Abstract
We aimed to investigate the role of oxidized low density lipoprotein (ox-LDL) in tumor necrosis factor-α (TNF-α) mediated chondrocyte death and explore the mechanisms. Ten osteoarthritis (OA) and normal control cartilage tissue and synovial fluid (SF) samples were collected, and the expression of lectin-like ox-LDL receptor-1 (LOX-1) and ox-LDL level was examined by real time quantitative PCR and enzyme-linked immunosorbent assay (ELISA). An in vitro chondrocyte model was established by the introduction of TNF-α and ox-LDL, cell death was analyzed by trypan blue assay and the mechanisms were explored based on the apoptosis related pathway and autophagy pathway. Significantly increased ox-LDL level (70.30±17.83 vs. 37.22±19.97, P<0.05) in SF sample and LOX-1 expression level (0.51±0.10 vs. 0.32±0.04, P<0.05) in cartilage tissue was found in OA patients compared to those corresponding samples from control subjects. Ox-LDL could facilitate TNF-α mediated chondrocyte death and this effect could be blocked by LOX-1 antibody neutralization. Autophagy inhibition by 3-MA and Atg-5 siRNA could reverse the cell death effect mediated by TNF-α and ox-LDL co-treatment on chondrocytes. Oxidized low density lipoprotein facilitates tumor necrosis factor-α mediated chondrocyte death via its interaction with LOX-1, and autophagy is involved in the mechanisms.
Keywords: oxidized low density lipoprotein, lectin-like ox-low density lipoprotein receptor-1, chondrocyte, tumor necrosis factor-α, autophagy
Introduction
Osteoarthritis (OA) represents the most prevalent type of joint disease especially in the elderly and it is estimated that about 3 million newly diagnosed OA could be presented each year (1,2). In current clinical practice, joint replacement remains the preferred treatment method for patients with advanced OA (3). However, due to the lacking of knowledge about the disease pathophysiology, only a few effective disease-modifying therapies has been proposed for OA treatment. Therefore, investigate the disease mechanism will give insights for the treatment.
OA is characterized by extracellular matrix destruction and chondrocyte function loss (apoptosis) and multiple risk factors could result in this phenomenon, including mechanical injury, aging and inflammation (4). Enhanced chondrocyte apoptosis is considered as the sign of cartilage joint degeneration in OA (5). Studies have shown that a variety of stimuli including tumor necrosis factor-α (TNF-α) (6), TNF-related apoptosis-inducing ligand (TRAIL) (7) and nitric oxide (8) were involved in the chondrocytes apoptosis process. Recent epidemiology and experimental studies have shown that lipid peroxidation was involved in the pathogenesis of OA (9,10). Associations were found between hypercholesterolemia or hypertension and OA (9,10). Joint manifestation are frequently presented in patients with familial hypercholesterolemia, which is characterized by a decreased removal of low-density lipoprotein (LDL), and treatment with a lipid-lowering diet attenuates the incidence of joint involvement (11). However, the few studies has explored the detailed events involved in these events.
Here, we aimed to investigate the role of oxidized low density lipoprotein (ox-LDL) in TNF-α mediated chondrocyte death and explore the mechanisms. Based on our patient data and cell model experiment data, we demonstrated that ox-LDL could enhance TNF-α mediated chondrocyte death via autophagy related pathway.
Materials and methods
OA cartilage and SF samples
OA (n=40) cartilage and synovial fluid (SF) samples were collected at the time of total knee arthroplasty at Shanghai Sixth People's Hospital, Shanghai, China. Normal cartilage and SF from patients (n=40) with no history of OA was obtained from Shanghai Sixth People's Hospital. Cartilage and SF samples were stored in −80°C before further processing. The demographic data of these patients, including age, gender, body mass index (BMI), disease duration, the total Western Ontario and McMasters University Osteoarthritis Index (WOMAC) score, ox-LDL level and Lox-1 level, were also collected and listed in Table I. Among these indexed, the total WOMAC score includes WOMAC pain score and WOMAC function score, and functional disability and pain were assessed by self-reported questionnaires according to previous publications (12,13). All research involving human participants was approved by the Institutional Review Board of Shanghai Jiaotong University School of Medicine, Shanghai, China. Written informed consent was collected from all the participants.
Table I.
Demographic data of the osteoarthritis patients.
| Healthy controls (n=40) | Patient (n=40) | |
|---|---|---|
| Age | 47.7±10.4 | 57.4±10.2 |
| Sex (female, %) | 22 (55%) | 21(52.5%) |
| BMI (kg/m2) | 27.5±4.5 | 26.2±3.9 |
| Disease duration | – | 52.2±38.1 |
| Total WOMAC score | – | 73.0±19.9 |
| LOX-1 level | 0.33±0.04 | 0.49±0.11 |
| Ox-LDL level (mU/ml) | 34.5±15.7 | 64.8±18.3 |
Ox-LDL, oxidized low density lipoprotein; LOX-1, lectin-like ox-low density lipoprotein receptor-1; BMI, mody mass index; WOMAC, Western Ontario and McMasters University Osteoarthritis Index.
