Abstract
Osteoporosis is an aging process of skeletal tissues with characteristics of reductions in bone mass and microarchitectural deterioration of bone tissue. The present study aimed to investigate the effects of glucocorticoid-induced osteoporosis on osteoblasts and to examine the roles of β-ecdysterone (β-Ecd) involved. In the present study, an in vivo model of osteoporosis was established through the subcutaneous implantation of prednisolone (PRED) into Sprague-Dawley rats, with or without a subcutaneous injection of β-Ecd (5 or 10 mg/kg body weight). Expression of Beclin-1 and microtubule-associated protein 1A/1B-light chain 3I/II and apoptosis in lumbar vertebrae tissues was measured by immunofluorescence and TUNEL assays, respectively. Serum concentration of calcium and phosphorus, and the activity of tartrate-resistant acid phosphatase (TRAP) and alkaline phosphatase (ALP) were measured by biochemical assay. Reverse transcription-quantitative polymerase chain reaction and western blotting was used for detect the expression of related genes and proteins. PRED treatment inhibited bone formation by decreasing bone mineral density, and suppressing the expression of Runt-related transcription factor 2 and bone morphogenetic protein 2, while enhancing the activity of alkaline phosphatase, upregulating the expression of receptor activator of nuclear factor-κB ligand, and increasing the serum content of calcium, phosphorus and tartrate-resistant acid phosphatase in rats. Additionally, PRED was revealed to inhibit autophagy through the downregulation of Beclin-1, autophagy protein 5 and microtubule-associated protein 1A/1B-light chain 3I/II expression, whereas it induced the apoptosis, through the activation of caspase-3 and the suppression of apoptosis regulator BCL2 expression. Notably, the PRED-induced alterations in bone formation, autophagy and apoptosis were revealed to be attenuated by β-Ecd administration. In conclusion, the findings of the present study suggested that β-Ecd may be a promising candidate for the development of therapeutic strategies for the treatment of osteoporosis, through the induction of autophagy and the inhibition of apoptosis in vivo.
Keywords: osteoporosis, β-ecdysterone, autophagy, apoptosis
Introduction
Osteoporosis is a silent aging process of skeletal tissues, characterized by reductions in bone mass and the microarchitectural deterioration of bone tissue, that results in enhanced bone fragility and, consequently, in increased risk of fractures (1,2). As the elderly population increases, the number of patients with osteoporosis is on the rise, and the prognosis of osteoporosis treatment using pharmacological agents is frequently affected by the poor bone quality of the elderly (3). Screening for osteoporosis is based on the assessment of bone mineral density (BMD) (4); however, the decrease in skeletal mechanical strength is not only caused by decreases in BMD, but also by alterations in the bone microstructure, which may be evaluated using non-invasive micro-computed tomography (CT) (5,6).
To the best of our knowledge, a number of methods have been employed to establish reliable osteoporosis models (7). Glucocorticoid-induced osteoporosis is the most common form of secondary osteoporosis, which results in reductions in BMD and an increase in fracture risk (8,9). Treatment with glucocorticoids leads to a decrease in the generation of osteoblasts and osteocytes, accompanied by an elongation in the lifespan of osteoclasts (10,11), which in turn increases bone resorption and inhibits the formation of new bone matrix. In the present study, the prednisolone (PRED)-induced osteoporosis model was used to study osteoporosis. Increased apoptosis and decreased autophagy of osteoblasts has been observed following treatment with a high dose of glucocorticoids in mice (12,13). A number of mechanisms have been suggested to be involved in glucocorticoid-induced apoptosis, including the upregulation of the proapoptotic factors apoptosis regulator BCL2 (Bcl-2)-like protein 11 and Bcl-2 homologous antagonist/killer, and the downregulation of the pro-survival factor Bcl-xL (14). Notably, the dosage of glucocorticoid treatment determines the fate of osteocytes: A low dose of glucocorticoids induces autophagy, whereas a high dose induces apoptosis (15).
