Abstract
Immonoglobulin G4-related ophthalmic disease (IgG4-ROD) is a IgG4-RD and exhibits two main characteristics: Fibrosis that is not necessarily marked histopathologically; and frequent formation of germinal centers (GCs). Follicular B helper T (Tfh) cells are now recognized as the true helper cells for B cells in antibody responses. In the present study, the profile and distribution of Tfh cells in involved tissues from patients with IgG4-ROD was compared to those of type 1 autoimmune pancreatitis (AIP) and patients with IgG4-related lymphadenopathy (IgG4-RL). A total of 7 patients with IgG4-ROD, 7 patients with type 1 AIP or IgG4-RL and 7 IgG4-negative controls were evaluated. The expression of Tfh-cell immunological proteins, the inducible T-cell costimulator, B-cell lymphoma 6 protein, C-X-C chemokine receptor type 5 (CXCR5) and interleukin-21 (IL-21) in affected tissues was analyzed using immunohistochemical staining and dual immunofluorescence. It was demonstrated that patients with IgG4-RD exhibited a significantly increased number of CD4+CXCR5+ Tfh cells compared with the IgG4-negative controls. Furthermore, CD4+CXCR5+ Tfh cells were detected in and outside of GCs in patients with IgG4-ROD and IgG4-RLF, whereas CD4+CXCR5+ Tfh cells were randomly distributed in areas demonstrating type 1 AIP. Fewer CD4+CXCR5+ Tfh cells were observed in patients with type 1 AIP compared with patients with IgG4-ROD and IgG4-RL. In addition, increased expression of IL-21 was observed in patients with IgG4-ROD and IgG4-RL compared with type 1 AIP. IL-21 expression was positively correlated with the IgG4/IgG ratio in immunohistochemically-positive cells. The results of the present study indicate that Tfh cells are involved in the histopathological pathogenesis of IgG4-RD and may serve a different role in IgG4-ROD and type 1 AIP. Tfh cells may serve a direct role in the IL-21-mediated pathogenesis of IgG4-ROD.
Keywords: immunoglobulin G4-related ophthalmic disease, type 1 autoimmune pancreatitis, immunoglobulin G4-related lymphadenopathy, follicular B helper T cells
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a chronic disorder of an unknown etiology with multiple organ involvement. It was first observed in the pancreas and is now known as type 1 autoimmune pancreatitis (AIP)/IgG4-related pancreatitis (1–3). This condition has been observed to affect virtually every organ: Orbital contents; bile ducts; salivary glands; retroperitoneum; pachymeninges; kidneys; lungs; lymph nodes; aorta; arteries; breast; prostate; thyroid; pericardium; and skin (3,4). Clinicopathological features demonstrate striking similarities among the involved organs, including a tendency to form tumefactive lesions, a dense lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells, storiform fibrosis, frequent elevations of serum IgG4 levels and good response to glucocorticoids (5,6). However, as a member of a broad spectrum of IgG4-RD diseases, IgG4-R ophthalmic (O)D (affects lacrimal glands, extraocular muscles, orbital nerve and eyelid) differs from the other members of the IgG4-RD group by two aspects: Fibrosis is not necessarily marked histopathologically, and germinal centers (GCs) are frequently observed (3,7). GCs are distinct structures within the B cell follicles in the secondary lymphoid tissues and are major sites of somatic hypermutation, class switching-associated recombination and affinity maturation of activated B cells, and the production of plasma cells and memory B cells (8,9). GCs depend on cluster of differentiation 4-positive (CD4+) T cells and it is now recognized that a subset of CD4+ T cells, termed follicular B helper T (Tfh) cells, are the true helper cells for B cells in antibody (Ab) responses (10,11).
Tfh cells were first described in the year 2000 when several groups reported that a significant proportion of C-X-C chemokine receptor type 5 (CXCR5)+CD4+ T cells involved in B cell help for Ab responses in tonsils was situated in GCs or follicles. These cells were subsequently termed Tfh cells (12,13). Following 17 years of research, there is now an improved understanding of the cellular and molecular mechanisms of helper functions of Tfh cells. The CXCR5 serves as a surface marker for Tfh cells, CD4+ T cells expressing CXCR5 can migrate in response to the follicular chemokine B lymphocyte chemoattractant (also known as CXCL13), and relocate to the border of T and B cell zone (T-B junction) and to the follicles in secondary lymphoid organs (14,15). B cell lymphoma 6 (Bcl-6) was strongly expressed in Tfh cells but not in T helper (Th)1 or 2 cells (16). Bcl-6 has been identified as a master regulator of Tfh cell differentiation in 2009 (17–19), in the absence of Bcl-6, Tfh cell differentiation ceases in vivo (17–19) while other CD4+ Th cell subsets are relatively unaffected (18,19). Tfh cells also express high levels of inducible T-cell costimulator (ICOS) and interleukin (IL)-21 (13,16,20). IL-21 is a Tfh cell-expressed helper cytokine (10) and the upregulation of ICOS is essential for the initiation and maintenance of Tfh differentiation (21,22).
The pathogenesis of IgG4-RD remains to be elucidated. Researchers in the field of immunology identified certain abnormal immunological mechanisms involved in the pathogenesis of IgG4-RD. The results suggested that Th2 cells and regulatory T cells (Tregs) as well as associated cytokines, including IL-4, IL-10, IL-13 and transforming growth factor β1, may serve key roles in the development of Mikulicz disease, IgG4-related pancreatitis and cholangitis (23,24). More recently, studies on the role of Th2 cytokines in IgG4-RD indicated that IL-18 and interferon-γ are implicated in the pathogenesis of IgG4-related dacryoadenitis and sialoadenitis (25,26). Currently, increasing attention is paid to Tfh cells; a higher frequency of GCs in patients with IgG4-ROD indicates an increased number of activated B cells, which may reflect the increase in Tfh cell number.