Enzyme-linked immunosorbent assay
SF samples were examined with enzyme-linked immunosorbent assay (ELISA) kit from Cell Biolabs Inc., (San Diego, CA, USA) and the absorbance value was measured at 450 nm to concentration measurement according to the manufacturer's instructions.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) assay
Total cellular RNA was extracted from of cartilage tissue with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RT-PCR was carried out using a One Step SYBR® PrimeScript™ RT-PCR kit (Takara Bio, Dalian, China) and an iQ5 Real-time PCR Detection system (Bio-Rad, Hercules, CA, USA). Expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was assayed simultaneously with samples as an internal control. Relative gene expression was determined by the 2−ΔΔCT method (14). Oligonucleotide primers specific for LOX-1 and GAPDH are listed in Table II.
Table II.
Primer sequences.
| Gene name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| LOX-1 | TTACTCTCCATG | AGCTTCTTCTGC |
| GTGGTGCC | TTGTTGCC | |
| GAPDH | CAAAGCCAGAG | GATGGTCTTGGT |
| TCCTTCAGA | CCTTAGCC |
LOX-1, lectin-like ox-low density lipoprotein receptor-1.
Cell culture and treatment
Human articular chondrocyte culture was purchased from cell culture collection of Fudan University, Shanghai, China, and cultured according to the manufacturer's instruction. Cell were plated at a density of 1×104/cm2 in a human chondrocyte medium containing chondrocyte growth supplement and penicillin/streptomycin (ScienCell, Carlsbad, CA, USA) and incubated at 37°C in a humidified 5% CO2 incubator. In the setting of LOX-1 neutralization, chondrocytes at 70–80% confluency were pre-incubated with or without anti-LOX-1 antibody (10 µg/ml) before processing for vehicle control or TNF-α (50 ng/ml) or ox-LDL (20 µg/ml, Yiyuan biotech, Guangzhou, China) or TNF-α (50 ng/ml) and ox-LDL (20 µg/ml) co-treatment for 24–48 h. In the experiment of autophagy inhibition, chondrocytes at 70–80% confluency were pre-treated with or without 3-MA (10 mM) for 24 h before processing for vehicle control or TNF-α (50 ng/ml) and ox-LDL (20 µg/ml) co-treatment for 24 h.
Lenti-virus mediated autophagy protein 5 (ATG5) knockdown
ATG5 siRNA lentivirus and control lentivirus were obtained from Shanghai Hanbio Co. Ltd., Shanghai, China. Chondrocytes cultured in a 6 well plate at 50% confluence were infected with 50 µl ATG5 siRNA and control lentivirus in 3 ml serum free human chondrocyte medium containing 8 µg/ml puromycin for 24 h. Medium change was performed after 24 h and the efficacy of ATG-5 knockdown was verified by western blotting. Chondrocytes were collected at 48 h after lentivirus infection, followed by TNF-α (50 ng/ml) and ox-LDL (20 µg/ml) co-treatment for 24 h.
Cell viability
Cell viability was determined by trypan blue exclusion assay.
Immunofluorescence
Cells were collected onto a clean glass slide by using centrifugation at 1,000 rpm for 5 min. The slide was then incubated with LC3 primary antibody for 1 h at 37°C, washed 3 times with PBS, incubated with FITC-conjugated secondary antibody for 1 h at room temperature and finally dropped with DAPI containing anti-quench reagents. Fluorescence images were observed and analyzed using Zeiss LSM 510 laser-scanning confocal microscope (Goettingen, Germany).
DNA fragmentation assay
After serum-starvation for 12–24 h, the medium was changed to serum-free medium containing Ox-LDL (20 µg/ml) or vehicle control for 24–48 h. Cells were washed with ice-cold PBS and lysed in a buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.3% SDS and 20 mM EDTA at 4°C for 30 min. After incubation with RNase A (20 µg/ml) and proteinase K (0.4 mg/ml), DNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1), dissolved in 30 µl Tris-EDTA buffer, and then subjected to 3% agarose gel electrophoresis. Fragmented DNA was visualized with the SYBR green I DNA staining system.