β-Ecdysterone (β-Ecd) is a naturally-occurring estrogen analog derived from Achyranthes bidentata Blumeand Cyanotis arachnoidea C.B. Clarke, and has been reported to stimulate protein synthesis, to promote carbohydrate and lipid metabolism, to alleviate hyperglycemia and hyperlipidemia, to modulate immune reactions, and to protect endothelial cells from apoptosis and induce their proliferation (16–18). β-Ecd has been revealed to attenuate the 1-methyl-4-phenylpyridinium-induced apoptosis of PC12 cells (19) and to protect SH-SY5Y cells against 6-hydroxydopamine-induced apoptosis, by interfering with p38 mitogen-activated protein kinase-p53 signaling (20). Treatment with β-Ecd additionally prevented the glucocorticoid-induced reduction in bone formation rate, the decrease in trabecular bone volume and cortical bone loss in mice in vivo (9).
The present study aimed to investigate whether β-Ecd was able to rescue the PRED-induced suppression of bone formation in rats in vivo. The present results suggested that treatment with β-Ecd may inhibit bone loss and prevent the deterioration of bone mechanical properties associated with PRED use, possibly through the maintenance of bone formation. In addition, autophagy was examined following treatment with PRED or β-Ecd, and the present findings suggested that autophagy may be among the mechanisms underlying the bone-anabolic effects of β-Ecd.
Materials and methods
Induction of osteoporosis and β-Ecd treatment
All animal care and experimental procedures in the present study complied with the protocols approved by the Institutional Animal Care and Use Committee of Xiao Shan TCM Hospital (Xiaoshan, China). A total of 24 specific pathogen-free Sprague-Dawley male rats (age, 4 weeks; weight, 180–220 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and housed in an animal facility at 25°C, with a relative humidity of 60–70%, under a 12-h light/dark cycle with free access to food and water. The rats were randomly divided into the 4 following groups (8 rats/groups): i) Control group (untreated rats); ii) PRED group, where a pellet of PRED (2.5 mg/pellet; Zhengzhou Lingrui Pharmaceutical Co., Ltd., Xinzheng, China) was subcutaneously implanted into the back of the rats every day for 4 weeks; iii) PRED + β-Ecd group, where rats received PRED subcutaneously similar to the PRED group, and were additionally subcutaneously injected with β-Ecd (5 or 10 mg/kg body weight; Shanghai Tauto Biotech Co., Ltd., Shanghai, China) 5 times for 4 weeks; and iv) PRED + alendronate (ALN) group, where rats received PRED subcutaneously similar to the PRED group, and were also subcutaneously injected with ALN (3 mg/kg body weight; Merck & Co., Inc., Whitehouse Station, NJ, USA) 5 times for 4 weeks.
Structural analysis using micro-CT and measurement of BMD
The fifth lumbar vertebrae (L5) were isolated following 4 weeks of treatment as previously described (21), fixed in 1% formalin overnight at room temperature and in 70% ethanol for 2 h at room temperature. The micro-CT apparatus and the SCANCO image processing language (version 5.08b) software used in the present study were obtained from Scanco Medical AG (Brüttisellen, Switzerland). The micro-CT contained a micro X-ray source (current, 85 mA; potential, 70 kV) directed toward the samples, according to the manufacturer's instructions. The BMD (g/cm3) of the L5 was measured using dual-energy C-ray absorptiometry with a DCS-600 Aloka bone densitometer (Hitachi, Ltd., Tokyo, Japan) using the small-animal scan mode.
Immunofluorescence
Paraffin-embedded lumbar vertebrae (L1-2) sections (4–7 µm) from rats (4 rats/group) were fixed in 10% formalin overnight at room temperature, separately dehydrated by 50, 70, 85, 95 and 100% ethanol for 2 h, deparaffinized by dimethylbenzene for 15 min and separately hydrated by 100, 95, 85 and 75% ethanol for 5 min at room temperature for histological assessment. For antigen retrieval, the tissue sections were incubated in sodium citrate buffer (JRDUN Biotechnology, Co., Ltd., Shanghai, China) at 37°C for 15 min and heated in a microwave oven at 92–98°C for 10–30 min. Following antigen retrieval, the tissue sections were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 20 min at room temperature. Following blocking with 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS for 1 h at room temperature, the tissue sections were incubated with anti-Beclin-1 (1:200, cat. no. ab62557; Abcam, Cambridge, MA, USA) and anti-microtubule-associated protein 1A/1B-light chain (LC) 3I/II (1:200, cat. no. ab128025; Abcam) antibodies at 4°C overnight, prior to incubation with the corresponding fluorescein isothiocyanate-labeled goat anti-rabbit immunoglobulin G (IgG) (H+L) secondary antibodies (1:500, cat. no. A0562; Beyotime Institute of Biotechnology, Haimen, China) for 1 h at room temperature. The nuclei were then stained with 1 µg/ml 4′,6-diamidino-2-phenylindole for 5 min at room temperature. Stained sections were observed under a CX41RF fluorescence microscope (Olympus Corporation, Tokyo, Japan) at ×200 magnification.
