Abstract
Fibroblast growth factor-21 (FGF-21) is a pleiotropic protein predominantly secreted in the liver, adipose tissue and pancreas. It has been reported that the metabolic hormone effects of FGF-21 on energy metabolism are essential for human vascular endothelial cells. The aim of the present study was to investigate the therapeutic effects and the underlying primary mechanism of FGF-21 on atherosclerosis in a rat model induced by vitamin D3 and a high fat diet. The rats with atherosclerosis were randomly divided into vehicle (PBS; negative control), FGF-21 (6 mg/kg/d) and atorvastatin (6 mg/kg/d; positive control) groups (n=40 in each group). The rats with atherosclerosis received continuous drug or PBS administration via intravenous injection for a treatment period of 30 days, following which all animals were sacrificed. The expression levels of FGF-21 were determined prior to and following treatment with the drug or PBS. Alterations in ultrastructure and histopathology in vascular endothelial cells were examined, and the expression of nuclear transcription factor kappa B (NF-κB) and levels of blood lipids in the thoracic aorta tissues were also determined. The results showed that typical atheromatous plaques formed, and the mRNA and protein expression levels of FGF-21 were lower in the vascular endothelial cells of the rats with atherosclerosis, compared with the normal rats. FGF-21 significantly reduced blood lipids and glucose in the rats with atherosclerosis, compared with those in the PBS and atorvastatin groups (P<0.01). The expression levels of Rho kinase and NF-κB were significantly lower in the FGF-21 group, compared with the normal control group (P<0.01). Statistically significant differences were found in atheromatous plaques and inflammatory factors in the FGF-21 group, compared with the PBS and atorvastatin groups (P<0.01). In conclusion, FGF-21 significantly downregulated the levels of blood lipids, Rho kinase and NF-κB, which contributed to atherosclerosis therapy in the model rats and indicated the potential mechanisms against atherosclerosis in the model rats.
Keywords: fibroblast growth factor-21, atherosclerosis, nuclear factor-ҝB pathway, blood lipids
Introduction
Cardiovascular disease is the most common cause of mortality worldwide, with >730,000,000 cases of coronary heart disease-associated mortality reported by the World Health Organization in 2016 (1). Clinical studies have shown that morbidity and mortality rates of cardiovascular disease in developing countries are increasing and are estimated to reach 2,330,000,000 by 2030 (2). Atherosclerosis is one of the most common causes of cardiovascular disease, which is characterized by subsequent myocardial ischemia, insufficient blood supply to the coronary arteries and hypoxia (3,4). Atherosclerosis characterized by lipid deposition is the most common and important type of arteriosclerosis in the intima of the involved artery, proliferation of fibrous tissue, calcium deposition, lesions in the middle layer of the artery and accumulation of complex carbohydrates (5). Clinically, coronary heart disease is a fundamental pathological phenotype of atherosclerosis (6,7). Despite an increasing number of drugs devoted to the treatment of atherosclerosis, the rates of atherosclerosis-associated morbidity and mortality remain at high levels (8). Therefore, the prevention and treatment of cardiovascular disease is a problem requiring urgent attention in order to improve the quality of life of patients with cardiovascular disease (9,10).
Fibroblast growth factor-21 (FGF-21) is an atypical member of the FGF family, and is a multifunctional protein predominantly secreted by adipose tissue, the pancreas and the liver (11,12). FGF-21 is predominantly identified as a momentous controller and regulator of glucose and lipid metabolism, and of long-term energy balance (13,14). FGF-21 has been found to be associated with various human diseases and metabolic syndromes, including ageing, obesity, type 2 diabetes mellitus and congenital hypothyroidism (15–17). A previous study showed that FGF-21 resulted in insulin resistance by inhibiting the activation of nuclear factor (NF)-κB (18). FGF-21 also functions as an endocrine hormone by inhibiting somatic growth leading to growth hormone resistance (19). FGF-21 has been reported to be associated with lipid metabolism and the incidence of cardiovascular disease (20). However, the mechanisms underlying the association of FGF-21 and cardiovascular disease remain to be fully elucidated.
In previous years, FGF-21 has been developed as a promising metabolic regulator and a potential drug for various human diseases, including ageing, type 2 diabetes and cardiovascular disease (21,22). Although the role of FGF-21 in metabolic regulation has been partially elucidated, the specific signaling pathways have not been reported or discussed in cardiovascular disease. A previous study showed that FGF-21 is beneficial for the regulation of inflammatory factors. Coincidentally, inflammatory aggravation is found in vessels in the progress of atherosclerosis, which is associated with blood lipid disorder. FGF-21 can regulate insulin phosphate metabolism and repair endothelial cells, which may contribute to recovery in cardiovascular disease. Endothelial dysfunction accounts for the most characteristic marker in patients with atherosclerosis, which is caused by oxidative stress. Therefore, FGF-21 may be associated with atherosclerosis.
The present study investigated the therapeutic effects and primary mechanism of FGF-21 on a rat model of atherosclerosis induced by vitamin D3 and a high-fat diet. The function of FGF-21 on the formation of neointimal cells and endothelial-dependent relaxation was also investigated, and the protein expression levels of Forkhead box O (FOXO) in animals in the atherosclerosis model treated with different doses of FGF-21 were examined. The resulting data indicated that FGF-21 significantly downregulated levels of blood lipids, Rho kinase and NF-κB, which contributed to atherosclerosis therapy and may be the mechanisms underlying anti-atherosclerotic effects in the model rats.