Western blot analysis
Cells treated according to abovementioned procedure were lysed in RIPA buffer, followed by high speed centrifugation and protein quantification using a bicinchoninic acid assay. Cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidenedifluoride membranes. After blocking, the membranes were incubated with primary monoclonal antibodies against LC3, Caspase-8 and Caspase-3 (Cell Signaling Technology, Cambridge, MA, USA). β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the loading control. Horseradish peroxidase-conjugated secondary antibodies were applied to detect labeled proteins. Protein bands were developed with SuperSignal Ultra Chemiluminescent Substrate (Pierce, Rockford, IL, USA) on X-ray films (Kodak, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out using SPSS v18 (SPSS, Chicago, IL, USA). Data were reported as means ± standard deviation (SD). Student's t-test or one-way analysis of variance was used to determine the significance of difference between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Increased level of ox-LDL and Lox-1 in OA patients
In order to clarify the possible role of ox-LDL and Lox-1 during the presence of osteoarthritis, we firstly collected SF and cartilage samples from 40 OA patients and 40 normal control subjects (please see Table II about the demographic data of these subjects) and determined the ox-LDL level in SF and Lox-1 expression in cartilage tissues by ELISA and qRT-PCR, respectively. The results showed that significantly increased ox-LDL level [(64.8±18.3) mU/ml vs. (34.5±15.7) mU/ml, P<0.05] (Fig. 1A) and Lox-1 expression (0.49±0.11 vs. 0.33±0.04, P<0.05) could be found in OA patients compared to normal controls (Fig. 1B). Moreover, we also found that correlations could be found between the ox-LDL (r=0.786, P<0.01) (Fig. 1C) or Lox-1 (r=0.805, P<0.01) (Fig. 1D) and the total WOMAC score, which is a common disease severity indexes used in OA. In addition, the association between ox-LDL and LOX-1 was also observed according to the correlation analyses (r=0.635, P<0.05) (Fig. 1E). These results suggested that increased level of ox-LDL and Lox-1 was presented in OA patients and according to the results from correlation analyses, ox-LDL and Lox-1 may be involved in the pathogenesis of OA.
Figure 1.
Increased level of ox-LDL and Lox-1 in patients with osteoarthritis are correlated with the total WOMAC score. A total of 40 cases of osteoarthritis (n=40) and 40 cases of normal controls (n=40) were included. (A) Relative Lox-1 expression and (B) levels of synovial fluid ox-LDL was determined by ELISA and quantitative real time polymerase chain reaction. Moreover, correlation analyses were performed between (C) ox-LDL level or (D) Lox-1 level and the total WOMAC score and (E) between ox-LDL level or Lox-1 level. *P<0.05 compared to normal control. WOMAC, Western Ontario and McMasters University Osteoarthritis Index; ox-LDL, oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL receptor-1.
Facilitation of TNF-α-mediated chondrocyte death by ox-LDL
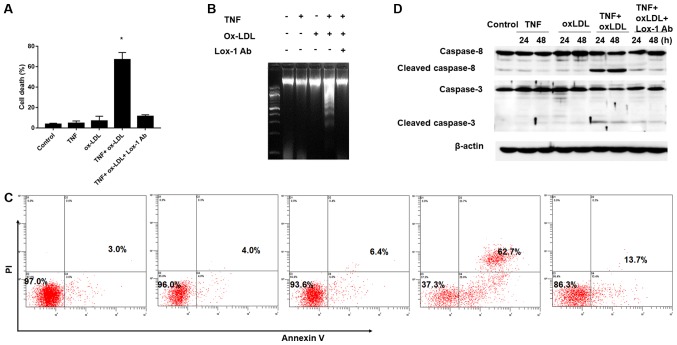
According to the previous description (15), low-grade inflammation with increased expression of proinflammatory cytokines (eg. TNF-α) in articular cartilage and synovium result in chondrocyte death and contribute to disease progression of OA. Since the increased ox-LDL level and Lox-1 expression was found in OA patients and they were considered to be involved in the disease process of OA, we further established an in vitro TNF-α mediate chondrocyte inflammation model to clarify the role of ox-LDL and LOX-1 during OA process. As shown in Fig. 2A, TNF-α and ox-LDL alone did not affect the cell death obviously compared to the control (TNF-α vs. ox-LDL vs. Control: 4.65±2.21% vs. 6.86±4.65% vs.3.60±1.04%, P>0.05), however, combine use TNF-α and ox-LDL could result in significantly increased chondrocyte death and these effects could be reversed by Lox-1 monoclonal antibody (TNF+oxLDL vs. control: 66.90±6.98% vs. 3.60±1.04%; TNF+oxLDL vs. TNF+oxLDL+Lox-1 Ab: 66.90±6.98% vs. 11.40±1.50%, P>0.05). In order to further confirmed the cell phenomenon during the TNF-α and ox-LDL combined treatment, we employed DNA fragmentation assay and flow cytometry assay to verification. Our results demonstrated obvious DNA fragmentation phenotype could be found during the combine use TNF-α and ox-LDL, but not in the condition of TNF-α and ox-LDL alone, and DNA fragmentation could be abolished by Lox-1 antibody (Fig. 2B); moreover, the flow cytometry results of cell apoptosis showed similar trend and the cell apoptosis rate under control, TNF-α, ox-LDL, TNF+oxLDL and TNF+oxLDL+Lox-1 Ab was 3.0, 4.0, 6.4, 62.7 and 13.7%, respectively (Fig. 2C). In addition, we also employed western-blotting to examine the apoptosis initiate caspase (caspase-8) and executioner caspase (caspase-3) and the results showed that increased level of cleaved caspase-8 and caspase-3 were found in the setting of TNF-α and ox-LDL combination, which could be abolished by adding in Lox-1 antibody (Fig. 2D). These results indicated that ox-LDL could facilitate the TNF-α effects on chondrocyte death by increasing cell apoptosis. Autophagy is involved in the cell death process mediated by TNF-α and ox-LDL.