An association between Tfh cells and autoimmunity has been suggested in many autoimmune diseases, including systemic lupus erythematosus (27,28), autoimmune thyroid disease (29) and myasthenia gravis (30,31). Since the major function of Tfh cells is to aid B cell activation and to stimulate Ab responses, patients with IgG4-RD display elevated IgG4 concentrations in sera and infiltrated IgG4-positive cells in associated tissues, suggesting the involvement of Tfh cells in the pathogenesis. In the studies performed with peripheral blood samples, Tfh cells were related to the pathogenic process of IgG4-RD (32,33). The profile and distribution of Tfh cells in involved tissue, which demonstrates the association between Tfh cells and IgG4-RD directly, has not been examined among patients with IgG4-RD and IgG4-ROD patients demonstrating GC formation.
In the following study, the expression of ICOS and Bcl-6 was increased in tissues of patients with IgG4-RD compared with IgG4-negative controls. It was also demonstrated that CD4+CXCR5+ Tfh cells were more abundant in patients with IgG4-RD compared with the IgG4-negative controls. Among patients with IgG4-RD, CD4+CXCR5+ Tfh cells were present in and outside GCs in patients with IgG4-ROD and IgG4-related lymphadenopathy (IgG4-RL) while staining for CD4 and CXCR5 in pancreatic lesions appeared as a randomly distributed pattern. Fewer CD4+CXCR5+ Tfh cells were observed in patients with type 1 AIP compared with patients with IgG4-ROD and IgG4-RL. The expression of IL-21 was coincidental with dual immunofluorescence of CD4 and CXCR5, and increased expression of IL-21 was observed in patients with IgG4-ROD and IgG4-RL. IL-21 expression in patients with Ig-G4-ROD was positively correlated with the IgG4/IgG ratio in immunohistochemically (IHC)-positive cells.
Materials and methods
Patients and samples
A total of 7 patients with IgG4-ROD (4 men and 3 women; mean age ± standard deviation age 60.9±7.5 years) referred to the Department of Ophthalmology at the Shanghai Changzheng Hospital (Shanghai, China) between January 2013 and June 2016 were included in the present study. A total of 7 patients with type 1 AIP or IgG4-RL (6 men and 1 women; mean age ± standard deviation age 56.0±7.0 Years) who were referred to Renji Hospital (Shanghai, China) between July 2012 and June 2016 were studied as disease positive controls. Cases with normal serum IgG4 and no IgG4-positive cells detected in tissue specimens (n=7) were designated as IgG4-negative controls. IgG4-RD was diagnosed using the following criteria (5,34): i) Clinical examination reveals diffuse/localized swelling or masses in single or multiple organs; ii) tissue biopsy meets at least two out of three major histopathologic features of the disease: A lymphoplasmacytic infiltrate, fibrosis (typically storiform); and obliterative phlebitis; and iii) infiltration of IgG4-positive plasma cells: Ratio of IgG4-positive cells to IgG-postive cells of >40% and >10 IgG4-positive cells/high power field (magnification, ×400).
The lesion specimens were collected from all patients with IgG4-RD from whom written informed consent was obtained, and tissues were used only for research purposes. The study was approved by the Ethics Committee of Renji Hospital.
IHC staining
The primary antibodies used for the IHC were as follows: Anti IgG4 (cat. no. ab109493; Abcam, Shanghai, China; at 1/500 dilution); IgG (cat. no. ab109489; Abcam, Shanghai, China; at 1/300 dilution); Bcl-6 (cat. no. ab41845; Abcam, Shanghai, China; at 1/50 dilution); ICOS (cat. no. ab105227; Abcam, Shanghai, China; at 1/50 dilution); IL-21 (cat. no. 118510; and Abcam, Shanghai, China; at 1/200 dilution).
IHC analyses
Paraffin sections (4 µm-thick) were deparaffinized by immersion in xylene, followed by a hydration in ethanol. An antigen retrieval was performed with EDTA pH 8.0 microwave repair method. Endogenous peroxide activity was quenched by 3% hydrogen peroxide in water. Then the sections were incubated for 30 min at room temperature with 3% bovine serum albumin (BSA) (cat. no. A8020; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) and incubated overnight at 4°C with primary antibody. Thereafter, the sections were incubated with a horseradish peroxidase-conjugated secondary antibody (cat. no. K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA; at 1/1 dilution) for 50 min at room temperature. The stain was prepared using diaminobenzidine substrate (cat. no. K5007; Dako; Agilent Technologies, Inc.). Sections were counterstained 3 min at room temperature with Harris hematoxylin and mounted prior to analysis. Between each step of the analysis, sections were rinsed with PBS three times, 5 min each. IHC images were captured using a light microscope equipped with a digital camera (Olympus Corporation, Tokyo, Japan). Stained IgG4- and IgG-positive cells were counted in three high-power fields with the highest density of positive cells and an average was calculated (5). Semiquantification of IL-21 was performed as previously described with a slight modification in the grade classification of staining intensity and percentage of positive cells (35). Depending on the proportion of positive stained cells (PP): <5% was scored 0, 6–25% was scored 1, 26–50% was scored 2, 51–75% was scored 3 and >75% was scored 4. According to the staining intensity (SI): No color was scored 0, light yellow was scored 1, light brown was scored 2, brown was scored 3. PP was multiplied by SI to obtain a total IHC score for any given sample.
