Abstract
General anesthesia is widely used in pediatric surgery, although the influence of general anesthesia on cerebellar information transmission and motor function is unclear. In the present study, neonatal mice received repeated inhalation of sevoflurane, and electrophysiological alterations in Purkinje cells (PCs) and the development of motor functions were detected. In addition, γ-aminobutyric acidA receptor ε (GABAA-R ε) subunit knockout mice were used to investigate the mechanism of action of sevoflurane on cerebellar function. In the neonatal mice, the field potential response of PCs induced by sensory stimulation and the motor function indices were markedly inhibited by sevoflurane, and the inhibitory effect was positively associated with the number of repetitions of anesthesia. In additional the GABAA-R ε subunit level of PCs was promoted by sevoflurane in a dose-dependent manner, and the inhibitory effects of sevoflurane on PC field potential response and motor function were alleviated in GABAA-R ε subunit knockout mice. The GABAA-R ε subunit was activated by sevoflurane, leading to inhibition of sensory information transmission in the cerebellar cortex, field potential responses of PCs and the development of cerebellar motor function. The present study provided experimental evidence for the safe usage of sevoflurane in clinical anesthesia, and suggested that GABAA-R ε subunit antagonists may be considered for combined application with general anesthesia with repeated inhalation of sevoflurane, for adverse effect prevention in the clinic.
Keywords: general anesthesia, sevoflurane, γ-aminobutyric acidA receptor ε subunit, Purkinje cells
Introduction
With the development of pediatric surgery, increasing numbers of children are exposed to various types of narcotic (1). If children are only given local anesthesia in pediatric surgery, the surgery may be more difficult or even fail because of the child moving therefore, general anesthesia has become commonly used in pediatric anesthesia (1). Previous studies suggested that the removal of a narcotic via the metabolism of the body causes patients to reawaken (2). However, the central nervous system is sensitive to anesthesia, and is readily damaged; long-term anesthesia may damage the central nervous system, particularly in children, causing neuronal degeneration, a reduction in the number of nerve cells or brain atrophy (3). It has been reported that general anesthetics (including nitrous oxide, ketamine, midazolam, pentobarbital sodium and isoflurane), used alone or in combination over an extended period of time, may lead to widespread neuronal degeneration during brain development, ultimately causing irreversible damage to the brain which has effects into adulthood (4). Sevoflurane is a commonly used anesthetic in clinical practice, although it is not clear whether sevoflurane is suitable for long term general anesthesia in children.
The cerebellum is an important motor center; once the external information is precision computed and synthesized, a variety of output instructions are issued by Purkinje cells (PCs) to facilitate the completion of numerous physiological activities (5,6). PCs, the only output neuron in the cerebellar cortex, are highly sensitive to narcotics (6). Repeated anesthesia may lead to PC degeneration, dendritic cell reduction and cerebellar degeneration in children (7). An in vitro study indicated that narcotics may affect central nervous system function primarily through the regulation of receptors and neurotransmitter delivery, including activation of the γ-aminobutyric acidA receptor (GABAA-R) and glutamate release (8). Anesthetic drugs may increase the synthesis and release of the presynaptic GABA transmitter in PCs, and subsequently increase the concentration of glutamate, resulting in a decrease in the excitability of PCs (9). A previous study reported that GABAA-R antagonists may inhibit glutamate-induced neuronal injury in the early stages of central nervous system development, and that the regulatory effect of the GABA-R on glutamate might be associated with the level of permeability to Cl− (10). Therefore, GABAA-Rs serve an important role in neuronal injury.
It has been demonstrated that GABAA-R-mediated inhibition serves a decisive role in the process of sensory information transmission in the cerebellar cortex; sensory stimulation may evoke PC-induced inhibitory postsynaptic potential (IPSP), and a subsequent pause in electrical discharge (11). However, the effects of anesthesia on neuronal activity, synaptic transmission and neural network activity in the mammalian cerebellar cortex are not clear, and the mechanism of sensory information transmission inhibition induced by anesthesia in the cerebellar cortex remains to be elucidated.
Since sevoflurane has the advantages of rapid induction, stability and rapid recovery, it has become a commonly used narcotic drug in pediatric anesthesia (12). However, it is unclear whether intermittent or multiple sevoflurane inhalations have an effect on the cerebellum and motor function in children. In the present study, field-clamp-clamp-field potential recording and pharmacological methods were used to observe the effects of sevoflurane at a sub-anesthetic concentration on sensory stimulation-induced cerebellar PC-layer potential activity in vivo. The expression levels of the GABAA-R ε subunit were detected, and GABAA-R ε subunit knockout mice were used to investigate the effects and mechanisms of repeated sevoflurane administration on neuronal activity, synaptic transmission, neural network, information transmission, the field potential response of PCs and motor function. Therefore, the present study sought to elucidate the role of sevoflurane in the regulation of the cerebellar cortical neural network and motor function, and to provide a theoretical basis for the future clinical application of sevoflurane at different doses and lengths of time in pediatric anesthesia.
Materials and methods
Animal experiment
GABAA-R ε−/− mice (female; 10–12 weeks old; weight 30±4 g) were purchased from Cyagen Biosciences (Santa Clara, CA, USA) and normal C57 wild-type (GABAA-R ε+/+) mice (male; 10–12 weeks old; weight 30±5 g) were provided by the Experimental Animal Center of Changzhi Medical College (Changzhi, China). As the reproductive performance of GABAA-R ε−/− mice is weak, the GABAA-R ε+/+ mice were mated to GABAA-R ε+/− mice, and the GABAA-R ε+/− mice were mated to each other to generate the GABAA-R ε−/− mice and the GABAA-R ε+/+ mice; GABAA-R ε−/− mice and GABAA-R ε+/+ mouse pups were used in the present study. The mice were housed at a temperature of 20–24°C and a relative humidity of 50–60%, and were maintained on a 12-h light/dark cycle, the mice had access to food that was sterilized by irradiation, and the water contained neomycin (4 g/l) and bacitracin (4 g/l). In the wild-type (GABAA-R ε+/+) mouse experiments, 220 neonatal mice were used; 200 neonatal mice were divided into 10 groups (n=20 mice/group) and the control group included 20 neonatal mice. In order to avoid confusion, each mouse was marked with an ear tag. For the experimental neonatal mice, the mice were given repeated inhalation of 1.5% sevoflurane for 1 h daily via a Julian anesthesia machine (Draeger, Inc., Telford, PA, USA), sustained for 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30 days, respectively. The control group received 1 l/min O2 and 1 l/min air for 1 h daily, sustained for 30 days.
