Abstract
Glioblastoma is the most common type of primary brain tumor in adults, with high mortality and morbidity rates. More effective therapeutic strategies are imperative. Previous studies have shown that the known p110-β-selective inhibitor TGX-221 blocks the activation of PKB/Akt in PTEN-deficient cells. We treated U87 and U251 glioblastoma cells with TGX-221 to determine the effect of TGX-221. We performed a Cell Counting Kit-8 (CCK-8) test, EDU staining and cell cycle distribution analysis and found that TGX-221 inhibited glioblastoma cell proliferation. Next, the effect of TGX-221 on cell apoptosis was investigated using flow cytometry. These results demonstrated that TGX-221 induced apoptosis in glioblastoma cells. Moreover, migration and invasion assays revealed that TGX-221 inhibited human glioblastoma cell migration and invasion. Collectively, our study revealed that TGX-221 could inhibit proliferation and induce apoptosis in glioblastoma cells.
Keywords: TGX-221, glioblastoma, apoptosis, proliferation
Introduction
Glioblastoma is the most common type of primary brain tumor in adults. This highly malignant tumor creates a serious social and economic burden and is associated with high mortality and morbidity (1). Although multimodal treatment consisting of surgery, radiation therapy and chemotherapy has been used, glioblastoma still exhibits a poor prognosis, with a less than 12-month survival period (2). In addition, less than 5% of patients with glioblastoma survive more than 5 years after diagnosis (3). Thus, more effective therapeutic strategies are imperative.
Class I phosphatidylinositol 3-kinases (PI3Ks) play critical roles in a variety of cellular processes, such as differentiation, metabolism, migration and survival (4). The PI3K family is subdivided into 3 classes, and class I PI3K is further divided into 4 members (p110α, p110β, p110γ and p110δ) (5). Previous studies have revealed that p110β can be activated by growth factor receptors and G protein-coupled receptors, and its overexpression is capable of transforming cells (6). In addition, the known p110-β-selective inhibitor TGX-221 blocked activation of PKB/Akt in PTEN-deficient cells (7,8). For example, p110β expression was significantly increased in malignant prostate tissues compared with that in their surrounding non-malignant counterparts, and its mRNA levels were correlated with disease progression in prostate cancer patients (9). Compared with the solvent control, TGX-221 significantly decreased xenograft tumor growth in nude mice (10). Furthermore, this result was supported by other groups using transgenic mouse models (11,12) and cell culture models (13).
Previous studies have revealed that PTEN restoration and PIK3CB knockdown synergistically suppressed glioblastoma growth in vitro and in xenografts (7). However, whether TGX-221 inhibits proliferation and induces apoptosis of glioblastoma cells has not been well studied. Thus, we treated U87 and U251 cells with TGX-221 to determine the effect of TGX-221 on glioblastoma cells.
Materials and methods
Cell culture
The human glioblastoma cell lines U251 and U87 were acquired from the State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbeccos modified Eagles medium (DMEM) containing 10% fetal bovine serum and incubated at 37°C in a humidified atmosphere containing 5% carbon dioxide. DMEM was acquired from GINOM Co., Ltd. (Guangzhou, China). TGX-221 was purchased from Selleckchem (Houston, TX, USA) and dissolved in dimethyl sulfoxide (DMSO), which was a product purchased from Sigma-Aldrich (St. Louis, MO, USA).
CCK-8 assay
Cell viability was assessed using Cell Counting Kit-8 (CCK-8) according to the manufacturer's instructions. CCK-8 was purchased from Dojindo China Co., Ltd. (Shanghai, China). Approximately 8×103 cells were seeded in a volume of 100 µl of DMEM into each well of a 96-well plate. TGX-221 was added to the medium and evaluated at different concentrations at a single time-point or at different time-points at a single concentration. In addition, 100 µl of fresh medium containing 5 µl of CCK-8 solution was added into each well and incubated at 37°C for 30 min. The absorbance at 450 nm was measured using a spectrophotometric plate reader. Each group was assessed in triplicate.