Figure 2.
Facilitation of TNF-α-mediated chondrocyte death by oxidized low density lipoprotein (ox-LDL). After 30 min preincubation with or without anti-LOX-1 antibody (10 μg/ml), Chondrocytes were co-treated with TNF-α (50 ng/ml) and ox-LDL (20 μg/ml), and harvested at 24 or 48 h for (A) cell death, (B) DNA fragmentation assay, (C) Flow cytometry analysis of the chrondrocyte apoptosis by Annexin V/PI staining and (D) western blot analysis of apoptosis related proteins. (A, B and C) Ox-LDL co-treatment could facilitate TNF-α-mediated chondrocyte death and this process could be blocked by Lox-1 monoclonal antibody pretreatment. (D) Western-blot analysis revealed that increased level of cleaved caspase-8 and caspase-3 in TNF-α and ox-LDL co-treated chondrocytes, and that this effect could be blocked by Lox-1 antibody pretreatment.*P<0.05 compared to control. ox-LDL, oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL receptor-1; TNF-α, tumor necrosis factor α; Ab, antibody; PI, propidium iodide.
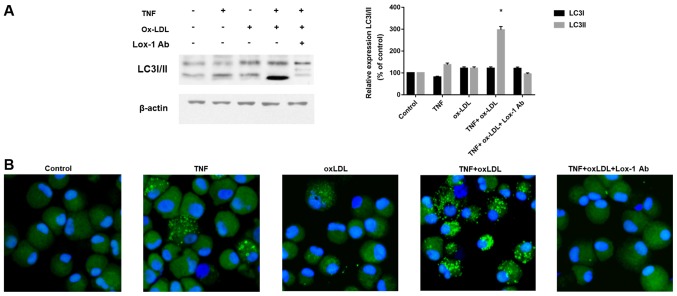
According to previous description (16), both apoptotic as well as non-apoptotic mechanisms were involved in the cell death affected by OA and autophagy related cell death was considered as one of novel mechanism involved in the chondrocyte death. We therefore examined the autophagy classical marker LC3 by western-blotting and immunofluorescence. As shown in Fig. 3A, we found that increased LC3-II, which is an indicator of autophagy activation, was found after TNF-α and ox-LDL combination treatment, and this process could be blocked by Lox-1 antibody neutralization. The immunofluorescence results in Fig. 3B also confirmed the effects of TNF-α and ox-LDL combination on autophagy activation by LC3 green fluorescence and Lox-1 antibody treatment could inhibit this process. These results indicated autophagy is involved in the cell death process mediated by TNF-α and ox-LDL.
Figure 3.
Autophagy is involved in the cell death process mediated by TNF-α and ox-LDL co-treatment. After 30 min preincubation with or without anti-LOX-1 antibody (10 µg/ml), chondrocytes were co-treated with TNF-α (50 ng/ml) and ox-LDL (20 µg/ml) for 24 h, and LC3 level and pattern was analyzed by (A) western blotting and (B) confocal microscopy, respectively. (A) Increased LC3 II was found by western blotting in TNF-α and ox-LDL co-treated chondrocytes and this effect could be blocked by Lox-1 antibody pretreatment. (B) Enhanced LC3 pattern was found in TNF-α and ox-LDL co-treated chondrocytes and this effect could be blocked by Lox-1 antibody pretreatment. *P<0.05 compared to control. ox-LDL, oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL receptor-1; LC3, microtubule-associated proteins 1A/1B light chain 3B.