In the GABAA-R ε−/− mouse experiments, 20 neonatal GABAA-R ε−/− mice were used as the control group, which received 1 l/min O2 and 1 l/min air; 40 neonatal GABAA-R ε−/− mice were used to investigate the role of GABAA-R ε, and all the mice were divided into 2 groups (n=20). The mice were given repeated inhalation of 1.5% sevoflurane for 1 h daily, sustained for 12 or 24 days, respectively. A total of 40 neonatal GABAA-R ε+/+ mice were used as the control for the GABAA-R ε−/− mice, and the mice were divided into 2 groups (n=20) and given repeated inhalation of 1.5% sevoflurane for 1 h daily, sustained for 12 and 24 days, respectively. Mice were placed into self-made inhalation anesthesia boxes, with a homemade water bath box at a temperature of 30–34°C. The concentration of sevoflurane in the inlet and outlet was continuously monitored using a gas monitor (Draeger, Lübeck, Germany) to control the experimental conditions. Following repeated anesthesia for 1 month, at 2 months following the birth of the mice, cerebellar electrophysiological examination was performed. The present study was approved by the Animal Experimental Ethics Committee of Changzhi Medical College.
Cerebellar electrophysiological examination
Following repeated anesthesia for 1 month, the mice were placed in a special brain stereotaxic device, with bilateral ear and maxillary fixing of the head. Craniotomy was performed using a precision dental drill with a diameter of 1–1.5 mm at the crus II of the cerebellum; the dura was carefully removed to expose the recording site. When the control electrode had been mounted, the mouse and brain stereotaxic apparatus were fixed onto the microscope (Nikon FN1; Nikon Corporation, Tokyo, Japan), to locate the operation site at the skull opening, avoiding blood vessels and perform the electrophysiological recording. The surgical site was perfused with artificial cerebrospinal fluid (124 mM Nacl, 26 mM NaHCO3, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 10 mM D-glucose, and the solution was aerated with 95% O2 and 5% CO2) using a peristaltic pump (MiniPuls 3; Gilson, Inc., Middleton, WI, USA). Body temperature was maintained at 37±0.2°C during the surgical procedure and was recorded through a body temperature maintainer and heating pad.
PC layer electrophysiological recording
A thin glass tube (outer diameter, 1.5 mm; Narishige Group, Tokyo, Japan) was drawn into a recording electrode using an automatic drawing apparatus (PB-10; Narishige Group). The electrode was filled with 20–30 µl artificial cerebrospinal fluid; following filling, the electrode impedance was 4–6 MΩ. The recording electrode was mounted on an electric micromanipulator (Sutter Medical Technologies USA, Atlanta, GA, USA) and the electrode movement was detected with a microscope (Nikon Eclipse E600FN; Nikon Corporation). PC discharge was recorded using axopatch-1D patch-clamp amplifiers (Molecular Devices, LLC, Sunnyvale, CA, USA) and Clampex 8.1 data acquisition and analysis software (Molecular Devices, LLC) was used. Following confirmation of the recording site under a microscope, the electrodes were placed on the brain surface of the recording area and slowly punctured into the pia mater from 200 to 400 µm to reach the PC layer of the cerebellar cortex, adjusting the amplitude of the discharge to a maximum of 0.5 mV. When the discharge frequency and amplitude were stable following 100 sec, the electrode was recorded. With the extracellular recording conditions, the confirmation of PC discharge was based on simple discharge (simple spike) and complex discharge (complex spike).
Immunofluorescence and immunohistochemistry
Upon completion of the electrode recording, the brain was rapidly extracted and placed on ice, and was briefly washed in ice-cold PBS (Beyotime Institute of Biotechnology, Haimen, China; 0.2 mol/l; pH 7.4; diluted in double distilled water). The cerebellum was subsequently isolated and fixed in 4% paraformaldehyde (Beyotime Institute of Biotechnology) diluted in PBS at 4°C for 24–48 h, followed by 30% sucrose (Sangon Biotech Co., Ltd., Shanghai, China; diluted in PBS) for an additional 24 hat 4°C. When the brain tissue had been fully immersed, it was embedded in Optimum Cutting Temperature embedding agent (Sangon Biotech Co., Ltd.) and frozen in a liquid nitrogen tank, removed and frozen at −20°C, and cut on a cryostat (Leica CM3050S; Leica Microsystems GmbH, Wetzlar, Germany) to a thickness of 15-µm. Brain slices were blocked with 10% goat serum (OriGene Technologies, Inc., Beijing, China) at room temperature for 30 min. The goat serum was diluted in a solution containing 0.3% Triton X-100 and 0.1 mol/l PBS; the Triton X-100 is used to perforate the cell membrane in order to promote antibody penetration into cells. Following blocking, the blocking solution was aspirated and the primary antibody, mouse immunoglobulin (Ig)G anti-GABAA-R ε (cat. no. sc-271668; 1:300; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or mouse IgG FluoroPan Neuronal Marker-Alexa488-conjugated (cat. no. MAB2300X; 1:100; EMD Millipore, Billerica, MA, USA), was added and incubated at 4°C overnight. PBS was used as a negative control. The secondary antibody, goat anti-mouse IgG fluorescein isothiocyanate (1:500; cat. no. F-2761; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) or goat anti-Mouse IgG phycoerythrin (1:500; cat. no. P-852; Invitrogen; Thermo Fisher Scientific, Inc.) was added, and incubated for 2 h at room temperature. DAPI (Sangon Biotech, Co., Ltd.) was added 10 min prior to the end of incubation. Following each step, the samples were washed in PBS for 10 min; antibody incubation and rinsing steps were performed on a shaker. Fluorescent encapsulated tablets (SouthernBiotech, Birmingham, AL, USA) were used for 10 min in the dark. Immunohistochemical staining was performed using an immunohistochemistry detection kit, according to the manufacturer's protocol (PowerVision™ Two-Step; OriGene Technologies, Inc.). All the samples were observed by fluorescence microscope (magnification, ×400) (Zeiss AG, Oberkochen, Germany).
