2S could enhance the osteogenic differentiation (18,19,35). These results were also in accordance with some studies illustrating that H2S could enhance RANKL-induced osteoclast differentiation in vitro and induce osteoclast differentiation in vivo (17,36).
It is generally known that remodeling and modeling of alveolar bone is considered as the most important aspect in orthodontic tooth movement (37,38). In the present study, non-invasive micro-CT was used to monitor the migration of tooth and the internal conditions of the alveolar bone, such as bone density, during orthodontic and H2S treatment. Data of the present study indicated that systemic administration of NaHS accelerated the OTM and reduced the alveolar bone density, providing evidence supporting that H2S could promote osteogenesis and osteoclastogenesis differentiation, and might facilitate the tooth movement during orthodontic treatment. In addition, it was observed that PAG decreased the OTM in the mouse model. This change might be associated with a reduction of endogenous H2S.
It is worth noting that the present study was based on an OTM mouse model, and is different from clinical work. The mechanism of how H2S regulates OTM is still unclear. However, the present study may provide a novel understanding on how to accelerate the tooth movement and shorten the treatment time.
In conclusion, the present study provided an in vivo evidence that H2S increased the rate of tooth movement by promoting osteogenesis and osteoclastogenesis differentiation in alveolar bone. These findings could encourage further studies looking for a potential therapeutic value of H2S for accelerating the orthodontic treatment.
Acknowledgements
This study was supported by the National Science Foundation of China (grant no. 81371177) and the Shanghai Science and Technology Committee (grant no. 17140903700).
References
- 1.Melsen B. Biological reaction of alveolar bone to orthodontic tooth movement. Angle Orthod. 1999;69:151–158. doi: 10.1043/0003-3219(1999)069<0151:BROABT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469.e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;129:458–468. doi: 10.1016/j.ajodo.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Zahrowski JJ. Optimizing orthodontic treatment in patients taking bisphosphonates for osteoporosis. Am Orthod Dentofacial Orthop. 2009;135:361–374. doi: 10.1016/j.ajodo.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G. RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training) in rat with glucocorticoid-induced osteoporosis. Histol Histopathol. 2013;28:1185–1196. doi: 10.14670/HH-28.1185. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi R, Loreto C, Talic N, Caltabiano R, Musumeci G. Immunolocalization of lubricin in the rat periodontal ligament during experimental tooth movement. Acta Histochem. 2012;114:700–704. doi: 10.1016/j.acthis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Cardile V, Musumeci G, Sicurezza E, Caggia S, Rusu MC, Leonardi R, Loreto C. Expression of TRAIL and its receptors DR5 and DcR2 in orthodontic tooth movement. Histol Histopathol. 2013;28:933–940. doi: 10.14670/HH-28.933. [DOI] [PubMed] [Google Scholar]
- 8.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta. 2014;1844:1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis; Proc Natl Acad Sci USA; 2009; pp. 21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 14.Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang G, Wang R, Perry SF. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- 15.Irie K, Ekuni D, Tomofuji T, Endo Y, Kasuyama K, Yaegaki K, Morita M. Combined effects of hydrogen sulfide and lipopolysaccharide on osteoclast differentiation in rats. J Periodontol. 2012;83:522–527. doi: 10.1902/jop.2011.110315. [DOI] [PubMed] [Google Scholar]
- 16.Irie K, Ekuni D, Yamamoto T, Morita M, Yaegaki K, Ii H, Imai T. A single application of hydrogen sulphide induces a transient osteoclast differentiation with RANKL expression in the rat model. Arch Oral Biol. 2009;54:723–729. doi: 10.1016/j.archoralbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Su Y, Liu D, Liu Y, Zhang C, Wang J, Wang S. Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells. J Periodontol. 2015;86:1276–1286. doi: 10.1902/jop.2015.150240. [DOI] [PubMed] [Google Scholar]