Double-labeled immunofluorescence for CD4, CXCR5 and IL-21
Sections were de-waxed by xylene, then hydrated through a graded ethanol series, and washed with distilled water. Antigen retrieval and serum block were performed using a similar method to the IHC staining. The primary antibodies used for the analysis of co-expression of CD4 and CXCR5 were anti-CD4 (cat. no. GB13064; Goodbio technology, Wuhan, Hubei, China; at 1/50 dilution), anti-CXCR5 (cat. no. GTX100351; GeneTex, Inc., Irvine, CA, USA; at 1/100 dilution). The anti-IL-21 goat polyclonal antibody was used to analyze the co-expression of IL-21 and CXCR5 (cat. no. GTX82914; GeneTex, Inc.; at 1/200 dilution while anti-CXCR5 at 1/200 dilution). Cy3-conjugated goat anti-mouse IgG (cat no. GB21301; Goodbio technology; at 1/300 dilution), Alexa 488-conjugated goat anti-rabbit IgG (cat no. GB25303; Goodbio technology; at 1/400 dilution), Cy3-conjugated donkey anti-goat IgG (cat no. GB21404; Goodbio technology; at 1/300 dilution) and Alexa 488-conjugated donkey anti-rabbit IgG (cat no. GB2540311; Goodbio technology; at 1/400 dilution) were used as fluorochrome-conjugated second antibodies. Then the sections were sequentially incubated with primary antibodies overnight at 4°C, corresponding second antibodies for 50 min at room temperature and DAPI for 10 min at room temperature. Between each step, sections were rinsed with PBS three times, 5 min each. Fluorescence images were captured using a fluorescence microscope (Nikon Corporation).
Statistical analysis
Differences in the IHC staining scores between more than two groups were determined using the Kruskal-Wallis test and differences between two groups were determined using the Mann-Whitney U test (two-tailed). Spearman's rank correlation coefficient was used to assess the correlation between IL-21 expression and the ratio of IgG4- to IgG-positive cells in IgG4-ROD samples. All statistical analyses were performed using SPSS software (version 20; IBM Corp., Armonk, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient profiles
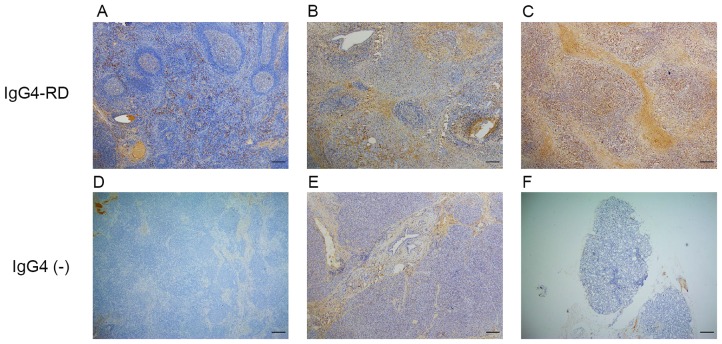
The clinical and serological characteristics of patients with IgG4-ROD are presented in Table I. Patients with IgG4-ROD were predominantly middle-aged to elderly males. IgG4-ROD affected the lacrimal gland in 6 patients the orbital soft tissue in 2 patients, including the orbital apex. Computed tomography or magnetic resonance imaging identified space-occupying lesions of lacrimal gland or orbital cavity. Serum IgG and IgG4 were elevated in all patients with a median level of 22.6 g/l (P25-P75: 16.2–31.3 g/l) and 17.5 g/l (P25-P75: 8.5–38.5 g/l), respectively. With the exception of 1 patient, the serum IgE value for all patients was elevated above the normal limit (<165 IU/ml). Case 2 exhibited paranasal sinusitis and case 5 exhibited bronchial asthma. Clinical and serological characteristics of patients with type 1 AIP or IgG4-RL and IgG4-negative controls are summarized in Table II. Immunohistochemistry results demonstrated that IgG4-positive cells mainly infiltrated the interfollicular areas in lymph nodes (Fig. 1A), areas around the acinar and ductal cells in pancreas (Fig. 1B) and diffusely in lacrimal glands (Fig. 1C).
Table I.
Clinical and serological characteristics of patients with immunoglobulin G4-related ophthalmic disease.
| Patient no. | Sex | Age at onset, years | Onset time | Onset signs and symptoms | Exophthalmos (mm) Right eye (orbital distance) left eye) | Side | IgG (g/l) | IgG4 (g/l) | IgE IU/ml |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | 1 year | Diplopia, proptosis, swelling | 15 (105) 14 | Bil | 33.7 | 38.5 | 368 |
| 2 | M | 57 | 8 years | Proptosis, swelling | 24.5 (113) 31 | Bil | 31.3 | 43.2 | 415 |
| 3 | F | 68 | 3 months | Proptosis | 20 (103) 24 | Bil | 9.77 | 3.26 | 415 |
| 4 | F | 59 | 6 months | Swelling | 13 (110) 13 | Bil | 22.6 | 20.2 | 1140 |
| 5 | M | 59 | 5 years | Swelling | 14 (110) 20 | Bil | 18.9 | 12.3 | 5 |
| 6 | F | 52 | 5 months | Swelling, ptosis | 14 (107)15 | Left | 16.2 | 8.48 | 495 |
| 7 | M | 57 | 7 years | Proptosis, swelling | 26 (99) 28 | Bil | 25.8 | 17.5 | 319 |
M, male; F, female; Bil, bilateral; IgG, immunoglobin G.
Table II.