Western blotting
Total proteins of the cerebellum were extracted using radioimmunoprecipitation assay lysis solution (Beyotime Institute of Biotechnology), proteins were quantified via a bicinchoninic acid assay (Beyotime Institute of Biotechnology), and western blot analysis samples were prepared, 30 µg protein/lane was separated by 15% SDS-PAGE. Subsequently, the sandwich structure composed of sponge, filter paper and polyvinylidene fluoride (PVDF) membrane was placed in the transfer machine at 200 mA for 90 min. The PVDF membrane was incubated with 5% bovine serum albumin (Beyotime Institute of Biotechnology) [diluted with PBS-Tween-20 (PBST)] at room temperature for 1 h. The primary antibodies anti-GABAA-R ε (cat. no. sc-271668; 1:300; Santa Cruz Biotechnology, Inc.) and anti-GAPDH (cat. no. sc-293335; 1:1,000; Santa Cruz Biotechnology, Inc.) were incubated at 4°C overnight, and then washed three times with PBST for 10 min each. The secondary antibody mouse immunoglobulin (Ig)G conjugated to CruzFluor™ 488 (cat. no. sc-516176; 1:3,000; Santa Cruz Biotechnology, Inc.) was incubated at 37°C for 1 h. The PVDF membrane was washed in PBST three times for 10 min each, and images were captured using an enhanced chemiluminescence solution (Beyotime Institute of Biotechnology), and the gray scales were scanned by Chemidoc XRS+ Imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the gray scale values were quantified analysis using Quantity One Analysis Software (version 4.6.9; Bio-Rad Laboratories, Inc.).
Gait kinematics detection and analysis
Subsequent to the repeated anesthesia and electrode recording for 1 month, 2 months following the birth of the mice, the footprints of mice in each group were recorded using a transparent table, and the CatWalk Automatic Gait Analysis system (Noldus Information Technology bv, Wageningen, The Netherlands) was used to automatically identify and analyze the footprints of the mice. A total of 97 indices, including walking cycle, step width, footprint area, speed, standard deviation of body angle, support duration, swing time, braking duration and propulsion time, were recorded and analyzed. Each mouse was subjected to at least three tests. For each test, the mice walked continuously for the length of the transparent glass plate. The experiment was performed in a darkroom environment. The gait analysis system was used to automatically extract and analyze gait video data, the gait kinematics parameters were obtained and the data were analyzed.
Statistical analysis
All electrophysiological data were analyzed using Clampfit 8.1 software (Molecular Devices, LLC). All data are expressed as the mean ± standard deviation. SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Independent t-tests were used for comparisons between groups, two-way analysis of variance was used for multi-group comparisons, and further pairwise comparisons were analyzed by the least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of field potential response of PCs induced by sensory stimulation in the mouse cerebellar cortex
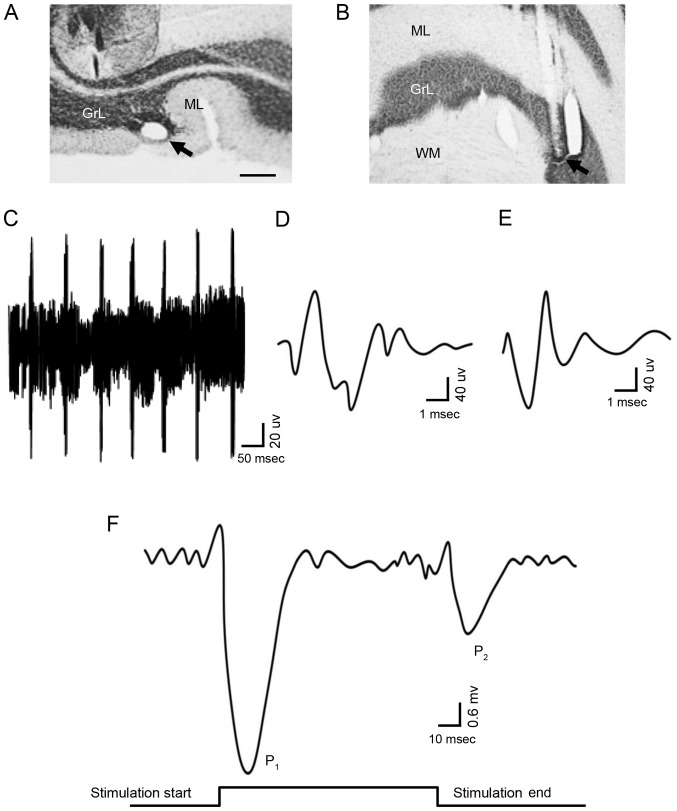
In order to record the potential responses of PCs, the recording electrodes were located at the specific histological region where the PCs are existence (Fig. 1A and B). Single-unit spike signals were excluded and the low noise signals, contributed to only by the PCs, were detected to record the time axle of electrophysiological behavior in the experiment. The PCs was identified depending on the complex spikes and simple spikes, via the waveform analysis algorithm. The criteria for spike identification of PCs (Fig. 1C) were as follows: i) Signal-to-noise ratio of individual action potentials >5:1; ii) existence of the complex spike (Fig. 1D); and iii) presence of the simple spike (Fig. 1E) followed by the complex spike. In the resting state, PCs did not exhibit synchronous excitatory activity; therefore, the field potential of the PCs was not altered. However, stimulation of the ipsilateral whisker pad (60 msec; 50–60 psi) was able to induce PCs to generate a pair of excitatory field potential responses (Fig. 1F); the presence of the first peak response (P1) followed by the commencement of stimulation, and the presence of the second peak (P2) followed by the end of the stimulation,=indicated that P1 was induced by the stimulation, while P2 was caused by the end of stimulation. The results of the present study indicated that sensory stimulation information may be integrally transmitted by cerebellar PCs, and that the information contains the onset and end of the stimulus. The above results suggested that the cerebellar PCs are able to transmit sensory information with high fidelity.
Figure 1.
Characteristics of field potential responses of PCs induced by sensory stimulation. The recording site in the PC layer is presented as (A) a cross section and (B) a longitudinal section; the arrows indicate the site (scale bar, 0.5 mm). (C) The signal-to-noise ratio was mediated to 5:1 in order to record the electric potential of PCs. The (D) complex spikes and (E) simple spike were considered to be markers of PCs. (F) The field potential responses of PCs induced by sensory stimulation is presented. There were two waves in the field potential of the PC layer: The P1 wave was caused by the onset of the stimulation, and the P2 wave was caused by the end of the stimulation. GrL, granular layer; ML, molecular layer; WM, white matter; PCs, Purkinje cells.