Materials and methods
Ethical approval
The present study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Affiliated Hospital of Nanjing University Medical School (Nanjing, China). All experimental protocols and animals were performed in accordance with National Institutes of Health and approved by the Committee on the Ethics of Animal Experiments Defence Research of Affiliated Hospital of Nanjing University Medical School (Nanjing, China) (23). All surgery and sacrifice were performed in a manner to minimize animal suffering.
Experimental animals
A total of 120 female Wistar rats (6–8 weeks old; 190±20 g) were purchased from Slack Experimental Animals Co., Ltd. (Shanghai, China). All animals were housed under pathogen-free conditions (license no. SCXK-2008-014). All rats were raised on a 12-h light/dark cycle. The animals had free access to water and the temperature was 20±2°C with a humidity of 50±5%. All rats were provided with a high-fat diet and intraperitoneal injections of vitamin D3 (160,000 U/kg) once a month for a total of five months, following a 7-day acclimatization period. The rats only received the high-fat diet in the next 2 months. The rats were randomly divided into three groups, in which animals received intravenous treatment with PBS, FGF-21 (6 mg/kg/d) or atorvastatin (4.8 mg/kg/d), respectively, once every 3 days. The rats were starved and sacrificed on day 30.
Tissue preparation
On day 30, the rats were sacrificed by intraperitoneal injection of 5% urethane (1,000 mg/kg). The abdominal aorta, full-length aorta and peripheral blood were obtained in the experimental rats. The tissues and serum samples were collected and stored to further analysis. Serum samples were obtained by centrifugation at 1,500 × g and 4°C for 15 min. The serum levels of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride and total cholesterol were measured according to the methods described in a previous study (24). The tissues were homogenized using electrically-driven tissue homogenizer (ABI, Grand Island, NY, USA) and prepared for reverse transcription-quantitative polymerase chain reaction (RT-qPCR), immunofluorescence and western blot analyses.
RT-qPCR analysis
Total RNA was obtained from on endothelial cells of the experimental rats using an RNAeasy Mini kit (Qiagen, Inc., Gaithersburg, MD, USA). The expression levels of FGF-21, chemokine (C-C motif) ligand (Ccl)2, Ccl5, intercellular adhesion molecule 1 (Icam1) and tumor necrosis factor (TNF)α in the cells were measured using β-actin as an endogenous control (25) (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The forward and reverse primers were synthesized by Invitrogen; Thermo Fisher Scientific, Inc. PCR amplification was preliminary denaturation at 94°C for 2 min, followed by 45 cycles of 95°C for 30 sec, annealing temperature reduced to 58°C for 30 sec, and 72°C for 10 min using a volume of 20 µl containing 50 ng of genomic DNA, 200 µM dNTP, 2.5 units of Taq DNA polymerase and 200 µM primers (Table I). The relative changes in mRNA expression were calculated using the 2−ΔΔCq method (25). The results are expressed as the n-fold change compared with the control.
Table I.
Sequences of primers used.
| Sequence | ||
|---|---|---|
| Gene name | Reverse | Forward |
| Ccl2 | 5′-GATCTCAGTGCAGAGGCTCG-3′ | 5′-TGCTTGTCCAGGTGGTCCAT-3′ |
| Ccl5 | 5′-GTGAGGAACAAGCCAGAG-3′ | 5′-TGACCAGAAGAAGGAATGC-3′ |
| Icam1 | 5′-GGAACCCATTGCCCGAGC-3′ | 5′-GGTGAGGATTGCATTAGGTC-3′ |
| TNFα | 5′-TCCAGACTTCCTTGAGACA-3′ | 5′-GGCGATTACAGACACAACT-3′ |
| β-actin | 5′-GTTGGTCTACCGGGACTCAA-3′ | 5′-CTGAACCCTAAGGCCAACCG-3′ |
Ccl, chemokine (C-C motif) ligand; Icam, intercellular adhesion molecule; TNF, tumor necrosis factor.
Western blot analysis
The endothelial cells in the experimental rats were homogenized in lysate buffer containing protease-inhibitor and were centrifuged at 6,000 × g at 4°C for 10 min. The supernatant of the mixture was used for analysis of the proteins of interest. For the detection of purpose protein, transmembrane proteins were extracted using a Transmembrane Protein Extraction kit (Qiagen, Inc.) according to the manufacturer's protocol. SDS assays were performed as previously described (26). For western blot analysis, primary antibodies: p65 (ab16502; 1:1,000; Abcam, Cambridge, UK), NF-κB (ab97726; 1:1,000; Abcam) were added following blocking in 5% skimmed milk for 1 h at 37°C. This was followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (1721019; 1:5,000; Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 24 h at 4°C. The results were visualized by using a chemiluminescence detection system (1705060; Bio-Rad Laboratories, Inc.).
Histological examination
Tissues were fixed for 48 h, dehydrated through a graded series of ethanol, embedded in paraffin wax, and cut into 4 µm tissue sections. The sections were stained with hematoxylin and eosin (H&E) and observed under a light microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan).
Immunofluorescence
The endothelial cells in the experimental rats were incubated with primary antibodies, followed by appropriate secondary antibodies: pro-inflammatory M1 (ab110273; 1:2,000; Abcam) and anti-inflammatory M2 (ab110274; 1:2,000; Abcam) were added as targets for primary antibodies for the immobilized cells for 12 h at 4°C. The horseradish peroxidase-conjugated anti-rabbit IgG (1721019; 1:5,000; Bio-Rad Laboratories, Inc.) was incubated with the endothelial cells following washing with PBS for 2 h at 37°C. In addition, the localization and levels of NF-κB were analyzed using fluorescein isothiocyanate secondary Ab and nuclear counterstaining (DAPI) using a fluorescence microscope (Leica DMi8; Leica Microsystems GmbH, Wetzlar, Germany) (27).