Clinical and serological characteristics of patients with type 1 AIP or IgG4-RL and IgG4-negative controls.
| A, Patients with type 1 AIP or IgG4-RL | ||||||
|---|---|---|---|---|---|---|
| Patient no. | Sex | Age at onset, years | Affected organ | IgG4 (g/l) | Onset time | Onset signs and symptoms |
| 8 | M | 57 | Pancreas, lymph node | N | 0.5 months | Asyptomatic, occupying |
| 9 | M | 67 | Pancreas | N | 10 days | Abdominal pain, swelling |
| 10 | M | 62 | Pancreas | N | 2 months | Abdominal pain, swelling |
| 11 | M | 49 | Pancreas, lymph node | 10.1 | 2 weeks | Asyptomatic, occupying |
| 12 | M | 54 | Lymph node | 28.1 | 1 years | Swelling |
| 13 | M | 56 | Lymph node | N | 0.5 months | Swelling |
| 14 | F | 47 | Lymph node | N | 4 years | Swelling |
| B, IgG4-negative controls | ||||||
| Patient no. | Sex | Age at onset, years | Affected organ | IgG4 (g/l) | Onset time | Onset signs and symptoms |
| 15 | F | 25 | Labial gland | 0.85 | 1.5 years | Dry mouth, dry skin |
| 16 | F | 52 | Orbital tissue | 0.35 | 10 years | Swelling |
| 17 | F | 44 | Peripancreatic lymph node | 0.90 | 1 years | Abdominal pain, swelling |
| 18 | M | 59 | Submandibular gland | 1.27 | 1 month | Swelling |
| 19 | M | 43 | Pancreas | 0.71 | 1 year | Abdominal pain |
| 20 | F | 55 | Orbital tissue | 1.25 | 7 months | Orbital pain |
| 21 | F | 63 | Bile duct | 0.74 | 2 months | Yellowing and pruritus of the skin |
M, male; F, female; N, not measured; type 1 AIP, autoimmune pancreatitis; IgG4-RL, immunoglobulin G4-related lymphadenopathy.
Figure 1.
Expression of IgG4 in IgG4-RD and IgG4-negative controls. (A) A large number of IgG4-positive cells infiltrated the lymph node of an IgG4-RL sample. (B) Pancreas sample from type 1 autoimmune pancreatitis. (C) Lacrimal gland sample from an IgG4-ROD patient. (D) Lymph node sample from the IgG4-negative control exhibiting no IgG4-positive cells. (E) Pancreatic specimen from the IgG4-negative control exhibiting no IgG4-positive cells. (F) Orbital tissue from the from the IgG4-negative control exhibiting no IgG4-positive cells. Scale bar, 200 µm; magnification, ×40. Ig, immunoglobulin; RD, related disease; RL, related lymphadenopathy; ROD, related ophthalmic disease.
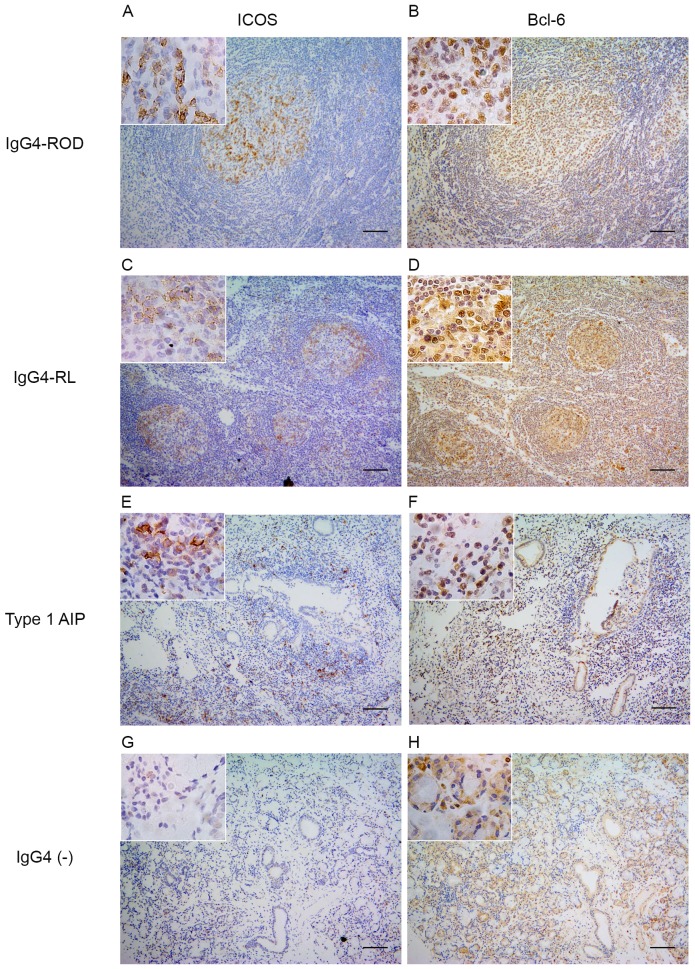
Expression of Bcl-6 and ICOS
The expression of Bcl-6, a master regulator transcription factor in Tfh cells and ICOS, a surface marker of Tfh cells, was examined (Fig. 2). In patients with IgG4-ROD, the nuclear expression of Bcl-6 and expression of ICOS were predominantly detected in GCs, and to a lesser extent in infiltrating lymphocytes outside the GCs (Fig. 2A and B). A small proportion of Bcl-6- and ICOS-positive cells were randomly distributed within the pancreatic lesions (Fig. 2E and F). In lymph nodes from patients with IgG4-RL positive staining for Bcl-6 and ICOS was only observed in GCs (Fig. 2C and D). By contrast, the nuclear expression of Bcl-6 was detected in relatively few lymphocytes in all IgG4-negative controls and ICOS-positive cells were rarely observed (Fig. 2G and H).
Figure 2.
Expression of Bcl-6 and ICOS. (A) ICOS-positive cells in the lacrimal gland of the patients with IgG4-ROD. (B) Bcl-6-positive cells in the lacrimal gland of the patients with IgG4-ROD. (C) ICOS-positive cells in the lymph node of the patients with IgG4-RL. (D) Bcl-6-positive cells in the lymph node of the IgG4-RL patients; (E) ICOS-positive cells in the pancreas of the patients with type 1 AIP. (F) Bcl-6-positive cells in the pancreas of the patients with type 1 AIP. (G) ICOS-positive cells in the labial gland lesions of IgG4-negative controls. (H) Nuclear expression of Bcl-6 with few mononuclear cells infiltrating the lesions in the labial gland of IgG4-negative controls. ICOS and Bcl-6 images were captured from almost the exact same fields. An enlarged part (inset) of each photograph is presented. Scale bars, 100 µm; magnification, ×100. ICOS, inducible T-cell costimulatory; Bcl-6, B cell lymphoma 6; Ig, immunoglobulin; ROD, related ophthalmic disease; type 1 AIP, type 1 autoimmune pancreatitis; ROD, related ophthalmic disease; RL, related lymphadenopathy.