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) staining
TUNEL staining of the fifth lumbar vertebrae from rats (4 rats/group) was performed using the In Situ Cell Death Detection kit, POD (cat. no. 11684817910; Sigma-Aldrich; Merck KGaA), according to the manufacturer's instructions. Each section (4–7 µm) was fixed in 10% formalin overnight and separately dehydrated by 50, 70, 85, 95 and 100% ethanol for 2 h at room temperature. Sections were incubated with TUNEL reagent for 1 h at 37°C and stained with DAB for 10 min and hematoxylin for 5 min at room temperature. Sections were visualized under a fluorescence microscope (CX41RF; Olympus Corporation, Tokyo, Japan) and the numbers of TUNEL-positive cells were counted at ×200 magnification in 30 fields of view/section.
Biochemical analysis
Serum was isolated from rats (4 rats/group) after 4 weeks treatment. Briefly, 10 ml of blood was collected in a serum separator tube and processed within 1 h. Separation of the serum was accomplished by centrifugation at 800 × g for 10 min at room temperature. The serum concentration of calcium and phosphorus, and the activity of tartrate-resistant acid phosphatase (TRAP) and alkaline phosphatase (ALP) were measured using a Hitachi 7070 analyzer (Hitachi, Ltd.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tibia tissues isolated and crushed from rats (4 rats/group) after 4 weeks treatment as previously described (22), using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's instructions. A total of 1 µg RNA was reverse transcribed to cDNA using a cDNA synthesis kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. qPCR was performed using SYBR-Green (Takara Biotechnology Co., Ltd., Dalian, China) on a StepOne Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.), with GAPDH as an internal control. The following thermocycling conditions were used for the PCR: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec, and a final extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec. The primers used in the present study were as follows: Bone morphogenetic protein 2 (BMP2) forward, 5′-CTGTCCCTACTGATGAGTTTC-3′ and reverse, 5′-CTAACCTGGTGTCCAATAGTC-3′; Beclin-1 forward, 5′-GAGTTGCCGTTGTACTGTTC-3′ and reverse, 5′-TGCCTCCAGTGTCTTCAATC-3′; Runt-related transcription factor (RUNX) 2 forward, 5′-ACTTCGTCAGCGTCCTATC-3′ and reverse, 5′-CATCAGCGTCAACACCATC-3′; autophagy protein (ATG) 5 forward, 5′-TGGCTGAGCGAGCATCTGAG-3′ and reverse, 5′-TGACTGCGGGTGGTTCCATC-3′; receptor activator of nuclear factor-κB ligand (RANKL) forward, 5′-CACAGCGCTTCTCAGGAGTT-3′ and reverse, 5′-GATGGTGAGGTGAGCAAACG-3′; and GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse, 5′-CCACCACCCTGTTGCTGTAG-3′. Relative gene expression was calculated according to the comparative Cq method (23) and the fold-change of target gene expression was normalized to the internal control GAPDH.