Repeated sevoflurane inhalation inhibits the field potential response of PCs induced by sensory stimulation in neonatal mice
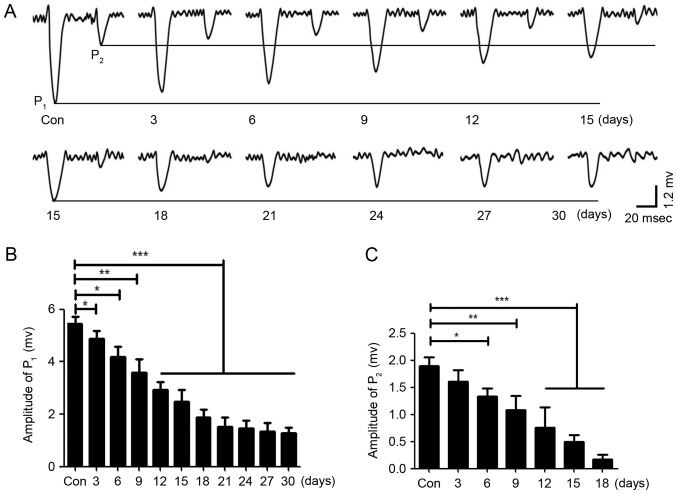
Repeated anesthesia was given for different lengths of time in neonatal mice, which were housed for 1 month. In the current clamp mode (I=0), the bimodal excitatory field potential responses of PCs were induced by the stimulation to the ipsilateral whisker pad and recorded (Fig. 2A). The results demonstrated that the average peak values of P1 and P2 decreased with an increased length of repeated anesthesia; the peak value of P1 no longer continued to decline when the neonatal mice received repeated anesthesia for 21 days (Fig. 2A and B), and the peak value of P2 almost disappeared when the neonatal mice received repeated anesthesia for 21 days (Fig. 2A) and statistical analysis was also performed (Fig. 2C). These results indicated that the central nervous system may be damaged by the repeated inhalation of sevoflurane in neonatal mice, and that the amount of sevoflurane may be negatively associated with neuronal activity and synaptic transmission, potentially leading to neuronal dysfunction. Therefore, sevoflurane may damage the central nervous system in a dose-dependent manner, although high concentrations of sevoflurane did not completely inhibit the response of PCs to stimulation; thus, sevoflurane may only partially inhibit the sensory information transmission of PCs.
Figure 2.
Sevoflurane inhibits the field potential response of PCs in a dose-dependent manner. (A) The field potential responses of PCs induced by sensory stimulation in neonatal mice were inhibited by repeated inhalation of sevoflurane, and the values of P1 and P2 were decreased by sevoflurane in a dose-dependent manner. Repeated inhalation of sevoflurane for 21 days may be the maximal effective concentration. The control was wild-type mice. (B) The statistical results of the value of P1 for neonatal mice with different lengths of repeated sevoflurane inhalation. (C) The statistical results of the value of P2. *P<0.05, **P<0.01, ***P<0.005. PCs, Purkinje cells; Con, control; P, peak.
Effect of repeated inhalation of sevoflurane on the movement behavior of neonatal mice
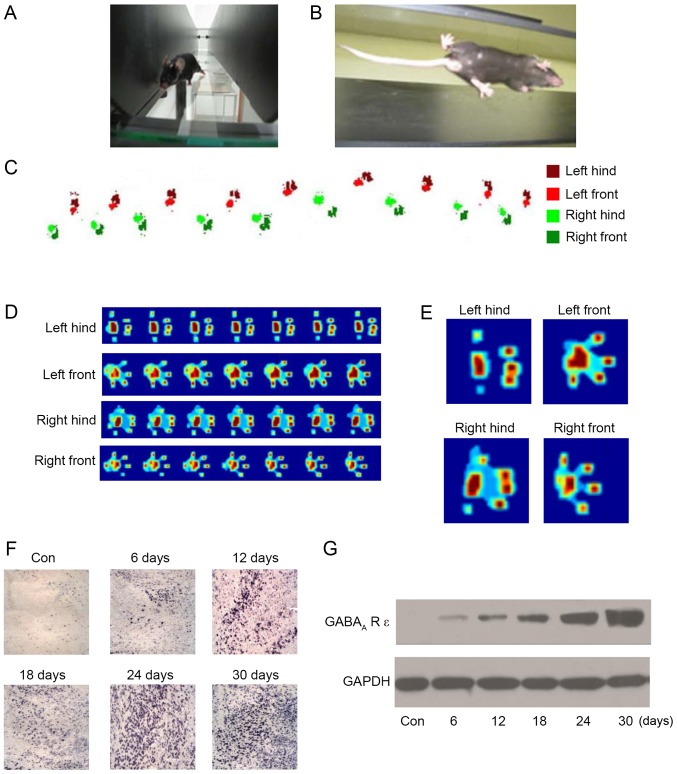
In order to evaluate the effect of sevoflurane on motor function in neonatal mice, the CatWalk Automatic Gait Analyzer (Fig. 3A and B) was used to quantitatively analyze the gait of mice following repeated inhalation of sevoflurane. The four feet were labeled with different colors (Fig. 3C), and the footprints were recorded. Additionally, the footprints were converted to a heat map, in order for the pressure at different sites to be determined (Fig. 3D and E). The results (Table I) demonstrated that the gait of the mice was abnormal following 6 days of repeated anesthesia and that the abnormality was more prominent with the increase in repeated anesthesia time, which primarily manifested in the support phase, swing phase, coordination, footprint average area, braking index and propulsion index. In addition, following 24 days of repeated anesthesia, the gait movement function of the mice was reduced to its worst state; continuing to increase the days of repeated anesthesia did not lead to a significant alteration in gait movement function. The results of the present study demonstrated that repeated inhalation of sevoflurane may lead to movement behavior disorder in neonatal mice. Gait indexes were negatively associated with repeated anesthesia time, which may explain the long-term alterations in motor function in neonatal mice.
Figure 3.