Oxidative stress
Intracellular superoxide in arterial tissues was analyzed using fluorescence microscopy combined with expressed as number of DHE-positive cells (DAPI staining) and superoxide-sensitive fluorescent dye dihydroethidium (2 mmol/l, DHE; Invitrogen; Thermo Fisher Scientific, Inc.). The activity of NADPH-dependent oxidase in the endothelial cell homogenates was measured using chemiluminescence by NADPH (100 mmol/l) and lucigenin (5 mmol/l).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical tests for data analysis included Fisher's exact test, log-rank test, χ2 test, and Student's two-tailed t-test. Multivariate statistical analysis was performed using a Cox regression model. Statistical analyses were performed using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Differences in the expression of FGF-21, blood glucose, blood lipids and body weight in rats with atherosclerosis
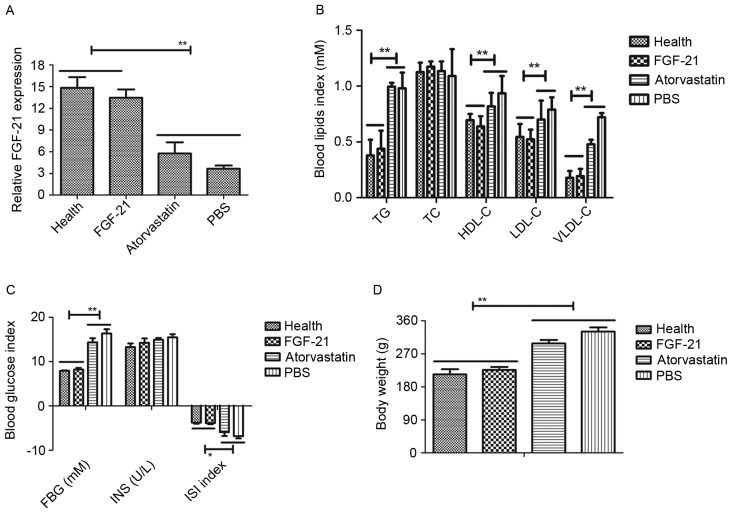
In order to examine the role of FGF-21 on atherosclerosis, the present study first investigated the expression level of FGF-21 in the peripheral blood of atherosclerosis rats, with healthy rats as a control. The results, as presented in Fig. 1A, showed that the expression levels of FGF-21 were downregulated in the rats with atherosclerosis, compared with the healthy rats. However, FGF-21 therapy improved recovery to normal levels of FGF-21. The present study also examined the blood glucose and blood lipids levels to determine the therapeutic effects of FGF-21 on atherosclerosis. As presented in Fig. 1B and C, blood glucose and blood lipids were increased in rats with atherosclerosis, compared with the rats administered with PBS or atorvastatin. The therapeutic effects of FGF-21 on body weight on the rats with atherosclerosis were also examined. The data indicated that FGF-21 inhibited the increase in body weight, compared with PBS or atorvastatin (Fig. 1D). Collectively, these data suggested that FGF-21 was downregulated in atherosclerosis, and that the restoration of FGF-21 had beneficial effects on blood glucose, blood lipids and body weight in the rats with atherosclerosis.
Figure 1.
Expression and function of FGF-21 in a rat model of atherosclerosis. (A) mRNA expression level of FGF-21 in rats with atherosclerosis and healthy rats. (B) Analysis of the efficacy of FGF-21 on blood lipids in rats with atherosclerosis. (C) Analysis of the efficacy of FGF-21 on blood glucose in rats with atherosclerosis. (D) FGF-21 inhibited the increase in body weight in the rats with atherosclerosis. **P<0.01 and *P<0.05 for intergroup analysis (n=3). FGF-21, fibroblast growth factor-21; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; FBG, fasting blood glucose; INS, insulin; ISI, international sensitivity index.
Effects of FGF-21 treatment on endothelial cell ultrastructure, endothelial-dependent relaxation and balloon-induced neointimal formation
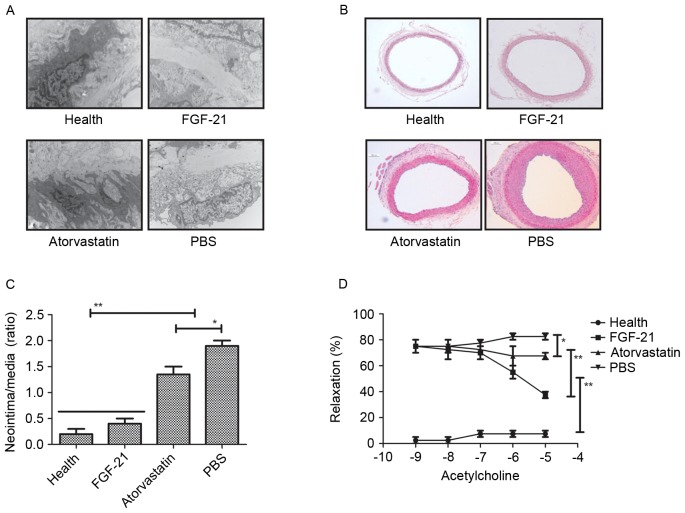
To analyze the effect of FGF-21 on the endothelial cells in the experimental rat, H&E staining was used to observe alteration in the endothelial cells. It was found that FGF-21 treatment markedly altered the ultrastructure of the endothelial cells in the aorta in the rats with atherosclerosis, compared with the rats administered with PBS or atorvastatin (Fig. 2A). In addition, the formation of neointima was analyzed in the carotid arteries of rats with atherosclerosis. The results revealed that FGF-21 significantly improved neointimal formation, and that the neointimal formation was higher, compared with that in either the PBS or atorvastatin-treated groups. (Fig. 2B and C). The present study also examined the effects of FGF-21 on endothelial-dependent relaxation in the experimental rats to confirm the improvement of vessel function. As shown in Fig. 2D, the results revealed that endothelial-dependent relaxation was increased in the injured carotid arteries of the rats with atherosclerosis. Taken together, the therapeutic effects of FGF-21 provided an advantage in the treatment of rats with atherosclerosis.