5-Ethynyl-2′-deoxyuridine (EdU) staining
The Cell-Light EdU DNA Cell Proliferation kit was purchased from RiboBio Co., Ltd. (Guangzhou, China) and used according to the manufacturer's instructions. Approximately 8×103 cells were seeded in a volume of 100 µl of DMEM into each well of a 96-well plate. The medium was mixed with TGX-221 at different concentrations, and the cells were evaluated 48 h after exposure to TGX-221. The cells were treated with 50 µmol/l EdU for 12 h at 37°C. After fixation with 4% paraformaldehyde for 15 min, the cells were treated with 0.5% Triton X-100 for 20 min and rinsed with phosphate-buffered saline (PBS) 3 times. Next, the cells were incubated with 100 µl of 1X Apollo® reaction cocktail for 30 min, and the cell nuclei were stained for 30 min with 5 µg/ml Hoechst 33342. Fluorescence images of the EdU and Hoechst in the cells were captured using a fluorescence microscope (Olympus, Tokyo, Japan). The number of EdU- and DAPI-positive cells was quantified using ImageJ software, and the EdU-labeling index was calculated as the ratio of the number of EdU-positive cells to the number of DAPI-positive cells.
Flow cytometry for cell apoptosis and cell cycle distribution analysis
The effects of TGX-221 on apoptosis and cell cycle distribution were determined using the Annexin V-FITC/propidium iodide (PI) apoptosis and cell cycle kit independently, according to the manufacturer's instructions from MultiSciences Biotech (Hangzhou, China). The cells were examined after 48 h following exposure to TGX-221 at different concentrations. At the end of the treatment period, 3×105 or more cells were trypsinized, collected by centrifugation at 1,000 rpm for 5 min and rinsed with cold PBS. Next, the corresponding dyes and solution were added and incubated according to the manufacturer's instructions. Cell apoptosis and cell cycle distribution were analyzed using a flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA), and the data were analyzed using FlowJo software (version 7.6).
Migration and invasion assays
Migration and invasion assays were performed using a Transwell chamber with an 8.0-µm pore polycarbonate membrane. The cells were seeded into the top chambers containing the membranes, which were either coated or not with Matrigel for migration and invasion assays, respectively. Then, the chambers were placed into a 24-well plate, and medium containing 10% fetal bovine serum was added. The cells were fixed and stained with crystal violet, which penetrated the underside surfaces of the membranes. Subsequently, the cells were quantified under a microscope. The assays were performed 3 times.
Western blot analysis
Cell proteins were extracted with RIPA lysis buffer and assessed using the standard BCA method (Beyotime Institute of Biotechnology, Jiangsu, China). Equal amounts of protein were separated using SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA, USA). The membranes were blocked in TBS containing 0.1% Tween-20 and 5% powdered milk, and incubated at 4°C overnight with primary antibodies against cleaved caspase-3, caspase-3, Bcl-2, Lc-3b, MMP9 and β-actin. Then, the membranes were incubated in the secondary antibody Alex Fluor 680/790-labeled goat anti-rabbit IgG (LI-COR Biosciences, Lincoln, NE, USA) for 1 h. Subsequently, the blots were visualized using the LI-COR Odyssey Infrared Imaging System.
Statistical analyses
All experimental results are expressed as the mean ± SD. A Student's t-test was performed to determine the significance between two mean values. The results were considered significant at P-values of <0.05.
Results
TGX-221 inhibits glioblastoma cell proliferation
To confirm the effect of TGX-221 on glioblastoma cell proliferation, we performed the CCK-8 assay using different concentrations of TGX-221 in U87 and U251 cells. As shown in Fig. 1A and B, TGX-221 significantly inhibited the viability of U87 and U251 cells in a dose-dependent manner. The IC50 values of TGX-221 in U87 and U251 cells were ~40 and 100 µM, respectively. We then performed the CCK-8 assay at different time-points with the IC50 values of TGX-221. Glioblastoma cell proliferation was inhibited significantly by TGX-221 in a time-dependent manner (Fig. 1C and D).
Figure 1.