Sevoflurane inhibits movement behavior and increases the expression level of the GABAA-R ε subunit. The movement behavior of mice was measured using the CatWalk Automatic Gait Analysis system; images were captured of (A) the mice walking and (B) the footprints under the glass. (C) The feet were labeled with different colors for footprint recordings. (D) The footprints were converted to heat maps and (E) the individual footprint heat maps were amplified and analyzed. (F) The cerebellar slices were subjected to an immunohistochemistry assay for GABAA-R ε subunit detection and the amount of GABAA-R ε subunit-positive cells was positively associated with the day of repeated inhalation of sevoflurane; 24 days was the maximal effect time. (G) The expression level of the GABAA-R ε subunit was measured by western blotting. GABAA-R, γ-aminobutyric acidA receptor; Con, control.
Table I.
Effect of repeated inhalation of sevoflurane on the movement behavior of wild-type neonatal mice.
| Group | Coordination of right rear to left front | Ipsilateral coordination of right front to left front | Swing phase-left front, sec | Swing phase-right front, sec | Support phase-left front, sec | Support phase-right front, sec | Propulsion index of left rear | Propulsion index of left front | Braking index of left rear | Average area of right rear footprint, mm2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Control, n=20 | 0.521±0.074 | 0.573±0.062 | 0.641±0.072 | 0.627±0.059 | 0.315±0.083 | 0.326±0.083 | 0.817±0.115 | 0.571±0.084 | 0.219±0.025 | 2.741±0.413 |
| 6 days, n=20 | 0.463±0.042a | 0.491±0.059a | 0.583±0.058a | 0.542±0.074a | 0.391±0.091a | 0.408±0.062a | 0.742±0.104a | 0.469±0.075a | 0.294±0.053a | 3.915±0.619a |
| 12 days, n=20 | 0.375±0.035a | 0.411±0.058a | 0.491±0.049a | 0.451±0.084a | 0.458±0.071a | 0.471±0.085a | 0.638±0.084a | 0.421±0.058a | 0.357±0.061a | 5.882±0.713a |
| 18 days, n=20 | 0.321±0.047a | 0.324±0.032a | 0.418±0.047a | 0.374±0.037a | 0.527±0.063a | 0.541±0.097a | 0.519±0.078a | 0.349±0.038a | 0.411±0.075a | 8.621±0.914a |
| 24 days, n=20 | 0.262±0.026a | 0.247±0.027a | 0.337±0.046a | 0.261±0.039a | 0.584±0.093a | 0.604±0.071a | 0.457±0.062a | 0.271±0.049a | 0.472±0.085a | 9.884±0.717a |
| 30 days, n=20 | 0.237±0.018a | 0.223±0.016a | 0.329±0.048a | 0.253±0.041a | 0.592±0.101a | 0.613±0.064a | 0.441±0.072a | 0.269±0.062a | 0.483±0.092a | 10.027±0.913a |
P<0.05 vs. control.
Effect of repeated inhalation of sevoflurane on the GABAA-R ε subunit
There have been reports that the GABAA-R ε subunit may be regulated by sevoflurane in cortical neurons (13,14). In order to investigate the role of the GABAA-R ε subunit in neonatal mice undergoing repeated inhalation of sevoflurane, the expression of the GABAA-R ε subunit in cerebellar tissue was detected using an anti-GABAA-R ε subunit antibody by immunohistochemistry. The results indicated that the level of expression of the GABAA-R ε subunit was increased by repeated inhalation of sevoflurane in the PC layer, and the number of GABAA-R ε subunit-positive PCs was increased with the increase in the number of days of repeated anesthesia; similarly to the effect of repeated inhalation of sevoflurane on movement behavior and the field potential response of PCs, the increase was stable at 24 days (Fig. 3F). In addition, the results of the western blot analysis demonstrated that the amount of GABAA-R ε subunit protein was increased by the repeated inhalation of sevoflurane, and was positively associated with the number of days of repeated anesthesia (Fig. 3G). The results of the present study indicated that the GABAA-R ε subunit was upregulated by the repeated inhalation of sevoflurane, and that the GABAA-R ε subunit may serve an important role in neonatal mice receiving repeated inhalation of sevoflurane.
Effects of repeated inhalation of sevoflurane on neonatal mice regulated by the GABAA-R ε subunit
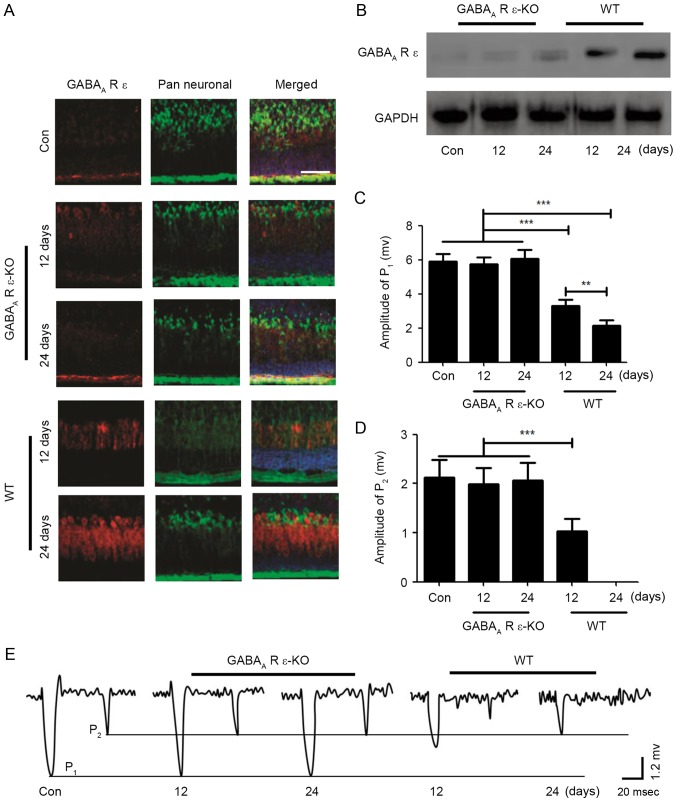
In order to determine whether the GABAA-R ε subunit served a role in the effects of repeated inhalation of sevoflurane in neonatal mice on the field potential response of PCs and movement behavior, GABAA-R ε subunit knockout mice were used in the present study. In the GABAA-R ε subunit knockout mice, following repeated inhalation of sevoflurane for 12 or 24 days, compared with the control group, the expression level of the GABAA-R ε subunit was not altered, whereas the expression level of the GABAA-R ε subunit was markedly increased in a time-dependent manner in the wild-type mice (Fig. 4A); the results of the western blot analysis confirmed this result (Fig. 4B). For the GABAA-R ε subunit knockout neonatal mice, the mice were subjected to repeated inhalation of sevoflurane for different lengths of time; when the mice were 2 months old, the excitatory field potential responses of PCs were induced by stimulation of the ipsilateral whisker pad in the current clamp mode (I=0). The results indicated that the values of P1 and P2 in GABAA-R ε subunit knockout mice were consistent with the control group, although in the wild-type mice, the values of P1 and P2 were significantly altered in a time-dependent manner (Fig. 4C-E). Additionally, the effect of repeated inhalation of sevoflurane on motor function in the GABAA-R ε subunit knockout mice was examined using the CatWalk Automatic Gait Analyzer. The results demonstrated that repeated inhalation of sevoflurane did not affect motor function in the GABAA-R ε subunit knockout mice, although motor function in wild-type mice was inhibited by repeated inhalation of sevoflurane (Table II). These results indicated that the GABAA-R ε subunit may serve an important role in the field potential response of PCs and the development of movement behaviors.