Figure 2.
Efficacy of FGF-21 on relaxation and neointimal formation in endothelial cells. (A) Changes in ultrastructure of endothelial cells of the aorta following FGF-21 treatment in rats with atherosclerosis. Magnification, ×400. (B) Morphological improvement of neointimal formation in FGF-21-treated endothelial cells of the aorta of rats with atherosclerosis. Magnification, ×400. (C) Increasing numbers of neointimal formation in endothelial cells of the aorta in rats with atherosclerosis following FGF-21 treatment. (D) FGF-21 improved the endothelial-dependent relaxation of injured carotid arteries in rats with atherosclerosis. **P<0.01 and *P<0.05 for intergroup analysis (n=3). FGF-21, fibroblast growth factor-21.
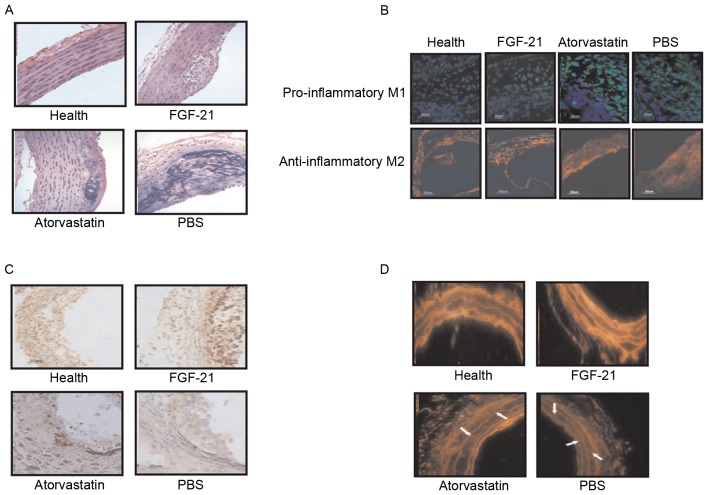
Effects of FGF-21 treatment on histopathological changes in atherosclerosis in rats
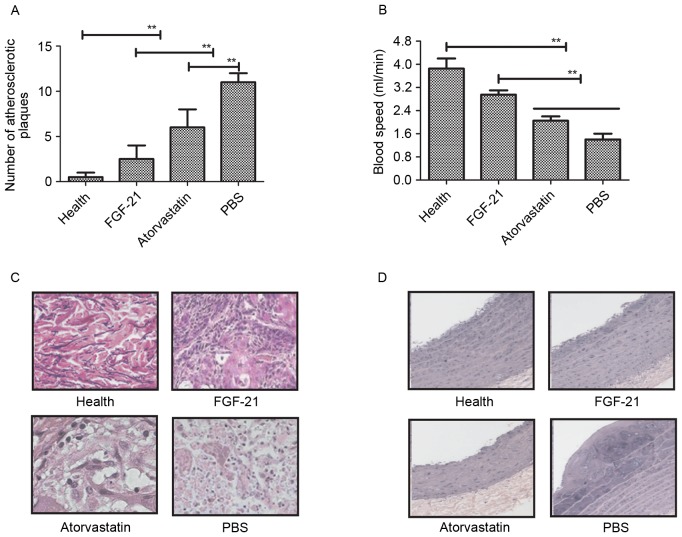
In order to identify the beneficial effects of FGF-21 on blood vessels, histopathologal changes were detected using H&E and immunohistochemical staining in the rat model of atherosclerosis. The data showed that the unit area of atherosclerotic plaques was significantly lower, compared with the model and atorvastatin groups (Fig. 3A). The blood velocity increased following FGF-21 treatment (Fig. 3B) and, as shown in Fig. 3C, adipocytes were decreased following FGF-21 treatment. There were marked pathological changes to the normal functioning in the aortas of the rats with atherosclerosis following FGF-21 treatment, compared with PBS and atorvastatin (Fig. 3D). Taken together, these findings suggested that FGF-21 treatment led to a visible decrease in pathological changes, including the vessel walls and atherosclerotic plaques, in rats with atherosclerosis.
Figure 3.
Histopathological changes in endothelial cells of the aorta in rats with atherosclerosis following FGF-21 treatment. (A) FGF-21 treatment decreased the number of atherosclerotic plaques in the endothelial cells of the aorta in rats with atherosclerosis. (B) FGF-21 treatment improved blood velocity in rats with atherosclerosis. (C) FGF-21 treatment decreased adipocytes in the endothelial cells of the aorta in rats with atherosclerosis. Magnification, ×400. (D) FGF-21 treatment improved the pathologic morphology of endothelial cells of the aorta in rats with atherosclerosis. Magnification, ×400. **P<0.01 for intergroup analysis (n=3). FGF-21, fibroblast growth factor-21.