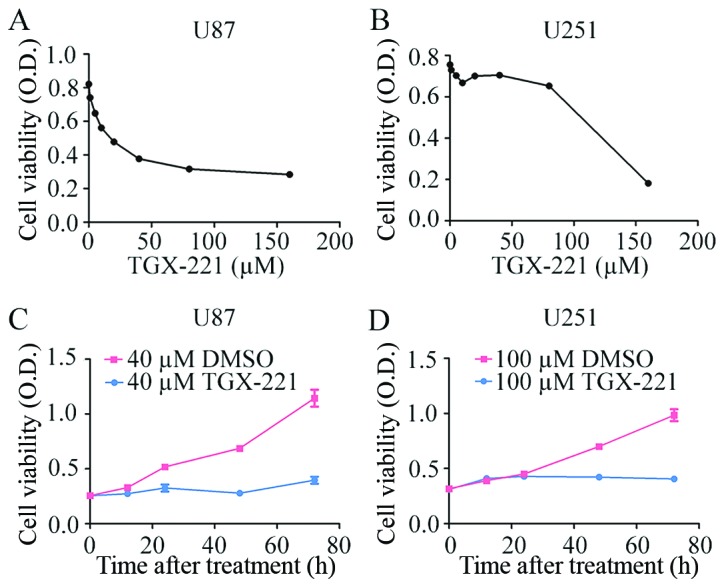
TGX-221 suppresses the viability of glioblastoma cells. (A) U87 cells were exposed to culture medium containing various concentrations of TGX-221 for 48 h, and (B) a similar protocol was performed using the U251 cells. (C) U87 cells were treated with 40 µM TGX-221 at different time-points. (D) U251 cells were treated with 100 µM TGX-221 at different time-points. The cell viability was assessed using the CCK-8 assay.
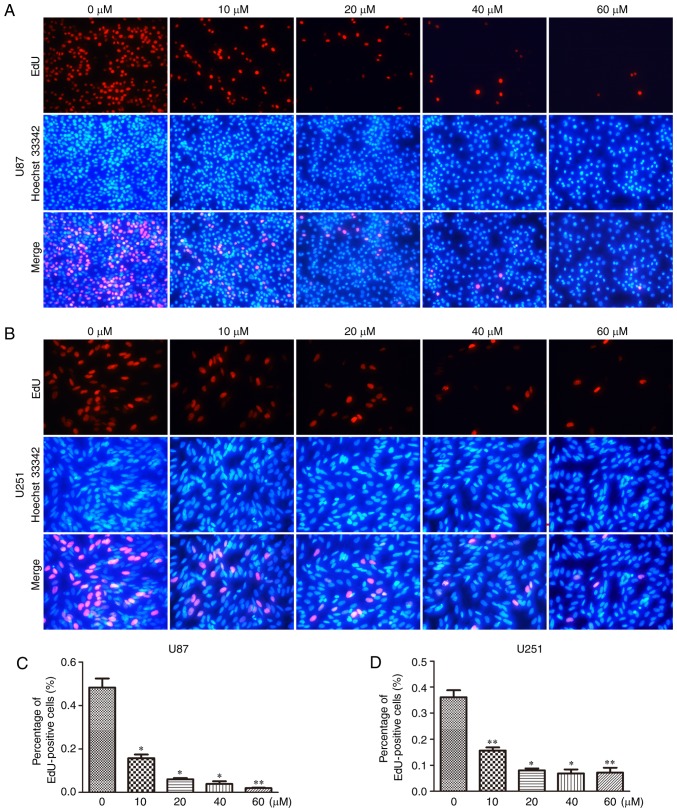
To further confirm the inhibitory effect of TGX-221 on cell proliferation in glioblastoma cells, we performed the EdU assay in both U87 and U251 cells (Fig. 2A and B). A significant inhibition of cell proliferation was observed in both U87 and U251 cells in a dose-dependent manner. With an increase in TGX-221 concentration, the number of cell nuclei with thymidine analog incorporation was decreased. The total percentage of stained nuclei in cells treated with TGX-221 was lower than that in the cells treated with DMSO (Fig. 2C and D).
Figure 2.
TGX-221 inhibits glioblastoma cell proliferation. Proliferating U87 cells were treated with different concentrations (0, 10, 20, 40 and 60 µM) of TGX-221. (A) Next, the cells were labeled with EdU, and the cell nuclei were stained with Hoechst 33342. (B) U251 cells were treated using the same protocol. (C and D) The percentage of EdU-positive U87 and U251 cells were quantified. *P<0.05, **P<0.01.