Figure 4.
GABAA-R ε subunit serves a role in the effects of repeated inhalation of sevoflurane on neonatal mice. (A) 15-µm cerebellar sections from GABAA-R ε subunit KO and WT mice were subjected to an immunofluorescence assay. GABAA-R ε subunit (red), pan neuronal marker (green) and DAPI were stained and merged. Scale bar, 50 µm. (B) The expression levels of the GABAA-R ε subunit were detected via western blotting. (C) The statistical results of the value of P1 in GABAA-R ε KO mice and WT mice were calculated. (D) The statistical results of value of P2 were calculated. (E) The field potential response of PCs induced by sensory stimulation is presented in GABAA-R ε KO mice and WT mice for different day of sevoflurane inhalation. **P<0.01, ***P<0.005. KO, knockout; WT, wild-type; P, peak; Con, control; GABAA-R, γ-aminobutyric acidA receptor.
Table II.
Effect of repeated inhalation of sevoflurane on the movement behavior of GABAA R ε knockout and wild-type neonatal mice.
| Group | Coordination of right rear to left front | Ipsilateral coordination of right front to left front | Swing phase-left front, sec | Swing phase-right front, sec | Support phase-left front, sec | Support phase-right front, sec | Propulsion index of left rear | Propulsion index of left front | Braking index of left rear | Average area of right rear footprint, mm2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Control, n=20 | 0.547±0.092 | 0.552±0.093 | 0.633±0.114 | 0.636±0.113 | 0.323±0.077 | 0.337±0.064 | 0.804±0.121 | 0.563±0.076 | 0.226±0.032 | 2.512±0.375 |
| GABAA-R ε knockout mice | ||||||||||
| 12 days, n=20 | 0.536±0.093 | 0.549±0.073 | 0.634±0.079 | 0.632±0.095 | 0.331±0.064 | 0.341±0.059 | 0.796±0.114 | 0.559±0.067 | 0.224±0.074 | 2.449±0.483 |
| 24 days, n=20 | 0.521±0.115 | 0.533±0.069 | 0.618±0.093 | 0.619±0.075 | 0.329±0.084 | 0.339±0.067 | 0.785±0.097 | 0.562±0.089 | 0.231±0.043 | 2.593±0.618 |
| Wild-type mice | ||||||||||
| 12 days, n=20 | 0.378±0.063a | 0.395±0.104a | 0.479±0.062a | 0.442±0.095a | 0.463±0.085a | 0.483±0.067a | 0.615±0.078a | 0.416±0.074a | 0.368±0.074a | 6.114±0.817a |
| 24 days, n=20 | 0.283±0.062a | 0.257±0.053a | 0.341±0.057a | 0.273±0.053a | 0.569±0.107a | 0.668±0.093a | 0.463±0.057a | 0.268±0.028a | 0.485±0.075a | 9.634±0.829a |
P<0.05 vs. control. GABAA-R ε, γ-aminobutyric acidA receptor ε subunit.
Discussion
The cerebellum is an important motor center; external information is precision computed and synthesized by the cerebellar cortex, and e PCs issue a variety of output instructions to complete physiological activities (15). PCs are the principal neurons in the cerebellar cortex, and their axons form the only efferent pathway of the cerebellar cortex (16). GABA is released via the axon terminals of PCs, which contribute to the inhibitory effect of PCs on the dominant nucleated neurons of the cerebellum (17). In the afferent fibers of the cerebellum, climbing fibers and mossy fibers may combine with PC dendrites to form excitatory synaptic connections (18). Excitatory afferents of climbing fibers may cause PC excitability, characterized by complex spikes and, subsequent to the formation of synaptic connection between the mossy fibers and granular cells, PC excitability is indirectly excited through the axons of granulosa cells and parallel fibers, and manifests as simple spikes (6,18). Basket cells (BCs) stellate cells (SCs) are the inhibitory interneurons of PCs; the long axon terminals of BCs are wrapped in the soma and axon segments of 3–5 PCs (19). The excitations of BCs may directly inhibit PCs via the soma, and the axons of SCs selectively combine with the dendrites of PCs to form inhibitory synaptic connections, contributing to the inhibition of PC dendrites and shunting the excitatory afferents of parallel fibers (20,21). It was previously suggested that one bundle of parallel fibers and its dominant PCs are excited by sensory information through the mossy fiber-granulosa-paracellular pathway (22). Subsequent studies demonstrated that sensory stimulation may stimulate PCs, in addition to leading to PC IPSP accompanied by discharge pause (23–25). Sensory stimulation may induce PC excitation following blocking of GABAA receptor activity, indicating that inhibitory interneurons serve a role in the process of cerebellar cortex sensory information transmission (11,26). In the present study, it was demonstrated that stimulation to the ipsilateral whisker pad may stimulate the high-fidelity response of PCs in the crus II region of the cerebellum and as the stimulus response at the beginning was greater than the stimulus response at the end, it indicates that the sensory stimulation information was preserved intact through mossy fiber-PC pathway transmission into cerebellar cortex. The integrity of the stimulation beginning and end information coding indicated that cerebellar PCs have a high fidelity for the transmission of sensory information.