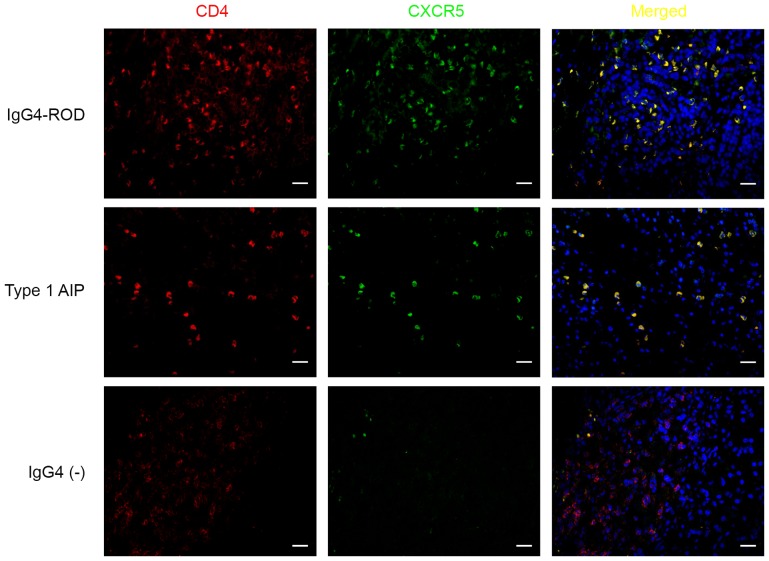
Increased number of CD4+CXCR5+ cells in involved tissues of patients with IgG4-RD
The above results indicated that expression of Bcl-6 and ICOS, which are the markers for Tfh cells, were increased in patients with IgG4-RD compared with patients with IgG4-negative. CD4+CXCR5+ cells were examined using dual immunofluorescence staining for CD4 and CXCR5 using paraffin-embedded specimens from patients with IgG4-RD and IgG4-negative patients (Figs. 3 and 4). In the IgG4-RD group, CD4+CXCR5+ cells were randomly distributed in areas of type 1 AIP (Figs. 3 and 4E) and were predominantly detected in GCs and T-B junctions in lymph nodes from IgG4-RL (Fig. 4C). CD4+CXCR5+ cells were scattered and distributed in GCs within the orbital lesions in patients with IgG4-ROD (Figs. 3 and 4A). In IgG4-negative controls, numerous CD4-positive cells were observed; however, few CD4+CXCR5+ cells could be identified (Fig. 3). The number of CD4+CXCR5+ cells among involved tissues from patients with IgG4-RD demonstrated significantly decreased CD4+CXCR5+ cells in patients with type 1 AIP compared with patients with IgG4-ROD and IgG4-RL (P=0.024 and 0.032, respectively), there was no significant difference between IgG4-ROD and IgG4-RL (Fig. 5).
Figure 3.
CD4+CXCR5+ cells in involved tissues of patients with IgG4-RD. Sections were stained for CD4 (red), CXCR5 (green), cells in yellow indicate the coexpression of CD4 and CXCR5. Upper row, lacrimal gland; middle row, pancreas; lower row, pancreas. Scale bars, 20 µm; magnification, ×400. IgG4-RD, immunoglobulin G4-related disease; CD4, cluster of differentiation 4; CXCR5, C-X-C chemokine receptor type 5.
Figure 4.
Distribution of CD4+CXCR5+ cells among patients with IgG4-RD. (A) Merged image of IgG4-RD lacrimal gland stained for CD4 and CXCR5. (B) IgG4-RD lacrimal gland with DAPI counterstain. (C) Merged image of IgG4-RD lymph node stained for CD4 and CXCR5. (D) IgG4-RD lymph node with DAPI counterstain. (E) Merged image of IgG4-RD pancreas stained for CD4 and CXCR5. (F) IgG4-RD pancreas with DAPI counterstain. Scale bars, 50 µm; magnification, ×200. IgG4-RD, immunoglobulin G4-related disease; CD4, cluster of differentiation 4; CXCR5, C-X-C chemokine receptor type 5.
Figure 5.

Frequency of CD4+CXCR5+ Tfh cells among patients with IgG4-RD. *P<0.05. IgG4-RD, immunoglobulin G4-related disease; IgG4-ROD, immunoglobulin G4-related ophthalmic disease; type 1 AIP, type 1 autoimmune pancreatitis; IgG4-RL, immunoglobulin G4-related lymphadenopathy.