Effects of FGF-21 treatment on atherosclerosis via the NF-κB signaling pathway
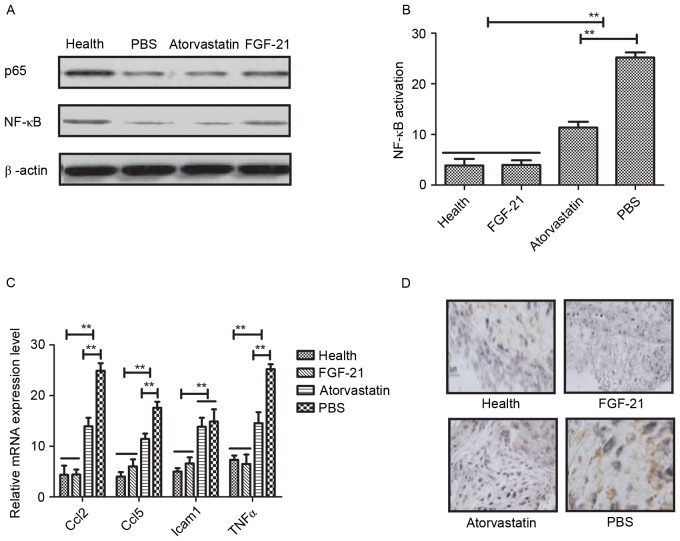
NF-κB is a crucial regulator in inflammatory responses, which controls the expression of several genes involved in atherogenesis (28). In the present study, the associations between FGF-21 and the NF-κB signaling pathway were analyzed in rats with atherosclerosis. It was observed that FGF-21 inhibited the nuclear import of activated NF-κB (p65) in the vascular endothelial cells of the experimental rats (Fig. 4A). The data indicated that FGF-21 inhibited the activation of NF-κB in vascular cells isolated from rats with atherosclerosis (Fig. 4B). In addition, it was found that FGF-21 treatment decreased the expression levels of anti-inflammatory genes involved in the NF-κB signaling pathway, including Ccl2, Ccl5, Icam1 and TNFα, determined using RT-qPCR analysis (Fig. 4C). The potential benefits of FGF-21 were also examined, which revealed marked NF-κB inactivation in the FGF-21-treated rats (Fig. 4D). Taken together, it was concluded that FGF-21 improved atherosclerosis via the NF-κB signaling pathway.
Figure 4.
FGF-21 mediated improvement of atherosclerosis via the NF-κB signaling pathway. (A) FGF-21 inhibited the nuclear import of activated NF-κB in vascular endothelial cells from experimental rats. (B) FGF-21 inhibited the activation of NF-κB in vascular cells isolated from rats with atherosclerosis. (C) FGF-21 treatment decreased the expression levels of anti-inflammatory genes involved in the NF-κB signaling pathway. (D) Inactivation of NF-κB was observed in FGF-21-treated rats. **P<0.01 for intergroup analysis (n=3). Magnification, ×400. FGF-21, fibroblast growth factor-21; NF-κB, nuclear factor-ҝB; Ccl; chemokine (C-C motif) ligand; Icam1, intercellular adhesion molecule 1; TNFα, tumor necrosis factor α.
FGF-21 treatment decreases oxidative stress and atherosclerotic plaques in rats with atherosclerosis
Further confirming the effects of FGF-21 treatment on rats with atherosclerosis, the data obtained showed that lesion size was reduced by FGF-21 treatment, in addition to a significant decrease in the number of macrophages within the atherosclerotic plaques (Fig. 5A). Regression analysis revealed a correlation of macrophage content with lesion area (r=0.724; P=0.0042) and NF-κB staining (r=0.746; P=0.0031) in the experimental model, indicating a higher inflammatory component in larger lesions. It was also observed that pro-inflammatory M1 and anti-inflammatory M2 were increased in the plaques of the FGF-21-treated rats (Fig. 5B). The results of immunofluorescence demonstrated a higher relative collagen and vascular smooth muscle cell (VSMC) content in the aortic lesions of the FGF-21-treated rats, compared with the PBS and atorvastatin groups (Fig. 5C). Furthermore, superoxide generation in the aortic root region of the experimental animals was analyzed using DHE staining. As demonstrated in Fig. 5D, FGF-21 treatment reduced the number of DHE-labeled nuclei in the plaques, suggesting that FGF-21 treatment inhibited oxidative stress through the NF-κB signaling pathway. Taken together, these data suggested that FGF-21 mediated inflammation and oxidative stress through the NF-κB signaling pathway in rats with atherosclerosis, which may be a potential drug candidate for the treatment of atherosclerosis.
Figure 5.
FGF-21 treatment decreases oxidative stress and atherosclerotic plaques in rats with atherosclerosis. (A) FGF-21 treatment decreased the number of macrophages within the atherosclerotic plaques. (B) Levels of pro-inflammatory M1 and anti-inflammatory M2 were increased in plaques of FGF-21-treated rats. (C) FGF-21 treatment increased relative collagen and VSMC content. (D) FGF-21 treatment reduced the number of DHE-labeled nuclei in plaques in the vascular endothelial cells from experimental rats. Arrow indicates thrombus layer. Magnification, ×400. FGF-21, fibroblast growth factor-21.