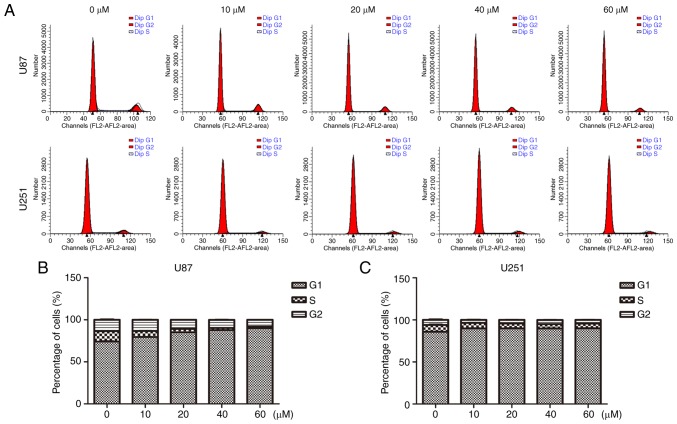
In addition, we performed flow cytometry to analyze the cell cycle distribution. As shown in Fig. 3, U87 and U251 cells were cultured with TGX-221 for 48 h. The percentage of cells in the G1 phase was increased compared with that in the control groups. The percentage of cells in the S and G2 phases was decreased, which suggested that TGX-221 inhibited glioblastoma cell proliferation. Importantly, the percentage of S and G2 phases decreased with an increase in TGX-221 concentration in glioblastoma cells.
Figure 3.
TGX-221 affects the cell cycle progression of glioblastoma cells. (A) U87 and U251 cells were treated with different concentrations (0, 10, 20, 40 and 60 µM) for 48 h, and the DNA content was analyzed using flow cytometry. (B and C) The percentage of cells in the S and G2 phases of the cell cycle was calculated.
TGX-221 induces apoptosis in glioblastoma cells
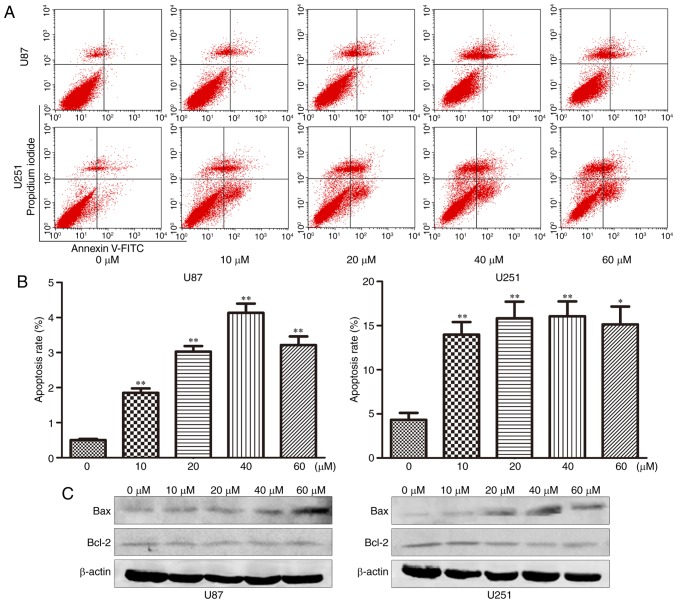
The effect of TGX-221 on cell apoptosis was investigated using flow cytometry. The apoptosis rates at 48 h after treatment at different concentrations (0,10, 20, 40 and 60 µM) are shown in Fig. 4A and B. We found that the apoptosis rate increased significantly with increasing TGX-221 concentrations.
Figure 4.
TGX-221 induces apoptosis in glioblastoma cells. (A) Apoptosis of U87 and U251 cells was analyzed using Annexin V-FITC/PI staining after a 48-h treatment with TGX-221 at different concentrations (0, 10, 20, 40 and 60 µM). (B) Apoptosis rates of U87 and U251 cells at different concentrations were compared. The data represent the mean ± SD; *P<0.05, **P<0.01. (C) After treatment of U87 and U251 cells with different concentrations (0, 10, 20, 40 and 60 µM), the expression of Bax was increased while the expression of Bcl-2 was decreased and with increasing TGX-221 concentrations, respectively.