Autophagy inhibition reverse the cell death process mediated by TNF-α and ox-LDL
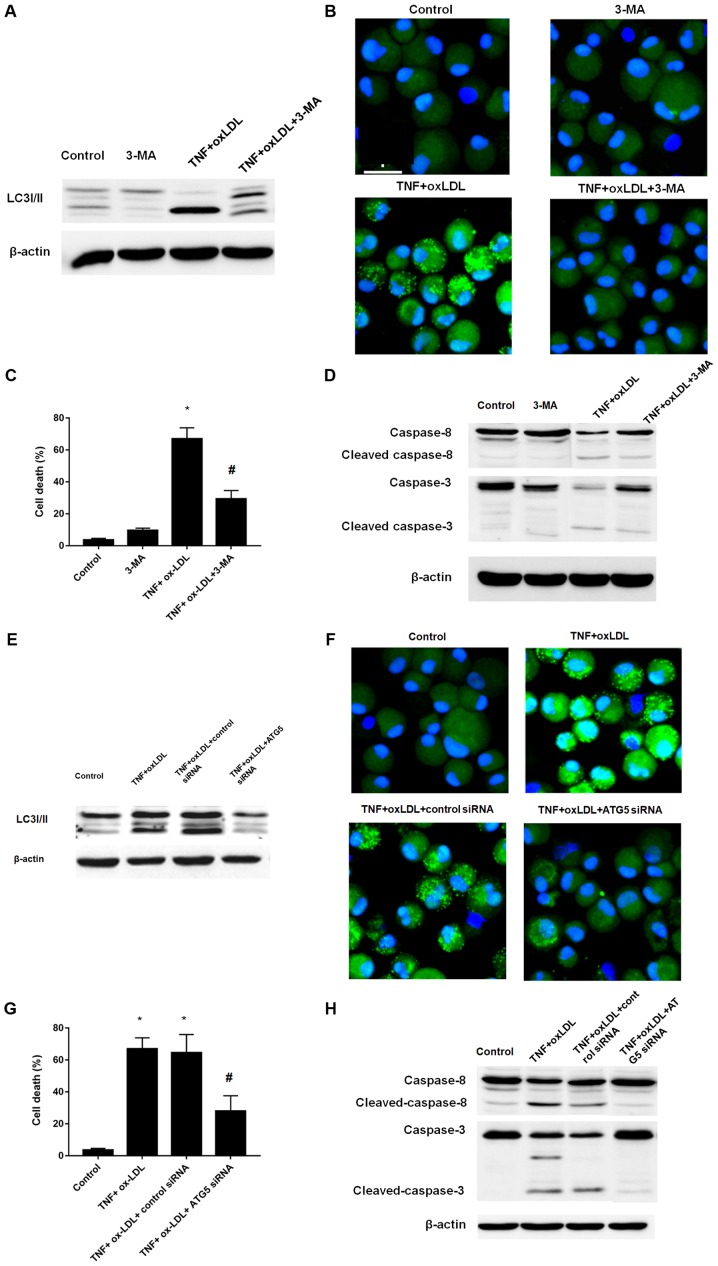
Since the above results suggested the involvement and enhancement of autophagy process in the cell death process mediated by TNF-α and ox-LDL, we here further determined the cell death by induction of autophagy inhibition by classical autophagy inhibitor 3-MA and ATG5 siRNA. According to the results, both 3-MA and ATG5 siRNA treatment could result in the decreased level of LC3II, which was verified by western-blotting (Fig. 4A for 3-MA; Fig. 4E for ATG5 siRNA) and immunofluorescence (Fig. 4B for 3-MA; Fig. 4F for ATG5 siRNA). We further employed the cell viability counting to verify the cell death phenotype, both 3-MA and ATG5 siRNA treatment could result in decreased cell death as shown in Fig. 4C and G, respectively. In addition, the western-blotting analysis of caspase-8 and caspase-3 also verified that both 3-MA and ATG5 siRNA treatment could decreased the level of cleaved caspase-8 and capase-3 (Fig. 4D for 3-MA; Fig. 4H for ATG5 siRNA). Taken together, by using small molecule inhibitor 3-MA and gene manipulating ATG5 knockdown, we confirmed the autophagy inhibition could reverse the cell death process mediated by TNF-α and ox-LDL.
Figure 4.
Autophagy inhibition by 3-MA or Atg-5 siRNA could reverse the effects mediated by TNF-α and ox-LDL co-treatment on chondrocytes. (A) Western blot analysis of the expression of LC3I/II. (B) LC3 pattern analysis by confocal microscopy. (C) Cell death analysis. (D) Western blot analysis of apoptosis related protein cleaved caspase-3 and caspase-8. 3-MA (5 mM) treatment could reverse the LC3II enhancement, punctuate LC3 pattern, increased cell death and cleaved caspase-3 and caspase-8 mediated TNF-α and ox-LDL co-treatment in chondrocytes. (E) Western blot analysis of the expression of LC3I/II. (F) LC3 pattern analysis by confocal microscopy. (G) Cell death analysis. (H) Western blot analysis of apoptosis related protein cleaved caspase-3 and caspase-8. Atg-5 siRNA treatment could reverse the LC3II enhancement, punctuate LC3 pattern, increased cell death and cleaved caspase-3 and caspase-8 mediated TNF-α and ox-LDL co-treatment in chondrocytes. *P<0.05 compared to control, #P<0.05 compared to TNF-α + ox-LDL group. ox-LDL, oxidized low density lipoprotein; Lox-1, lectin-like ox-LDL receptor-1; si, small interfering; TNF-α, tumor necrosis factor α; LC3, microtubule-associated proteins 1A/1B light chain 3B; 3-MA, 3-methyladenine autophagy inhibitor; Atg-5, autophagy protein 5.