Discussion
Atherosclerosis is one of the leading causes of cardiovascular disease-associated mortality worldwide, and is characterized as a chronic and multifactorial disease occurring in the arterial wall (29,30). A previous study indicated that atherosclerotic plaques are the most important clinical features, characterized by leukocyte infiltration and lipid accumulation (6). The continuous inflammation in the artery leads to atherosclerotic plaques and thrombus formation, which further exacerbates the disease and the difficulty of treatment (31). Leukocyte infiltration and lipid accumulation have been reported to contribute to different process in plaque formation in the development of atherosclerosis through a serious inflammatory responses and metabolic disorders (32). Therefore, controlling leukocyte infiltration and lipid accumulation may be beneficial for recovery in patients with atherosclerosis. In the present study, data indicated that lower levels of leukocyte infiltration and lipid accumulation were observed in FGF-21-treated rats with atherosclerosis, resulting in improvement through a decrease in the number of atherosclerotic plaques.
FGF-21 has been demonstrated to be associated with glucose metabolism, lipid metabolism, hyperglycemia and thermogenesis, which are performed in a hormone-like manner (33). The upstream and downstream signaling pathways of FGF-21 remain to be fully elucidated and are the focus of numerous investigations. In the present study, the therapeutic effects of FGF-21 on atherosclerosis were investigated in a rat model of atherosclerosis. Previously, the association between FGF-21 concentration and restoration has been reported to be associated with insulin sensitivity and metabolic syndrome, and that FGF-21 injection presents a potential multifunctional regulator in improving insulin resistance and obesity-associated metabolic disorders (34). A number of reports on FGF-21 have focused on its efficacy in diagnosing and treating type 2 diabetes (34,35). Fewer reports have described the association between FGF-21 and atherosclerosis in preclinical and clinical studies. It is known that the expression of FGF-21 is significantly upregulated when cells are in a state of fasting/starvation, and regulates glucose and lipid metabolism (36). The hormone-like FGF-21 is involved in the regulation of diverse metabolic pathways, including the NF-κB signaling pathway. In the present study, FGF-21 was shown to regulate leukocyte infiltration and lipid accumulation via the NF-κB signaling pathway in rats with atherosclerosis.
The interaction between NF-κB and atherosclerosis in vascular inflammation and atherosclerosis has been investigated and discussed in previous studies (37,38). The activation of NF-κB has been detected in human atherosclerotic plaques, and the modulation of NF-κB inflammatory activity has been shown to limit disease progression in mice (39). NF-κB is a major regulator of inflammation, which controls the expression of several functional genes involved in atherogenesis (40). In addition, several cellular assembly and molecular inflammatory responses are involved in the progress of atherosclerosis, including the release of pro-inflammatory and inflammatory cytokines, dysfunction of VSMCs, and activation of leukocyte infiltration and migration (41). The NF-κB signaling pathway is also a crucial factor regulating the expression of genes in different processes of atherosclerotic formation, including lipid modification, adhesion of leukocytes, monocyte differentiation and VSMC proliferation (42). NF-κB is also vital in the initiation and development of the atherosclerotic process through mediating pro-inflammatory transcription factors in endothelial cells in the artery (43). The aberrant activation of NF-κB is observed in the majority of human cardiovascular diseases (44,45). Evidence also suggests that poor survival rates and outcomes of the atherosclerotic process are associated with aberrant activation of the NF-κB signaling pathway (46,47).
In conclusion, the findings of the present study suggested that FGF-21 improved and maintained the morphology of the vascular endothelium in rats with atherosclerosis by activating the NF-κB signaling pathway. Data showed that FGF-21 treatment downregulated the levels of inflammatory factors and inhibited oxidative stress. It was also demonstrated that FGF-21 inhibited the nuclear importing of activated NF-κB (p65) in vascular endothelial cells and downregulated the levels of physiological NF-κB to protect the vascular endothelium in the experimental rats, which may offer potential as an alternative for the treatment of atherosclerosis.