Western blot analysis
The tibia tissue samples obtained from rats (4 rats/group) after 4 weeks of treatment were homogenized in ice-cold lysis buffer [150 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.4), 20 mM EGTA, 1 mM DTT, 1 mM Na3VO4 and protease inhibitor cocktail tablet] and total cell lysates were prepared using radioimmunoprecipitation assay lysis buffer supplemented with protease inhibitor (Beyotime Institute of Biotechnology). The total protein concentration in each sample was measured using a Lowry protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of extracted protein samples (50 µg) were separated by SDS-PAGE on a 10% gel and transferred onto polyvinylidene difluoride membranes (Roche Diagnostics GmbH, Mannheim, Germany). Membranes were blocked in fat-free milk overnight at 4°C and incubated with primary antibodies, anti-Runx2 (1:1,000; cat. no. ab76956), anti-RANKL (1:1,000; cat. no. ab45039), anti-Beclin-1 (1:600; cat. no. ab55878), anti-ATG5 (1:5,000; cat. no. ab108327), anti-BMP2 (1:800; cat. no. ab14933), and anti-caspase-3 (1:500; cat. no. ab44976) (all from Abcam), anti-Bcl-2 (1:400, cat. no. Sc-492; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-LC3 (1:1,000; cat. no. 2775s), and anti-GAPDH (1:1,500; cat. no. 5174) (both from Cell Signaling Technology, Inc., Danvers, MA, USA), for 2 h at 25°C. The membranes were subsequently incubated for 1 h at 37°C with horseradish peroxidase-conjugated IgG secondary antibodies (1:1,000, cat. nos. A0208, A0181, A0216; Beyotime Institute of Biotechnology). Protein bands were visualized using Western Lightning Plus Enhanced Chemiluminescence reagent (PerkinElmer, Inc., Waltham, MA, USA), and blots were semi-quantified by densitometry using Quantity One software version 4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation of at least three independent replicates and were analyzed using unpaired, two-tailed Student's t-test, and one-way analysis of variance followed by Tukey's post hoc test. Statistical analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of β-Ecd on PRED-induced alterations in bone microarchitecture
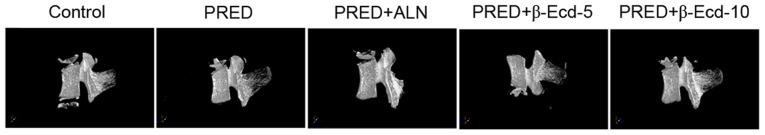
Bone loss was observed in all PRED-treated rats, as detected using micro-CT analysis (Fig. 1 and Table I). In the PRED group, the BMD values of L5 were significantly decreased compared with in the control group. Micro-CT evaluation revealed that the 2-D model bone surface density (BS/TV) of the trabecular bones was significantly decreased in the PRED group compared with in control rats (29.1% decrease; Table I). 3D model analysis demonstrated that the trabecular number (Tb.N) was significantly lower following PRED treatment compared with in control rats (27.3% decrease; Table I). In addition, a significantly increased trabecular plate separation (Tb.Sp) was revealed in the PRED treatment group compared with in the control group (59.8% increase; Table I). The trabecular bone tissue volume density (BV/TV) was revealed to be 30.4% lower following treatment with PRED. However, no significant difference was detected in the trabecular thickness (Tb.Th) and structure model index (SMI) between PRED-treated and control rats. Notably, treatment with 10 mg/kg β-Ecd significantly increased the levels of BMD, BS/TV, BV/TV and Tb.N, whereas it decreased Tb.Sp and SMI compared with the PRED group; treatment with 3 mg/kg ALN produced similar results (Table I). Additionally, treatment with 5 mg/kg β-Ecd significantly increased the levels of BMD, BS/TV, BV/TV and Tb.N, whereas it decreased Tb.Th and Tb.Sp levels compared with the PRED group (Table I).
Figure 1.
3D-reconstructed micro-CT images of the 5th lumbar vertebrae isolated from rats in the various treatment groups. Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. CT, computed tomography; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone.
Table I.