Discussion
In present study, we first found that significantly increased ox-LDL level in SF sample and LOX-1 expression level in cartilage tissue was found in OA patients compared to those corresponding samples from control subjects. Based on this phenotype, we further explored the effect of ox-LDL on TNF-α mediated chondrocyte death, and results showed that ox-LDL could facilitate TNF-α mediated chondrocyte death and this effect could be blocked by LOX-1 antibody neutralization. Moreover, we also found that autophagy inhibition by 3-MA and Atg-5 siRNA could reverse the cell death effect mediated by TNF-α and ox-LDL co-treatment on chondrocytes. Therefore, we concluded that ox-LDL facilitates tumor necrosis factor-α mediated chondrocyte death via its interaction with LOX-1 and autophagy is involved as the mechanisms. To the best of our knowledge, this the first report to link ox-LDL, chondrocyte death and autophagy.
According to recent studies, peroxidation of serum LDL was observed during the inflammation and infection process (17). During the osteoarthritis or rheumatoid arthritis, accelerated vascular porosity could be initiated by inflammation, thereby resulting in invasion of various inflammatory cells and permeation of the biological activators, such as oxLDL, into joints. Furthermore, ox-LDL has suggested to play an important role in the pathogenesis of some ageing related disorders (eg. atherosclerosis) (18–20). In addition, both hypercholesterolemia and hypertension was identified as the risk factor for OA (9,10). Taken together, these evidences suggested that ox-LDL may be involved in the OA. Therefore, we first collected the SF to perform the quantitative analysis of ox-LDL and significantly elevated ox-LDL was found in OA patients compared to normal controls.
LOX-1, firstly cloned from cultured bovine aortic endothelial cells, is identified as the receptor of ox-LDL (21). Previous studies have shown that the expression of LOX-1 on non-phagocytes, such as vascular endothelial cells, smooth muscle cells, platelets and cardiomyocytes (22,23), and inducible expression of LOX-1 could be found during the inflammation. Notably, it has been reported that expression of LOX and association of ox-LDL was found in chondrocytes of a rat model of arthritis (24). Moreover, treatment of these arthritis rats with anti-LOX-1 monoclonal antibody could suppress articular cartilage degeneration. In vitro chondrocytes model showed that enhanced LOX-1 expression could be found by treatment with ox-LDL and pro-inflammatory cytokine (eg.interleukin-1β) (25). Here, we examined the expression of LOX-1 in cartilage tissue and our results showed that increased level of LOX-1 in cartilage tissue from OA patients compared to normal controls.
Two mechanisms are proposed to be involved in the TNF-α mediated chondrocyte apoptosis, including direct apoptosis induction and indirect apoptosis priming by Fas ligand presentation (26,27). The proto-oncogenes of Bcl-2/Bax family are involved in the cellular signaling pathway of TNF-α mediated chondrocyte apoptosis, and activation of effector caspases (such as caspase-3 and caspase-8) are proposed as the downstream signaling events (28). We here found that ox-LDL could enhance the TNF-α mediated chondrocyte apoptosis as evidenced by increased level of cleaved caspases. Here, our found that cell death under the treatment of TNF-α is not significant higher than the control (Fig. 2A) and this result was consistent with several previous studies (29–32). However, the further investigate might be needed to prove the exact role of TNF-α on chondrocyte biology. Autophagy is a considered as the catabolic pathways for intracellular macromolecules degradation. At the beginning of autophagy, autophagosome was formed by sequestration of cytoplasmic organelles in a membrane vacuole. Then, fusion of the autophagosomes with lysosomes could result in degradation and recycling of the cellular materials. Recent studies have shown that increased autophagic activity could induce cell death (33,34). According to previous studies, autophagy is an important cell survival mechanism under various forms of stress (35). Autophagy serves not only to regulate the final stages of the chondrocyte lifecycle, but also the rate at which chondrocytes enter the maturation process (36). Autophagy in normal adult articular cartilage is an important mechanism for cellular homeostasis (16). Catabolic and nutritional stresses could also increase autophagy in OA, and during the initial degenerative phase at least, autophagy is increased in OA chondrocytes and cartilage, with increased level of autophagy related molecules, including LC3 and Beclin-1 in OA chondrocytes (37). Here, we used TNF to induce inflammation to mimic the OA chondrocytes. Moreover, ox-LDL was shown to induce apoptosis in multiple cells (such as endothelial cells (38) and macrophages) (39) and we observed increased level of ox-LDL in