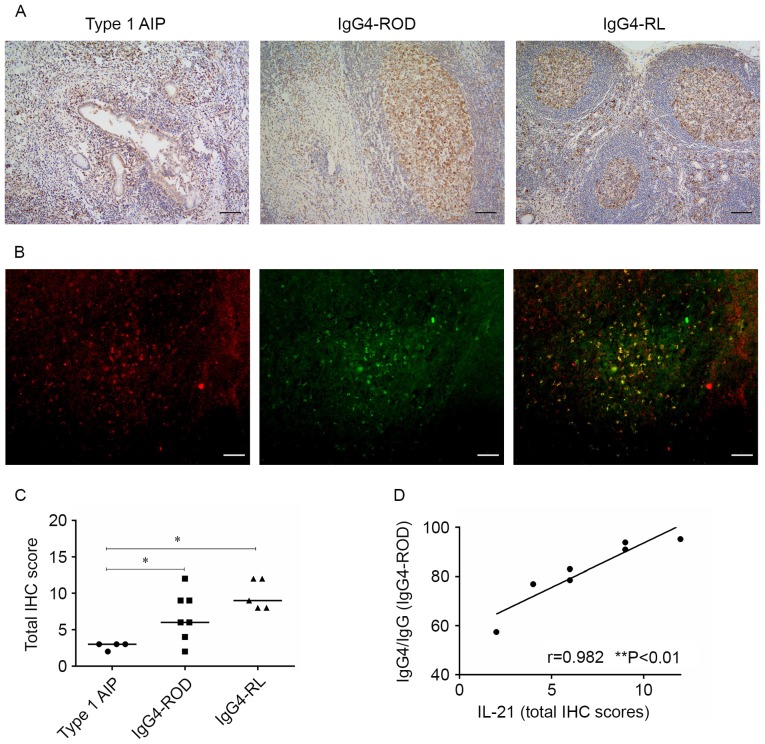
Expression of IL-21 in involved tissues of patients with IgG4-RD
IL-21 is the main effector cytokine produced by Tfh cells (10) and the present study, using immunohistochemical analyses, determined whether IL-21 is expressed in samples from patients with IgG4-RD. All 16 IgG4-RD samples expressed IL-21 (Fig. 6A). IL-21 was prominent in GCs and less evident in infiltrating lymphocytes outside the GCs in orbital lesions and lymph nodes. Staining for IL-21 in pancreatic lesions appeared as a scattered pattern, primarily in infiltrating lymphocytes around the acinar and slightly detected in ductal cells. Fluorescence microscopy revealed that the IL-21 expression in involved tissues from patients with IgG4-RD coincided with their CXCR5 expression (Fig. 6B). While the scores from the IHC staining exhibit decreased expression of patients with IL-21 in type 1 AIP compared with patients with IgG4-ROD and IgG4-RL (P=0.042 and 0.016, respectively), the scores were comparable between IgG4-ROD and IgG4-RL patients (Fig. 6C).
Figure 6.
Expression of IL-21 in involved tissues of patients with IgG4-RD. (A) Representative micrographs stained for IL-21 of pancreas (left), orbital soft tissue (middle), and lymph node (right) with IgG4-RD (scale bar, 100 µm; magnification, ×100). (B) Representative micrographs were from the orbital soft tissue in IgG4-ROD patients. Immunofluorescent double staining of IL-21-positive cells (red) and CXCR5+ cells (green), cells in yellow indicate the coexpression of IL-21 and CXCR5 (scale bars, 50 µm; magnification, ×200). (C) Total IHC scores for IL-21 for patients with IgG4-RD. *P<0.05. (D) Correlation between IgG4 production and IL-21 IHC scores in orbital specimens from IgG4-ROD as determined by Spearman's rank test. IgG4-RD, immunoglobulin G4-related disease; IgG4-ROD, immunoglobulin G4-related ophthalmic disease; type 1 AIP, type 1 autoimmune pancreatitis; IgG4-RL, immunoglobulin G4-related lymphadenopathy; CXCR5, C-X-C chemokine receptor type 5; IL-21, interleukin 2; IHC, immunohistochemistry.
Association between IgG4 production and expression of IL-21 in patients with IgG4-ROD
The association between IgG4 production and the levels of IL-21 IHC scores in orbital lesions was examined. Spearman's rank correlation analysis demonstrated that the IL-21 IHC scores in orbital lesions were positively correlated with the ratio of IgG4-positive cells to IgG-positive cells in patients with IgG4-ROD (Fig. 6D).
Discussion
The present study analyzed the expression of the key Tfh cell immunological proteins ICOS, Bcl-6, CXCR5 and IL-21 in the affected tissues of patients with IgG4-RD, using IHC and dual immunofluorescence. The results demonstrated increased expression of ICOS and Bcl-6 in the involved tissues of patients with IgG4-RD compared with the IgG4-negative control group. The CD4+CXCR5+ Tfh cells were more prevalent in IgG4-RD and scarce in the IgG4-negative control group. Among patients with IgG4-RD, CD4+CXCR5+ Tfh cells were detected inside and ouside GCs in patients with IgG4-ROD and IgG4-RL, whereas staining for CD4 and CXCR5 in pancreatic lesions appeared as a randomly distributed pattern. A decreased number of CD4+CXCR5+ Tfh cells was observed in patients with type 1 AIP compared with IgG4-ROD and IgG4-RL patients. The expression of IL-21 was almost coincidental with the dual immunofluorescence of CD4 and CXCR5 and elevated expression of IL-21 was observed in patients with IgG4-ROD and IgG4-RL. IL-21 expression was positively correlated with the IgG4/IgG ratio in IHC positive cells.
Naive CD4+ T cells may differentiate into one of several lineages of Th cells and exert a variety of immunological functions. These include clearing viruses, helminths and fungi by Th1, Th2 and Th17 cells, respectively, and suppressing immune responses by induced Treg cells (36). Tfh cells are a subset of CD4+ T cells that provide help for B cells during the GC reaction (10). A large GC is frequently formed within involved organs in individuals with IgG4-ROD (7,37) and is presumably the source of IgG4-positive plasma cells. Based on this observation, the present study targeted IgG4-ROD and investigated the Tfh cell abundance in affected tissues.
Tfh cells depend on the expression of the master regulator transcription factor Bcl-6 and distinguishing features of Tfh cells are the expression of ICOS, CXCR5, IL-21, programmed cell death protein 1 and SLAM-associated protein (10). The analysis of the expression of Bcl-6 and ICOS in patients with IgG4-RD and IgG4-negative controls, revealed elevated expression of ICOS and Bcl-6 in the involved tissues of patients with IgG4-RD. Tfh cells require upregulation of Bcl-6 to develop and perform their function in B cell maturation in GCs (38). Bcl-6 binding is associated with the control of Tfh cell migration and repression of alternative cell fates (39). The upregulation of ICOS is essential for the initiation and maintenance of Tfh differentiation, and CD4+CXCR5+ cells were scarce in mice with B cell-specific deletion of ICOS ligand (11,21). Increased expression of both Bcl-6 and ICOS in involved tissues of patients with IgG4-RD indicated the presence of Tfh cells.