TGX-221 inhibits glioblastoma cell migration and invasion
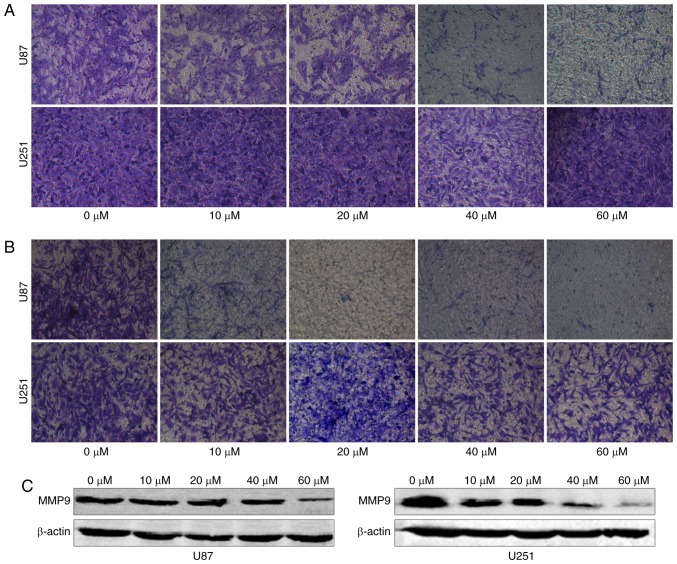
To examine whether TGX-221 inhibits the migration and invasion of glioblastoma cells, we performed a migration and invasion assay in U87 and U251 cells at different concentrations (0, 10, 20, 40 and 60 µM). We found that TGX-221 inhibited glioblastoma cell migration and invasion (Fig. 5A and B). These results were further confirmed using western blot analyses. Furthermore, we demonstrated that MMP9 gradually decreased with increasing concentrations of TGX-221 (Fig. 5C).
Figure 5.
TGX-221 inhibits glioblastoma cell migration and invasion. (A) The migration ability gradually decreased in U87 and U251 cells with increasing concentrations of TGX-221. (B) The invasion ability of glioblastoma cells gradually decreased in a dose-dependent manner. (C) MMP9 significantly decreased with increasing concentrations of TGX-221 in U87 and U251 cells.
Discussion
The PI3K family can be divided into 3 classes according to their homology and function (14). Class I PI3Ks consists of two groups (A and B), and previous research has shown that only Class IA enzymes are expressed in human types of cancer. Class IA is a heterodimeric protein consisting of a p110-kDa catalytic subunit and a p85-kDa regulatory subunit. The p85 regulatory subunit inhibits the catalytic activity of the p110 subunit in quiescent cells (15). Previous studies have demonstrated that activating point mutations or amplifications in the PIK3CA gene are present in many types of human cancer (16–21). Moreover, these findings revealed that an aberration of PIK3CA affects the occurrence and development of human types of cancer. Furthermore, PIK3CB has been demonstrated to play an important role in PI3K/AKT signaling in glioblastomas (7). Recent studies examining mutant PIK3CA also revealed that p110α was the most effective therapeutic target in many human tumors. However, PTEN-deficient types of cancer appear to be dependent on PIK3CB, but not PIK3CA. Several studies have confirmed these findings in many human tumor cells, including prostate, glioma, breast and endometrial cancer cells (22–25). In a previous study, we used the combined treatment of PTEN restoration and PIK3CB-siRNA and demonstrated that it was an effective gene therapy approach for PTEN-deficient glioblastomas (7).
We examined the role of the PI3K p110β isoform in signaling pathways and found that TGX-221 inhibited p110β based on a detailed structure and function analysis of LY294002. TGX-221 exhibited a >1,000-fold selectivity for PI3K p110β over a broad range of protein kinases. Similar to LY294002, the concentration of ATP affected the inhibitory effects of TGX-221 (26). Furthermore, TGX-221 consists of a chiral center with an aniline moiety, and uses racemic material to exert its functions (27). TGX-221 has been successfully used to inhibit p110β activity in some human tumors. Recent studies also demonstrated that TGX-221 effectively blocked tumor growth in prostate cancer xenograft mouse (10), transgenic mouse (11,12) and cell culture models (13). In the present study, we investigated U87 and U251 cells treated with TGX-221 to examine the effect of TGX-221 in glioblastoma cells. We hypothesized that TGX-221 inhibited proliferation and induced apoptosis in human glioblastoma cells based on the findings obtained in previous studies (10–13).
Our results indicated that TGX-221 inhibited proliferation, migration and invasion, and induced apoptosis. In addition, we found that U87 cells were more sensitive to TGX-221 than U251 cells. A previous study revealed that PIK3CB knockdown suppressed glioblastoma growth with PTEN restoration in vitro and in xenografts (7). Thus, TGX-221 could be more effective in U87 cells potentially due to their lack of PTEN expression.