OA patients, therefore, we add ox-LDL to the OA chondrocyte model and examined its effects. Increased LC3 represents the increased autophagy level, while increased caspase-3 and caspase-8 represents the executioner caspase and initiator caspase, respectively, during the cell apoptosis. Our results was consistent with the conclusion that both apoptotic as well as non-apoptotic mechanisms were involved in the cell death affected by OA, which were found by previous studies (16,40–42). According to previous description (40–42), both apoptotic as well as non-apoptotic mechanisms were involved in the cell death affected by OA and they also concluded that demonstrated that cell death of chondrocytes within OA undergoes changes different from the classical apoptosis and they considered that this type of death is a combination between the classical apoptosis and autophagy. Our results here supported these descriptions. Moreover, although the role of autophagy in cell death has been elucidated in multiple experimental and physiological system, controversial are existed on the positive or negative role of autophagy on cell death due to both cytoprotective and cell death functions are implicated during autophagy process (43–45). The results here suggested that autophagy inhibition could facilitate the chondrocytes survival during the challenge of TNF-α and ox-LDL. Here, we did not examine the effects of TNF-α inhibitors on change of LOX-1 expression level because we are unable to obtain the monoclonal antibodies for TNF-alpha inhibitor (46), but these examinations could be the potential future work.
In conclusion, we demonstrated here that ox-LDL could interaction with LOX-1 on chondrocyte and promote TNF-α mediated chondrocyte death via autophagy related mechanisms.
Acknowledgements
The present study was funded by National Nature Science Foundation of China to Yaozeng Xu (81472077 and 81672238).
References
- 1.Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134–138. doi: 10.1016/j.rehab.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmander LS, Roos EM. Clinical update: Treating osteoarthritis. Lancet. 2007;370:2082–2084. doi: 10.1016/S0140-6736(07)61879-0. [DOI] [PubMed] [Google Scholar]
- 4.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Mani SB, He Y, Hall AM, Xu L, Li Y, Zurakowski D, Jay GD, Warman ML. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J Clin Invest. 2016;126:2893–2902. doi: 10.1172/JCI83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Q, Sun Y, Zhang P, Zheng J. Simultaneously blocking necrosis and apoptosis to protect TMJ chondrocytes from TNF-alpha induced death: A preliminary study. Int J Clin Experiment Med. 2016;9:2202–2210. [Google Scholar]
- 7.Jang KW, Buckwalter JA, Martin JA. Inhibition of cell-matrix adhesions prevents cartilage chondrocyte death following impact injury. J Orthop Res. 2014;32:448–454. doi: 10.1002/jor.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intekhab-Alam NY, White OB, Getting SJ, Petsa A, Knight RA, Chowdrey HS, Townsend PA, Lawrence KM, Locke IC. Urocortin protects chondrocytes from NO-induced apoptosis: A future therapy for osteoarthritis? Cell Death Dis. 2013;4:e717. doi: 10.1038/cddis.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic syndrome, its components, and knee osteoarthritis: The framingham Osteoarthritis study. Arthritis Rheumatol. 2017;69:1194–1203. doi: 10.1002/art.40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haugen IK, Ramachandran VS, Misra D, Neogi T, Niu J, Yang T, Zhang Y, Felson DT. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: Data from the Framingham Heart Study. Ann Rheum Dis. 2015;74:74–81. doi: 10.1136/annrheumdis-2013-203789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain S Monira, Wang Y, Cicuttini FM, Simpson JA, Giles GG, Graves S, Wluka AE. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: A prospective cohort study. Semin Arthritis Rheum. 2014;43:429–436. doi: 10.1016/j.semarthrit.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 13.Roorda LD, Jones CA, Waltz M, Lankhorst GJ, Bouter LM, Van der Eijken JW, Willems WJ, Heyligers IC, Voaklander DC, Kelly KD, Suarez-Almazor ME. Satisfactory cross cultural equivalence of the Dutch WOMAC in patients with hip osteoarthritis waiting for arthroplasty. Ann Rheum Dis. 2004;63:36–42. doi: 10.1136/ard.2002.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted role of plasminogen activator inhibitor-1 in regulating early remodeling of vein bypass grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–1787. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 2010;15:631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 17.Balci B. The modification of serum lipids after acute coronary syndrome and importance in clinical practice. Curr Cardiol Rev. 2011;7:272–276. doi: 10.2174/157340311799960690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: Players and layers. Circ Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrins CJ, Bobryshev YV. Current advances in understanding of immunopathology of atherosclerosis. Virchows Archiv. 2011;458:117–123. doi: 10.1007/s00428-010-1006-5. [DOI] [PubMed] [Google Scholar]