- 18.Liao C, Hua Y. Effect of hydrogen sulphide on the expression of osteoprotegerin and receptor activator of NF-κB ligand in human periodontal ligament cells induced by tension-force stimulation. Arch Oral Biol. 2013;58:1784–1790. doi: 10.1016/j.archoralbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Hua Y. Hydrogen sulfide promotes osteogenic differentiation of human periodontal ligament cells via p38-MAPK signaling pathway under proper tension stimulation. Arch Oral Biol. 2016;72:8–13. doi: 10.1016/j.archoralbio.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Kou X, Yang R, Liu D, Wang X, Song Y, Feng L, He D, Gan Y, Zhou Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res. 2014;93:1163–1169. doi: 10.1177/0022034514551769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Y, Liu F, Kou X, Liu D, Yang R, Wang X, Song Y, He D, Gan Y, Zhou Y. T cells are required for orthodontic tooth movement. J Dent Res. 2015;94:1463–1470. doi: 10.1177/0022034515595003. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Long H, Pyakurel U, Wang Y, Liao L, Zhou Y, Lai W. Interventions for accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2013;83:164–171. doi: 10.2319/031512-224.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimeri G, Kau CH, Abou-Kheir NS, Corona R. Acceleration of tooth movement during orthodontic treatment-a frontier in orthodontics. Prog Orthod. 2013;14:42. doi: 10.1186/2196-1042-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kau CH, Kantarci A, Shaughnessy T, Vachiramon A, Santiwong P, de la Fuente A, Skrenes D, Ma D, Brawn P. Photobiomodulation accelerates orthodontic alignment in the early phase of treatment. Prog Orthod. 2013;14:30. doi: 10.1186/2196-1042-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kau CH. A radiographic analysis of tooth morphology following the use of a novel cyclical force device in orthodontics. Head Face Med. 2011;7:14. doi: 10.1186/1746-160X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura M, Chiba M, Ohashi T, Sato M, Shimizu Y, Igarashi K, Mitani H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008;133:572–583. doi: 10.1016/j.ajodo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 28.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 29.Ciacli C, Puşchiţă M. RANKL/RANK/OPG molecular complex-control factors in bone remodeling in psoriatic arthritis. Rev Med Chir Soc Med Nat Iasi. 2011;115:354–360. [PubMed] [Google Scholar]
- 30.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 31.Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13:3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 32.Souza LK, Araújo TS, Sousa NA, Sousa FB, Nogueira KM, Nicolau LA, Medeiros JV. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric Oxide. 2017;64:1–6. doi: 10.1016/j.niox.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Ivanciuc T, Sbrana E, Ansar M, Bazhanov N, Szabo C, Casola A, Garofalo RP. Hydrogen sulfide is an antiviral and antiinflammatory endogenous gasotransmitter in the airways. Role in respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2016;55:684–696. doi: 10.1165/rcmb.2015-0385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, Shi S. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin J, Hua Y. Effects of hydrogen sulfide on the expression of alkaline phosphatase, osteocalcin and collagen type I in human periodontal ligament cells induced by tension force stimulation. Mol Med Rep. 2016;14:3871–3877. doi: 10.3892/mmr.2016.5680. [DOI] [PubMed] [Google Scholar]
- 36.Itou T, Maldonado N, Yamada I, Goettsch C, Matsumoto J, Aikawa M, Singh S, Aikawa E. Cystathionine γ-lyase accelerates osteoclast differentiation: Identification of a novel regulator of osteoclastogenesis by proteomic analysis. Arterioscler Thromb Vasc Biol. 2014;34:626–634. doi: 10.1161/ATVBAHA.113.302576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viecilli RF, Katona TR, Chen J, Hartsfield JK, Jr, Roberts WE. Orthodontic mechanotransduction and the role of the P2X7 receptor. Am J Orthod Dentofacial Orthop. 2009;135:694.e1–16; discussion 694–695. doi: 10.1016/j.ajodo.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res. 2009;88:597–608. doi: 10.1177/0022034509338914. [DOI] [PubMed] [Google Scholar]