Previous studies have provided some clues regarding the mechanism of TGX-221 (27). First, TGX-221 is an inhibitor of p110β, which participates in the PI3K/Akt signaling pathway (5). Thus, we proposed that the effect of TGX-221 may potentially involve the PI3K/Akt signaling pathway. Akt regulates cell apoptosis and survival (4,28), and Akt may exert its effects via an NF-κB signaling pathway to affect cell survival. The mechanistic effects observed were similar to our results. Thus, TGX-221 may induce apoptosis and inhibit proliferation in glioblastoma cells via the PI3K/Akt signaling pathway. Many studies have illustrated that P110β plays a role in thrombosis and stenosis reduction (29–31). The effect of TGX-221 in thrombosis and stenosis potentially occurs via the regulation of ERK phosphorylation (31). ERK can affect cell proliferation via the MAPK signaling pathway. Thus, TGX-221 may also affect cell proliferation via the MAPK signaling pathway. Previous studies have proposed that p110β plays an important role in insulin signaling (32–34). Moreover, some studies have also demonstrated that p110β can be activated by GPCRs (35–36). Furthermore, p110β exhibited different requirements for Ras activation (6). On the basis of these studies, we found that p110β affected cell apoptosis, the cell cycle, cell proliferation and cell survival via several pathways. Thus, TGX-221 may inhibit p110β to affect the biological behaviors of glioblastoma cells.
Although we obtained some results that can explain the effects observed with TGX-221, the present study has several limitations. First, we only demonstrated the effect of TGX-221, but we did not examine its underlying mechanism. Furthermore, we only performed our studies using U87 and U251 cells, and did not examine the effect of TGX-221 in vivo. Finally, we did not have sufficient clinical evidence to demonstrate our results. However, our results are credible and aligned with our expectations.
Collectively, our study illustrated that TGX-221 can inhibit proliferation and induce apoptosis in human glioblastoma cells, which may represent a promising strategy for the treatment of glioblastoma.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (nos. 81372683 and 81572489) (to Q.-X.C.), and (no. 81502175) (to B.-H.L.).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: Surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85:485–491. doi: 10.1002/(SICI)1097-0142(19990115)85:2<485::AID-CNCR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: The path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase; Proc Natl Acad Sci USA; 2006; pp. 1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Mei L, Zhou L, Shen X, Guo C, Zheng Y, Zhu H, Zhu Y, Huang L. PTEN restoration and PIK3CB knockdown synergistically suppress glioblastoma growth in vitro and in xenografts. J Neurooncol. 2011;104:155–167. doi: 10.1007/s11060-010-0492-2. [DOI] [PubMed] [Google Scholar]
- 8.Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, Friedman LS, Belvin M. Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res. 2010;70:1164–1172. doi: 10.1158/0008-5472.CAN-09-2525. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, Youn H, Tang J, Tawfik O, Dennis K, Terranova PF, Du J, Raynal P, Thrasher JB, Li B. Phosphoinositide 3-OH kinase p85alpha and p110beta are essential for androgen receptor transactivation and tumor progression in prostate cancers. Oncogene. 2008;27:4569–4579. doi: 10.1038/onc.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Zhao Y, Huang Y, Yang Q, Zeng X, Jiang W, Liu J, Thrasher JB, Forrest ML, Li B. Nanomicellar TGX221 blocks xenograft tumor growth of prostate cancer in nude mice. Prostate. 2015;75:593–602. doi: 10.1002/pros.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Poulogiannis G, Pyne S, Jia S, Zou L, Signoretti S, Loda M, Cantley LC, Roberts TM. A constitutively activated form of the p110beta isoform of PI3-kinase induces prostatic intraepithelial neoplasia in mice; Proc Natl Acad Sci USA; 2010; pp. 11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Chen S, Asara JM, Balk SP. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J Biol Chem. 2010;285:14980–14989. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 15.Wee S, Lengauer C, Wiederschain D. Class IA phosphoinositide 3-kinase isoforms and human tumorigenesis: Implications for cancer drug discovery and development. Curr Opin Oncol. 2008;20:77–82. doi: 10.1097/CCO.0b013e3282f3111e. [DOI] [PubMed] [Google Scholar]