- 20.Twardowski L, Cheng F, Michaelsen J, Winter S, Hofmann U, Schaeffeler E, Müller S, Sonnenberg M, Steuer K, Ott G, et al. Enzymatically modified low-density lipoprotein is present in all stages of aortic valve sclerosis: Implications for pathogenesis of the disease. J Am Heart Assoc. 2015;4:e002156. doi: 10.1161/JAHA.115.002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: Biological functions and pharmacological modifiers. Cell Mol Life Sci. 2013;70:2859–2872. doi: 10.1007/s00018-012-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misaka T, Suzuki S, Sakamoto N, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Saitoh S, Sawamura T, Ishibashi T, Takeishi Y. Significance of soluble lectin-like oxidized LDL receptor-1 levels in systemic and coronary circulation in acute coronary syndrome. Biomed Res Int. 2014;2014:649185. doi: 10.1155/2014/649185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa T, Akagi M, Hoshikawa H, Chen M, Yasuda T, Mukai S, Ohsawa K, Masaki T, Nakamura T, Sawamura T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates leukocyte infiltration and articular cartilage destruction in rat zymosan-induced arthritis. Arthritis Rheum. 2002;46:2486–2494. doi: 10.1002/art.10504. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura S, Akagi M, Yoshida K, Hayakawa S, Sawamura T, Munakata H, Hamanishi C. Oxidized low-density lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1 (LOX-1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF-kappaB. Osteoarthritis Cartilage. 2004;12:568–576. doi: 10.1016/j.joca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Yumoto K, Nifuji A, Rittling SR, Tsuchiya Y, Kon S, Uede T, Denhardt DT, Hemmi H, Notomi T, Hayata T, et al. Osteopontin deficiency suppresses tumor necrosis factor-α-induced apoptosis in chondrocytes. Cartilage. 2012;3:79–85. doi: 10.1177/1947603511421502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Xu M, Xo R, Mates A, Wilson GL, AW IV Pearsall, Grishko V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis Cartilage. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caramés B, López-Armada MJ, Cillero-Pastor B, Lires-Dean M, Vaamonde C, Galdo F, Blanco FJ. Differential effects of tumor necrosis factor-alpha and interleukin-1beta on cell death in human articular chondrocytes. Osteoarthritis Cartilage. 2008;16:715–722. doi: 10.1016/j.joca.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 30.López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Lee SW, Song YS, Lee SY, Yoon YG, Lee SH, Park BS, Yun I, Choi H, Kim K, Chung WT, Yoo YH. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163. doi: 10.1371/journal.pone.0019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang LB, Meng DH, Lee SM, Liu SH, Xu QT, Wang Y, Zhang J. Dihydroartemisinin inhibits catabolism in rat chondrocytes by activating autophagy via inhibition of the NF-κB pathway. Sci Rep. 2016;6:38979. doi: 10.1038/srep38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, Bonewald L, Jiang JX, Lane NE. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–3376. doi: 10.1096/fj.11-182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: The role of autophagy in skeletal biology. Autophagy. 2014;10:7–19. doi: 10.4161/auto.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M, Kuroda R. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 38.Hong D, Bai YP, Gao HC, Wang X, Li LF, Zhang GG, Hu CP. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis. 2014;235:310–317. doi: 10.1016/j.atherosclerosis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Zheng H, Cui D, Quan X, Yang W, Li Y, Zhang L, Liu E. Lp-PLA2 silencing protects against ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR signaling pathway in human THP1 macrophages. Biochem Biophys Res Commun. 2016;477:1017–1023. doi: 10.1016/j.bbrc.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Del Carlo M, Jr, Loeser RF. Cell death in osteoarthritis. Curr Rheumatol Rep. 2008;10:37–42. doi: 10.1007/s11926-008-0007-8. [DOI] [PubMed] [Google Scholar]
- 41.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: Friend by day, foe by night. Curr Opin Genet Dev. 2011;21:113–119. doi: 10.1016/j.gde.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, et al. Autophagy: Cancer's friend or foe? Adv Cancer Res. 2013;118:61–95. doi: 10.1016/B978-0-12-407173-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marx J. Autophagy: Is it cancer's friend or foe? Science. 2006;312:1160–1161. doi: 10.1126/science.312.5777.1160. [DOI] [PubMed] [Google Scholar]
- 46.Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors-state of knowledge. Arch Med Sci. 2014;10:1175–1185. doi: 10.5114/aoms.2014.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]