Experimental animal behavior analysis is widely used in studies of motor function, learning, memory and other higher central nervous system functions (27). Compared with the analysis of electrophysiological, biochemical and other parameters, experimental animal behavior analysis may fully reflect the overall state of animals, and gait analysis is commonly used in the study of motor behavior (28). The gait of mice was detected using the CatWalk Gait Analysis system following repeated inhalation of sevoflurane, which may effectively reflect the severity of brain injury and movement functions, and guarantee objectivity (29). Therefore, the CatWalk Gait Analysis system may be used as a method to evaluate animal behavior in the repeated inhalation of sevoflurane model. The parameters associated with cerebellar injury in gait analysis are: Ipsilateral coordination, contralateral coordination, support phase, swing phase, propulsion index, braking index and average footprint (30). In the present study, the results indicated that the gait analysis indexes were significantly associated with cerebellar injury in mice undergoing repeated inhalation of sevoflurane with different time. When the anesthesia time increased, the gait analysis indexes deteriorated, which indicated that the cerebellar injury was positively associated with the length of time of repeated inhalation of sevoflurane.
In the present study, the results indicated that repeated inhalation of sevoflurane exerted inhibitory effects on cerebellar sensory information transmission and motor function development, and there was a certain dose-dependent effect; however, long-time repeated inhalation of sevoflurane was unable to completely inhibit the sensory stimulation-induced response of PCs and motor function development. It was additionally demonstrated that the GABAA-R ε subunit was significantly activated, suggesting that repeated inhalation of sevoflurane may inhibit the transmission of sensory information in PCs and motor function development by enhancing GABAA-R ε subunit activity. In the GABAA-R ε subunit knockout mice, the inhibitory effects of repeated inhalation of sevoflurane on the transmission of sensory information in PCs and motor function development were completely inhibited. The results of the present study indicated that the GABAA-R ε subunit served a role in the field potential response of PCs and the development of movement behavior.
The results of the present study may be associated with a number of factors. Repeated inhalation of sevoflurane may affect central nervous system function by regulating receptor and neurotransmitter transmittance, contributing to GABAA-R ε subunit activation (31). Therefore, the repeated inhalation of sevoflurane may directly activate the GABAA-R ε subunit in PCs, which may result in a sustained inhibition of the field potential response in the PCs, contributing to inhibition of information transmission and the development of motor function. Increasing the concentration of sevoflurane in the cerebellum may synergize with the endogenous GABAA-R ε subunit, thereby enhancing the inhibitory effect of the endogenous GABAA-R ε subunit on the field potential response of PCs, delaying or inhibiting the transmission of movement information and, consequently, the development of motor function (32). Sevoflurane increases the excitability of Golgi cells by inhibiting the activity of Na+/K+ ATPase, thereby enhancing the paroxysmal inhibition of PCs and increasing the leakage of the GABAA-R ε subunit around PCs, resulting in an increase in the GABAA-R ε subunit concentration in the cerebellum and further increasing the sustained inhibition of information transmission in PCs, affecting motor function and development (33–35). Therefore, an increase in sevoflurane concentration in the cerebellar cortex may excite Golgi cells, increase the release of the GABAA-R ε subunit, increase the concentration of GABAA-R ε subunits around the PCs, and enhance the sustained inhibitory effect on the sensory stimulus-induced responses of PCs and the transmission of motion information, delaying motor function development.
In conclusion, repeated inhalation of sevoflurane exerted an effect on GABAA-R ε subunit activity in neonatal mice. The field potential response of the PCs was markedly inhibited by sevoflurane through GABAA-R ε subunit activation. Sevoflurane was additionally able to inhibit sensory information transmission in the cerebellar cortex, further delaying the cerebellar motor function development. These results demonstrated that repeated inhalation of sevoflurane may damage the cerebellum, and revealed a partially dose-dependent effect, suggesting that repeated inhalation of anesthesia may require careful consideration in children; GABAA-R ε subunit antagonists may be applied in combination, in order to reduce the incidence of side-effects associated with repeated inhaled anesthesia.
References
- 1.Brandt ML, Harmon CM, Helmrath MA, Inge TH, McKay SV, Michalsky MP. Morbid obesity in pediatric diabetes mellitus: Surgical options and outcomes. Nat Rev Endocrinol. 2010;6:637–645. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- 2.Mallineni SK, Yiu CK. Dental treatment under general anesthesia for special-needs patients: Analysis of the literature. J Investig Clin Dent. 2016;7:325–331. doi: 10.1111/jicd.12174. [DOI] [PubMed] [Google Scholar]
- 3.Parekh S, Gardener C, Ashley PF, Walsh T. Intraoperative local anaesthesia for reduction of postoperative pain following general anaesthesia for dental treatment in children and adolescents. Cochrane Database Syst Rev. 2014;12:CD009742. doi: 10.1002/14651858.CD009742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 5.Hirata Y, Katagiri K, Tanaka Y. Direct causality between single-Purkinje cell activities and motor learning revealed by a cerebellum-machine interface utilizing VOR adaptation paradigm. Cerebellum. 2012;11:455–456. doi: 10.1007/s12311-012-0385-3. [DOI] [PubMed] [Google Scholar]
- 6.Barmack NH, Yakhnitsa V. Climbing fibers mediate vestibular modulation of both ‘complex’ and ‘simple spikes’ in Purkinje cells. Cerebellum. 2015;14:597–612. doi: 10.1007/s12311-015-0725-1. [DOI] [PubMed] [Google Scholar]
- 7.Huang JJ, Yen CT, Tsao HW, Tsai ML, Huang C. Neuronal oscillations in Golgi cells and Purkinje cells are accompanied by decreases in Shannon information entropy. Cerebellum. 2014;13:97–108. doi: 10.1007/s12311-013-0523-6. [DOI] [PubMed] [Google Scholar]