- 16.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 17.Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, Liu JM, Yang DM, Yang WK, Shen CY. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19:2739–2744. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 19.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM, Trink B, Ladenson PW, Sidransky D, Xing M. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 2005;90:4688–4693. doi: 10.1210/jc.2004-2281. [DOI] [PubMed] [Google Scholar]
- 21.Phillips WA, Russell SE, Ciavarella ML, Choong DY, Montgomery KG, Smith K, Pearson RB, Thomas RJ, Campbell IG. Mutation analysis of PIK3CA and PIK3CB in esophageal cancer and Barrett's esophagus. Int J Cancer. 2006;118:2644–2646. doi: 10.1002/ijc.21706. [DOI] [PubMed] [Google Scholar]
- 22.Pu P, Kang C, Zhang Z, Liu X, Jiang H. Downregulation of PIK3CB by siRNA suppresses malignant glioma cell growth in vitro and in vivo. Technol Cancer Res Treat. 2006;5:271–280. doi: 10.1177/153303460600500308. [DOI] [PubMed] [Google Scholar]
- 23.An HJ, Cho NH, Yang HS, Kwak KB, Kim NK, Oh DY, Lee SW, Kim HO, Koh JJ. Targeted RNA interference of phosphatidylinositol 3-kinase p110-beta induces apoptosis and proliferation arrest in endometrial carcinoma cells. J Pathol. 2007;212:161–169. doi: 10.1002/path.2158. [DOI] [PubMed] [Google Scholar]
- 24.Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, Taketani Y, Kuramoto H, Knight ZA, Shokat KM, et al. PIK3CA cooperates with other phosphatidylinositol 3′-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 2008;68:8127–8136. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 25.Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C. PTEN-deficient cancers depend on PIK3CB; Proc Natl Acad Sci USA; 2008; pp. 13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, et al. PI 3-kinase p110β: A new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Erhard K, Hardwicke MA, Luengo JI, Mack JF, McSurdy-Freed J, Plant R, Raha K, Rominger CM, Sanchez RM, et al. Synthesis and structure-activity relationships of imidazo[1,2-a]pyrimidin-5(1H)-ones as a novel series of beta isoform selective phosphatidylinositol 3-kinase inhibitors. Bioorg Med Chem Lett. 2012;22:2230–2234. doi: 10.1016/j.bmcl.2012.01.092. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 29.Sturgeon SA, Jones C, Angus JA, Wright CE. Advantages of a selective beta-isoform phosphoinositide 3-kinase antagonist, an anti-thrombotic agent devoid of other cardiovascular actions in the rat. Eur J Pharmacol. 2008;587:209–215. doi: 10.1016/j.ejphar.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Bird JE, Smith PL, Bostwick JS, Shipkova P, Schumacher WA. Bleeding response induced by anti-thrombotic doses of a phosphoinositide 3-kinase (PI3K)-β inhibitor in mice. Thromb Res. 2011;127:560–564. doi: 10.1016/j.thromres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP. Role of phosphoinositide 3-kinase β in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem J. 2010;429:369–377. doi: 10.1042/BJ20100166. [DOI] [PubMed] [Google Scholar]
- 32.Roche S, Downward J, Raynal P, Courtneidge SA. A function for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in fibroblasts during mitogenesis: Requirement for insulin- and lysophosphatidic acid-mediated signal transduction. Mol Cell Biol. 1998;18:7119–7129. doi: 10.1128/MCB.18.12.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooshmand-Rad R, Hájková L, Klint P, Karlsson R, Vanhaesebroeck B, Claesson-Welsh L, Heldin CH. The PI 3-kinase isoforms p110(alpha) and p110(beta) have differential roles in PDGF- and insulin-mediated signaling. J Cell Sci. 2000;113(Pt 2):1–214. doi: 10.1242/jcs.113.2.207. [DOI] [PubMed] [Google Scholar]
- 34.Asano T, Kanda A, Katagiri H, Nawano M, Ogihara T, Inukai K, Anai M, Fukushima Y, Yazaki Y, Kikuchi M, et al. p110beta is up-regulated during differentiation of 3T3-L1 cells and contributes to the highly insulin-responsive glucose transport activity. J Biol Chem. 2000;275:17671–17676. doi: 10.1074/jbc.M910391199. [DOI] [PubMed] [Google Scholar]
- 35.Kubo H, Hazeki K, Takasuga S, Hazeki O. Specific role for p85/p110beta in GTP-binding-protein-mediated activation of Akt. Biochem J. 2005;392:607–614. doi: 10.1042/BJ20050671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]