Dual immunofluorescence of paraffin-embedded specimens revealed increased numbers of CD4+CXCR5+ Tfh cells in all cases of IgG4-RD irrespective of the organs involved, and an enhanced expression of Bcl-6 and ICOS, which histopathologically indicated that this cell population was involved in the pathogenesis of IgG4-RD. The frequency and distribution of CD4+CXCR5+ Tfh cells differed in patients with IgG4-ROD and type 1 AIP. CD4+CXCR5+ Tfh cells were observed inside and outside GCs in patients with IgG4-ROD, however they were randomly distributed and fewer in areas of type 1 AIP compared with IgG4-ROD. The distribution of CD4+CXCR5+ Tfh cells corresponded with the expression of Bcl-6 and ICOS. CXCR5 is the canonical Tfh marker and in the present study, CD4+CXCR5+ cells which localized in GCs were considered as GC Tfh cells and predecessors of those cells with Tfh-like characteristics (localized in extrafollicles) were termed Pre-Tfh cells (10,11,40). Human autoimmune diseases predominantly affect nonlymphoid tissues, which frequently contain large numbers of infiltrating, activated lymphocytes, as well as lymphoid-like tissues with a GC. Tfh cells have been identified in nonlymphoid tissues, particularly in autoimmune diseases (41). High frequency of GC formation and an increased number of Tfh cells in IgG4-ROD may suggest that IgG4-ROD is an autoimmune disease and may also indicate that Tfh cells serve a different role in IgG4-ROD and type 1 AIP, since GCs are not commonly observed in type 1 AIP (42). Previous studies considered blood CD4+CXCR5+ cells as equivalents of Tfh cells. Akiyama et al (32) demonstrated that the proportion of circulating Tfh cells was significantly increased in patients with IgG4-RD compared with patients with allergic rhinitis or with healthy controls, and these results are consistent with those obtained in the present study (11,15).
Tfh cells are a predominant source of IL-21 (43). In the present study, IL-21 was abundant in GCs in orbital lesions and lymph nodes, whereas staining for IL-21 in pancreatic lesions appeared as a scattered pattern, primarily in infiltrating lymphocytes around the acinar and ductal cells. Expression of IL-21 was weaker in Type 1 AIP patients compared with IgG4-ROD and IgG4-RL patients. The IL-21 expression in involved tissues from patients with IgG4-RD was coincidental with their CXCR5 expression. Concurrent expression of IL-21 and CXCR5, together with the distribution of IL-21-positive cells indicates that the IL-21 in affected tissues of IgG4-ROD is mainly produced by Tfh cells and the high expression of IL-21 in IgG4-ROD is due to the increased number of Tfh cells. IgG4 production was positively correlated with the IL-21 IHC scores in patients with IgG4-ROD. IL-21 can promote CD40L-mediated GC B cell proliferation and drive human B cell differentiation into Ig-secreting cells in vitro (44,45). Additionally, IL-21 controls the maintenance and optimal affinity maturation of the GC reaction by maintaining the Bcl-6 expression in GC B cells in vivo (46,47). IL-21 can also contribute to IgG4 production in Mikulicz's disease (48) and the data obtained in the present study demonstrates that Tfh-derived IL-21 can promote B cell differentiation into IgG4-secreting cells in patients with IgG4-ROD.
Based on the available data (32,33,41), it can be hypothesized that chronic stimulation of orbital tissues by an unknown antigen induces production of CXCL13, which in turn recruits circulating Tfh and B cells. Interaction between Tfh and B cells triggers GC formation and B cell differentiation into IgG4-producing cells through secretion of IL-21 in IgG4-ROD.
Steroids represent an effective short-term treatment of IgG4-RD, their effect typically becomes evident within weeks; however, £40% of patients relapse within the 1st year (49). Rituximab is a promising medication but resistant cases have been reported (50). The aforementioned results of the present study suggest that the use of T cell activation inhibitors for Tfh cells, including abatacept, could represent an effective treatment of IgG4-RD. Yamamoto et al (51) recently reported a rituximab-resistant IgG4-RD patient who showed a good response to treatment with abatacept. Since abatacept may affect Tfh cells in the GCs (51), it may exert greater effect on patients with IgG4-ROD. Understanding of the pathgenetic relevance of IL-17 in rheumatoid arthritis and collagen-induced arthritis led to the development of a semi-specific immunotherapy based on cytokine antagonism for the treatment of these disorders (52,53). Identification of IL-21 as a potentially key cytokine in IgG4-ROD may enable the use of specific IL-21 antagonists in the treatment of IgG4-ROD.
However, the significance of the present study is limited by the number of patients analyzed. As clinicians are becoming increasingly aware of IgG4-RD and the administration of glucocorticoid therapy is more prevalent, invasive medical procedures are less prevalent, decreasing the availability of samples from IgG4-RD patients, especially those with IgG4-ROD. Larger cohort studies performed in the future may further elucidate the underlying mechanism of Tfh cell action in affected tissues.
In summary, in the present study CD4+CXCR5+ Tfh cells were highly prevalent in IgG4-RD, and the frequency and distribution of CD4+CXCR5+ Tfh cells differed between patients with IgG4-ROD and type 1 AIP, which may suggest different roles for Tfh cells in IgG4-ROD and type 1 AIP. IL-21 was highly expressed in patients with IgG4-ROD and Tfh-derived IL-21 can promote B cell to differentiate into IgG4-secreting cells in IgG4-ROD, suggesting that Tfh cells may have a direct role in the pathogenesis of IgG4-ROD. These results make Tfh cells and IL-21 an important focus of potential therapeutic methods to treat IgG4-ROD.