References
- 1.Tarugi P, Averna M, Di Leo E, Cefalù AB, Noto D, Magnolo L, Cattin L, Bertolini S, Calandra S. Corrigendum to ‘Molecular diagnosis of hypobetalipoproteinemia: An ENID review’ [Atherosclerosis 195 (2) (2007) 19–27] Atherosclerosis. 2016;253:e1. doi: 10.1016/j.atherosclerosis.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Rapid progression of coronary atherosclerosis: A review. Thrombosis. 2015;2015:634983. doi: 10.1155/2015/634983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen ME, Singh D, Menon BK, Frayne R. A systematic literature review of the effect of carotid atherosclerosis on local vessel stiffness and elasticity. Atherosclerosis. 2015;243:211–222. doi: 10.1016/j.atherosclerosis.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Kousios A, Kouis P, Panayiotou AG. Matrix metalloproteinases and subclinical atherosclerosis in chronic kidney disease: A systematic review. Int J Nephrol. 2016;2016:9498013. doi: 10.1155/2016/9498013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayyappan Plakkal J, Paul A, Goo YH. Lipid droplet-associated proteins in atherosclerosis (Review) Mol Med Rep. 2016;13:4527–4534. doi: 10.3892/mmr.2016.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gholami S, Salavati A, Houshmand S, Werner TJ, Alavi A. Assessment of atherosclerosis in large vessel walls: A comprehensive review of FDG-PET/CT image acquisition protocols and methods for uptake quantification. J Nucl Cardiol. 2015;22:468–479. doi: 10.1007/s12350-015-0069-8. [DOI] [PubMed] [Google Scholar]
- 7.Crickx E, Saussine A, Vignon-Pennamen MD, Cordoliani F, Mouly F, Bagot M, Rybojad M. Diffuse dermal angiomatosis associated with severe atherosclerosis: Two cases and review of the literature. Clin Exp Dermatol. 2015;40:521–524. doi: 10.1111/ced.12565. [DOI] [PubMed] [Google Scholar]
- 8.Merashli M, Ster IC, Ames PR. Subclinical atherosclerosis in Behcet's disease: A systematic review and meta-analysis. Semin Arthritis Rheum. 2016;45:502–510. doi: 10.1016/j.semarthrit.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Shah NR, Mahmoudi M. The role of DNA damage and repair in atherosclerosis: A review. J Mol Cell Cardiol. 2015;86:147–157. doi: 10.1016/j.yjmcc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Olubamwo OO, Onyeka IN, Miettola J, Kauhanen J, Tuomainen TP. Hepatitis C as a risk factor for carotid atherosclerosis-a systematic review. Clin Physiol Funct Imaging. 2016;36:249–260. doi: 10.1111/cpf.12229. [DOI] [PubMed] [Google Scholar]
- 11.Eto K. FGF-21, a newcomer in the field of hypertension research. J Hum Hypertens. 2013;27:343–344. doi: 10.1038/jhh.2012.68. [DOI] [PubMed] [Google Scholar]
- 12.Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143–2150. doi: 10.1210/jc.2012-1221. [DOI] [PubMed] [Google Scholar]
- 13.Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK, Tyni T, Kiuru-Enari S, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5:e15534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren G, Yin J, Wang W, Li L, Li D. Fibroblast growth factor (FGF)-21 signals through both FGF receptor-1 and 2. Sci China Life Sci. 2010;53:1000–1008. doi: 10.1007/s11427-010-4035-z. [DOI] [PubMed] [Google Scholar]
- 16.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 17.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehi MH, Kamalidehghan B, Houshmand M, Aryani O, Sadeghizadeh M, Mossalaeie MM. Association of fibroblast growth factor (FGF-21) as a biomarker with primary mitochondrial disorders, but not with secondary mitochondrial disorders (Friedreich Ataxia) Mol Biol Rep. 2013;40:6495–6499. doi: 10.1007/s11033-013-2767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gahete MD, Córdoba-Chacón J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154:263–269. doi: 10.1210/en.2012-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu D, Sun CY, Sun GP, Ren GP, Ye XL, Zhu SL, Wang WF, Xu PF, Li SJ, Wu Q, et al. The synergistic effect of FGF-21 and insulin on regulating glucose metabolism and its mechanism. Yao Xue Xue Bao. 2014;49:977–984. (In Chinese) [PubMed] [Google Scholar]
- 21.Matuszek B, Lenart-Lipińska M, Duma D, Solski J, Nowakowski A. Evaluation of concentrations of FGF-21-a new adipocytokine in type 2 diabetes. Endokrynol Pol. 2010;61:50–54. [PubMed] [Google Scholar]
- 22.Yu Y, Bai F, Wang W, Liu Y, Yuan Q, Qu S, Zhang T, Tian G, Li S, Li D, Ren G. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav. 2015;133:122–131. doi: 10.1016/j.pbb.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 23.In: Guidance for the Description of Animal Research in Scientific Publications. Washington, DC: 2011. [PubMed] [Google Scholar]
- 24.Sartang Mohammadi M, Mazloomi SM, Tanideh N, Zadeh Rezaian A. The effects of probiotic soymilk fortified with omega-3 on blood glucose, lipid profile, haematological and oxidative stress and inflammatory parameters in streptozotocin nicotinamide-induced diabetic rats. J Diabetes Res. 2015;2015:696372. doi: 10.1155/2015/696372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao S, Wang J, Xiao N. MicroRNAs as noninvasive biomarkers in bladder cancer detection: A diagnostic meta-analysis based on qRT-PCR data. Int J Biol Markers. 2016;31:e276–e285. doi: 10.5301/jbm.5000199. [DOI] [PubMed] [Google Scholar]
- 26.Wai-Hoe L, Wing-Seng L, Ismail Z, Lay-Harn G. SDS-PAGE-based quantitative assay for screening of kidney stone disease. Biol Proced Online. 2009;11:145–160. doi: 10.1007/s12575-009-9007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahav G, Hirshberg A, Salomon O, Amariglio N, Trakhtenbrot L, Fixler D. Fluorescence lifetime imaging of DAPI-stained nuclei as a novel diagnostic tool for the detection and classification of B-cell chronic lymphocytic leukemia. Cytometry A. 2016;89:644–652. doi: 10.1002/cyto.a.22890. [DOI] [PubMed] [Google Scholar]