- 8.Rostain JC, Lavoute C, Risso JJ, Vallée N, Weiss M. A review of recent neurochemical data on inert gas narcosis. Undersea Hyperb Med. 2011;38:49–59. [PubMed] [Google Scholar]
- 9.Irie T, Kikura-Hanajiri R, Usami M, Uchiyama N, Goda Y, Sekino Y. MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar Purkinje cells via activation of presynaptic CB1 receptor. Neuropharmacology. 2015;95:479–491. doi: 10.1016/j.neuropharm.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: Function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015;40:402–409. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- 11.Kueh SL, Dempster J, Head SI, Morley JW. Reduced postsynaptic GABAA receptor number and enhanced gaboxadol induced change in holding currents in Purkinje cells of the dystrophin-deficient mdx mouse. Neurobiol Dis. 2011;43:558–564. doi: 10.1016/j.nbd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Gueli SL, Lerman J. Controversies in pediatric anesthesia: Sevoflurane and fluid management. Curr Opin Anaesthesiol. 2013;26:310–317. doi: 10.1097/ACO.0b013e328360e94f. [DOI] [PubMed] [Google Scholar]
- 13.Ando N, Sugasawa Y, Inoue R, Aosaki T, Miura M, Nishimura K. Effects of the volatile anesthetic sevoflurane on tonic GABA currents in the mouse striatum during postnatal development. Eur J Neurosci. 2014;40:3147–3157. doi: 10.1111/ejn.12691. [DOI] [PubMed] [Google Scholar]
- 14.Ishizeki J, Nishikawa K, Kubo K, Saito S, Goto F. Amnestic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology. 2008;108:447–456. doi: 10.1097/ALN.0b013e318164cfba. [DOI] [PubMed] [Google Scholar]
- 15.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16:79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcaggi P. Cerebellar endocannabinoids: Retrograde signaling from purkinje cells. Cerebellum. 2015;14:341–353. doi: 10.1007/s12311-014-0629-5. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela CF, Jotty K. Mini-review: Effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex-recent advances. Cerebellum. 2015;14:438–446. doi: 10.1007/s12311-014-0639-3. [DOI] [PubMed] [Google Scholar]
- 18.Barmack NH, Yakhnitsa V. Topsy turvy: Functions of climbing and mossy fibers in the vestibulo-cerebellum. Neuroscientist. 2011;17:221–236. doi: 10.1177/1073858410380251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney ER, Kemper TL, Rosene DL, Bauman ML, Blatt GJ. Density of cerebellar basket and stellate cells in autism: Evidence for a late developmental loss of Purkinje cells. J Neurosci Res. 2009;87:2245–2254. doi: 10.1002/jnr.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Zhao SN, Zhao GY, Sun L, Chu CP, Qiu DL. N-methyl-d-aspartate inhibits cerebellar Purkinje cell activity via the excitation of molecular layer interneurons under in vivo conditions in mice. Brain Res. 2014;1560:1–9. doi: 10.1016/j.brainres.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Lennon W, Hecht-Nielsen R, Yamazaki T. A spiking network model of cerebellar Purkinje cells and molecular layer interneurons exhibiting irregular firing. Front Comput Neurosci. 2014;8:157. doi: 10.3389/fncom.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka S, Kawaguchi SY, Shioi G, Hirano T. Long-term potentiation of inhibitory synaptic transmission onto cerebellar Purkinje neurons contributes to adaptation of vestibulo-ocular reflex. J Neurosci. 2013;33:17209–17220. doi: 10.1523/JNEUROSCI.0793-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gmaz JM, McKay BE. Toluene decreases Purkinje cell output by enhancing inhibitory synaptic transmission in the cerebellar cortex. Neurosci Lett. 2014;560:1–6. doi: 10.1016/j.neulet.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Ono Y, Saitow F, Konishi S. Differential modulation of GABAA receptors underlies postsynaptic depolarization- and purinoceptor-mediated enhancement of cerebellar inhibitory transmission: A non-stationary fluctuation analysis study. PLoS One. 2016;11:e0150636. doi: 10.1371/journal.pone.0150636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batchelor PE, Skeers P, Antonic A, Wills TE, Howells DW, Macleod MR, Sena ES. Systematic review and meta-analysis of therapeutic hypothermia in animal models of spinal cord injury. PLoS One. 2013;8:e71317. doi: 10.1371/journal.pone.0071317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimolin V, Galli M. Summary measures for clinical gait analysis: A literature review. Gait Posture. 2014;39:1005–1010. doi: 10.1016/j.gaitpost.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund's adjuvant-induced arthritis using the CatWalk system. BMC Musculoskelet Disord. 2013;14:14. doi: 10.1186/1471-2474-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozkurt A, Scheffel J, Brook GA, Joosten EA, Suschek CV, O'Dey DM, Pallua N, Deumens R. Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and CatWalk gait analysis. Behav Brain Res. 2011;219:55–62. doi: 10.1016/j.bbr.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Stojanovic T, Capo I, Aronica E, Adle-Biassette H, Höger H, Sieghart W, Kovacs GG, Milenkovic I. The α1, α2, α3, and γ2 subunits of GABAA receptors show characteristic spatial and temporal expression patterns in rhombencephalic structures during normal human brain development. J Comp Neurol. 2016;524:1805–1824. doi: 10.1002/cne.23923. [DOI] [PubMed] [Google Scholar]
- 32.Li ZX, Yu HM, Jiang KW. Tonic GABA inhibition in hippocampal dentate granule cells: Its regulation and function in temporal lobe epilepsies. Acta Physiol (Oxf) 2013;209:199–211. doi: 10.1111/apha.12148. [DOI] [PubMed] [Google Scholar]
- 33.Groundwater PW, Hamid K, Ng I, Tallapragada VJ, Hibbs DE, Hanrahan J. The differential effects of resveratrol and trans-ε-viniferin on the GABA-induced current in GABAA receptor subtypes expressed in xenopus laevis oocytes. J Pharm Pharm Sci. 2015;18:328–338. doi: 10.18433/J3QW3K. [DOI] [PubMed] [Google Scholar]
- 34.Chou WH, Wang D, McMahon T, Qi ZH, Song M, Zhang C, Shokat KM, Messing RO. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci. 2010;30:13955–13965. doi: 10.1523/JNEUROSCI.0270-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisinger BE, Zhao C, Driessen TM, Saul MC, Gammie SC. Large scale expression changes of genes related to neuronal signaling and developmental processes found in lateral septum of postpartum outbred mice. PLoS One. 2013;8:e63824. doi: 10.1371/journal.pone.0063824. [DOI] [PMC free article] [PubMed] [Google Scholar]