Acknowledgements
The present study was supported by the Science and Technology Commission Foundation of Shanghai (grant no. 13ZR1424800).
References
- 1.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 2.Hamanou H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/S0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): General concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.3109/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 6.Stone JH, Zen Y, Deshpande V. IgG4-Related Disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 7.Goto H, Takahira M, Azumi A, Japanese Study Group for IgG4-Related Ophthalmic Disease Diagnostic criteria for IgG4-related ophthalmic disease. Jpn J Ophthalmol. 2015;59:1–7. doi: 10.1007/s10384-015-0376-2. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 9.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 10.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 11.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 12.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. Cxc chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitfeld D, Ohl L, Kremmer EK, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T Cells express Cxc chemokine receptor 5, Localize to B cell follicles and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 16.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T Follicular helper cells express a distinctive transcriptional profile, reflecting their role as Non-Th1/Th2 effector cells that provide help for B Cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 21.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, Vu Van D, Mages HW, Haftmann C, Riedel R, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med. 2015;212:217–233. doi: 10.1084/jem.20141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zen Y, Fujii M, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, Shinozaki S, Kubo Y, Nakamura S. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254–263. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 25.Komori T, Kondo S, Wakisaka N, Nakanishi Y, Nakanishi-Yagi S, Tsuji A, Endo K, Murono S, Yoshizaki T. IL-18 is highly expressed in inflammatory infiltrates of submandibular glands in patients with immunoglobulin G4-related disease. Hum Pathol. 2015;46:1850–1858. doi: 10.1016/j.humpath.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Maehara T, Mattoo H, Ohta M, Mahajan VS, Moriyama M, Yamauchi M, Drijvers J, Nakamura S, Stone JH, Pillai SS. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis. 2017;76:377–385. doi: 10.1136/annrheumdis-2016-209139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 28.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, Borba EF, Goncalves CR, Costa PR, Kallas EG, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis Rheumatol. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, Tang X, Xu H, Lu L, Wang S. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Zhou Y, Guo J, Li H, Tian F, Gong L, Wang X, Lan M, Li Z, Zhang W. Thymic TFH cells involved in the pathogenesis of myasthenia gravis with thymoma. Exp Neurol. 2014;254:200–205. doi: 10.1016/j.expneurol.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Zhang CJ, Gong Y, Zhu W, Qi Y, Yang CS, Fu Y, Chang G, Li Y, Shi S, Wood K, et al. Augmentation of Circulating Follicular Helper T Cells and Their Impact on Autoreactive B Cells in Myasthenia Gravis. J Immunol. 2016;197:2610–2617. doi: 10.4049/jimmunol.1500725. [DOI] [PubMed] [Google Scholar]
- 32.Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, Kondo H, Kassai Y, Miyazaki T, Morita R, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 2015;67:2476–2481. doi: 10.1002/art.39209. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama M, Yasuoka H, Yamaoka K, Suzuki K, Kaneko Y, Kondo H, Kassai Y, Koga K, Miyazaki T, Morita R, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016;18:167. doi: 10.1186/s13075-016-1064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.3109/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 35.Friedrichs K, Gluba S, Eidtrnann H, Jonat W. Overexpression of p53 and Prognosis in Breast Cancer. Cancer. 1993;72:3641–3647. doi: 10.1002/1097-0142(19931215)72:12<3641::AID-CNCR2820721215>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato Y, Ohshima K, Ichimura K, Sato M, Yamadori I, Tanaka T, Takata K, Morito T, Kondo E, Yoshino T. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int. 2008;58:465–470. doi: 10.1111/j.1440-1827.2008.02257.x. [DOI] [PubMed] [Google Scholar]
- 38.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: Lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 42.Zen Y. The Pathology of IgG4-Related Disease in the Bile Duct and Pancreas. Semin Liver Dis. 2016;36:242–256. doi: 10.1055/s-0036-1584319. [DOI] [PubMed] [Google Scholar]
- 43.Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Good KL, Bryant VL, Tangye SG. Kinetics of Human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 45.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into ig-secreting cells: Predominant Role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 46.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maehara T, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Tanaka A, Shinozaki S, Kubo Y, Nakamura S. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Ann Rheum Dis. 2012;71:2011–2019. doi: 10.1136/annrheumdis-2012-201477. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Valle F, Fernandez-Codina A, Pinal-Fernandez I, Orozco-Galvez O, Vilardell-Tarres M. IgG4-related disease: Evidence from six recent cohorts. Autoimmun Rev. 2017;16:168–172. doi: 10.1016/j.autrev.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto M, Awakawa T, Takahashi H. Is rituximab effective for IgG4-related disease in the long term? Experience of cases treated with rituximab for 4 years. Ann Rheum Dis. 2015;74:e46. doi: 10.1136/annrheumdis-2015-207625. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto M, Takahashi H, Takano K, Shimizu Y, Sakurai N, Suzuki C, Naishiro Y, Yajima H, Awakawa T, Himi T, Nakase H. Efficacy of abatacept for IgG4-related disease over 8 months. Ann Rheum Dis. 2016;75:1576–1578. doi: 10.1136/annrheumdis-2016-209368. [DOI] [PubMed] [Google Scholar]
- 52.Bai F, Tian H, Niu Z, Liu M, Ren G, Yu Y, Sun T, Li S, Li D. Chimeric anti-IL-17 full-length monoclonal antibody is a novel potential candidate for the treatment of rheumatoid arthritis. Int J Mol Med. 2014;33:711–721. doi: 10.3892/ijmm.2013.1611. [DOI] [PubMed] [Google Scholar]
- 53.Yoshiga Y, Goto D, Segawa S, Ohnishi Y, Matsumoto I, Ito S, Tsutsumi A, Taniguchi M, Sumida T. Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. Int J Mol Med. 2008;22:369–374. [PubMed] [Google Scholar]