- 28.Mallavia B, Recio C, Oguiza A, Ortiz-Muñoz G, Lazaro I, Lopez-Parra V, Lopez-Franco O, Schindler S, Depping R, Egido J, Gomez-Guerrero C. Peptide inhibitor of NF-κB translocation ameliorates experimental atherosclerosis. Am J Pathol. 2013;182:1910–1921. doi: 10.1016/j.ajpath.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Wong MC, Zhang DX, Wang HH. Rapid emergence of atherosclerosis in Asia: A systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr Opin Lipidol. 2015;26:257–269. doi: 10.1097/MOL.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 30.Singh TP, Vangaveti VN, Malabu UH. Dipeptidyl peptidase-4 inhibitors and their potential role in the management of atherosclerosis-A review. Diabetes Metab Syndr. 2015;9:223–229. doi: 10.1016/j.dsx.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Wu GC, Leng RX, Lu Q, Fan YG, Wang DG, Ye DQ. Subclinical atherosclerosis in patients with inflammatory bowel diseases: A systematic review and meta-analysis. Angiology. 2017;68:447–461. doi: 10.1177/0003319716652031. [DOI] [PubMed] [Google Scholar]
- 32.Cuspidi C, Sala C, Tadic M, Gherbesi E, Grassi G, Mancia G. Nondipping pattern and carotid atherosclerosis: A systematic review and meta-analysis. J Hypertens. 2016;34:385–392. doi: 10.1097/HJH.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 33.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoenphandhu N, Suntornsaratoon P, Krishnamra N, Sa-Nguanmoo P, Tanajak P, Wang X, Liang G, Li X, Jiang C, Chattipakorn N, Chattipakorn S. Fibroblast growth factor-21 restores insulin sensitivity but induces aberrant bone microstructure in obese insulin-resistant rats. J Bone Miner Metab. 2017;35:142–149. doi: 10.1007/s00774-016-0745-z. [DOI] [PubMed] [Google Scholar]
- 35.Fan H, Sun X, Zhang H, Liu J, Zhang P, Xu Y, Pan Q, Wang G. Effect of metformin on fibroblast growth factor-21 levels in patients with newly diagnosed type 2 diabetes. Diabetes Technol Ther. 2016;18:120–126. doi: 10.1089/dia.2015.0261. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Liu J, Yang N, Hu Y, Zhang H, Miao L, Yao Z, Xu Y. Levothyroxine treatment restored the decreased circulating fibroblast growth factor 21 levels in patients with hypothyroidism. Eur J Intern Med. 2016;31:94–98. doi: 10.1016/j.ejim.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Zhao H, Ren X. Estrogen and progestogen inhibit NF-κB in atherosclerotic tissues of ovariectomized ApoE (−/-) mice. Climacteric. 2016;19:357–363. doi: 10.3109/13697137.2016.1167867. [DOI] [PubMed] [Google Scholar]
- 38.Hsueh TP, Sheen JM, Pang JH, Bi KW, Huang CC, Wu HT, Huang ST. The anti-atherosclerotic effect of naringin is associated with reduced expressions of cell adhesion molecules and chemokines through NF-κB Pathway. Molecules. 2016;21 doi: 10.3390/molecules21020195. pii: E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu L, Xu R, Wang S, Li S, Sheng H, Wu J, Qu Y. Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IkappaB/NF-κB, in atherosclerotic cell model. Exp Mol Med. 2015;47:e171. doi: 10.1038/emm.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KM, Choi JY, Yoo SE, Park MY, Lee BS, Ko YH, Sung SH, Shin HM, Park JE. HMCO5, herbal extract, inhibits NF-kappaB expression in lipopolysaccharide treated macrophages and reduces atherosclerotic lesions in cholesterol fed mice. J Ethnopharmacol. 2007;114:316–324. doi: 10.1016/j.jep.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Asare Y, Shagdarsuren E, Schmid JA, Tilstam PV, Grommes J, El Bounkari O, Schütz AK, Weber C, de Winther MP, Noels H, Bernhagen J. Endothelial CSN5 impairs NF-κB activation and monocyte adhesion to endothelial cells and is highly expressed in human atherosclerotic lesions. Thromb Haemost. 2013;110:141–152. doi: 10.1160/TH13-02-0155. [DOI] [PubMed] [Google Scholar]
- 42.Sun R, Xiao L, Duan S. High expression of ubiquitin conjugates and NF-κB in unstable human intracranial atherosclerotic plaques. J Cell Physiol. 2012;227:784–788. doi: 10.1002/jcp.22790. [DOI] [PubMed] [Google Scholar]
- 43.Sigala F, Savvari P, Liontos M, Sigalas P, Pateras IS, Papalampros A, Basdra EK, Kolettas E, Papavassiliou AG, Gorgoulis VG. Increased expression of bFGF is associated with carotid atherosclerotic plaques instability engaging the NF-κB pathway. J Cell Mol Med. 2010;14:2273–2280. doi: 10.1111/j.1582-4934.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan S, Lei L, Chen S, Li H, Yan F. Rosiglitazone impedes Porphyromonas gingivalis-accelerated atherosclerosis by downregulating the TLR/NF-κB signaling pathway in atherosclerotic mice. Int Immunopharmacol. 2014;23:701–708. doi: 10.1016/j.intimp.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Ye X, Jiang X, Guo W, Clark K, Gao Z. Overexpression of NF-κB p65 in macrophages ameliorates atherosclerosis in apoE-knockout mice. Am J Physiol Endocrinol Metab. 2013;305:E1375–E1383. doi: 10.1152/ajpendo.00307.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazaro I, Oguiza A, Recio C, Mallavia B, Madrigal-Matute J, Blanco J, Egido J, Martin-Ventura JL, Gomez-Guerrero C. Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes. 2015;64:3600–3613. doi: 10.2337/db14-1926. [DOI] [PubMed] [Google Scholar]
- 47.Bhat OM, Kumar PU, Giridharan NV, Kaul D, Kumar MJ, Dhawan V. Interleukin-18-induced atherosclerosis involves CD36 and NF-κB crosstalk in Apo E−/− mice. J Cardiol. 2015;66:28–35. doi: 10.1016/j.jjcc.2014.10.012. [DOI] [PubMed] [Google Scholar]