Alterations in BMD and microarchitectural parameters of the fifth lumbar vertebra of rats in the various treatment groups.
| Group | |||||
|---|---|---|---|---|---|
| Parameters | Control | PRED | PRED + ALN | PRED + β-Ecd, 5 mg/kg | PRED + β-Ecd, 10 mg/kg |
| BMD, g/cm3 | 0.299±0.007 | 0.150±0.031a | 0.259±0.015c | 0.229±0.006b | 0.263±0.006c |
| BS/TV, mm−1 | 3.78±0.212 | 2.68±0.213a | 3.81±0.139c | 3.74±0.238c | 3.74±0.046c |
| BV/TV, % | 35.68±0.379 | 24.83±1.627a | 32.07±0.558c | 30.49±0.761c | 32.46±0.001c |
| Tb.N, mm−1 | 1.313±0.049 | 0.954±0.079a | 1.275±0.058c | 1.250±0.064c | 1.276±0.009c |
| Tb.Th, mm | 0.272±0.007 | 0.260±0.005 | 0.252±0.007 | 0.244±0.006b | 0.254±0.002 |
| Tb.Sp, mm | 1.037±0.048 | 1.657±0.155a | 1.067±0.011c | 1.122±0.071c | 1.061±0.030c |
| Structure model index | −3.90±0.345 | −4.32±0.344 | −3.21±0.261b | −3.36±0.543 | −3.48±0.193c |
Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. Data are expressed as the median ± standard deviation.
P<0.01 vs. control
P<0.05
P<0.01 vs. PRED. BMD, bone mineral density; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone; BS/TV, bone surface density; BV/TV, trabecular bone tissue volume density; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular plate separation.
Effects of β-Ecd on PRED-induced alterations in biochemical indices
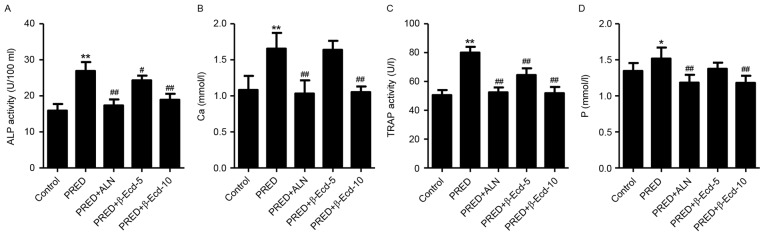
In the present study, treatment with PRED was demonstrated to significantly increase the serum levels of calcium and phosphorus by 69.2 and 58.2%, respectively, and enhance the serum activity of ALP and TRAP by 52.8 and 12.9%, respectively, compared with the control group (Fig. 2). However, treatment with ALN or 10 mg/kg β-Ecd significantly decreased the serum calcium and phosphorus levels and suppressed the serum activity of ALP and TRAP. Conversely, treatment with 5 mg/kg β-Ecd was revealed to attenuate the PRED-induced increase in ALP and TRAP levels; however, it had no effect on the serum levels of calcium and phosphorus.
Figure 2.
Biochemical parameters in rats from various experimental groups. The serum activity or expression of (A) ALP, (B) Ca, (C) TRAP and (D) P were detected using a biochemical analyzer. Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. Data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. PRED. ALP, alkaline phosphatase; TRAP, tartrate-resistant acid phosphatase; Ca, calcium; P, phosphorus; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone.
Effects of β-Ecd on PRED-inducedalterations inautophagy and apoptosis
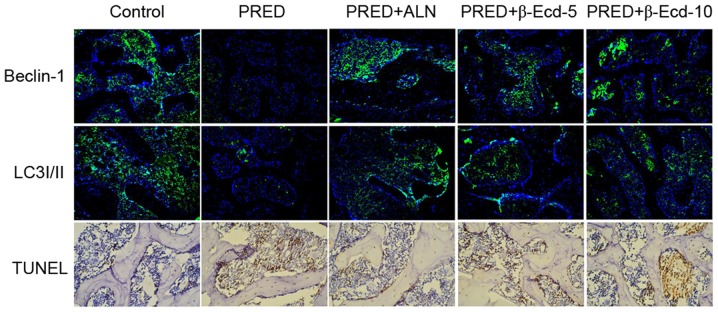
As presented in Fig. 3, immunofluorescence staining demonstrated that treatment with PRED markedly downregulated the expression of Beclin-1 and LC3I/II compared with the control group. However, ALN and β-Ecd administration appeared to inhibit the PRED-induced decreases in Beclin-1 and LC3I/II expression. In addition, TUNEL-positive cells in PRED-treated rats were markedly increased compared with control rats, whereas treatment with ALN and β-Ecd was revealed to markedly reduce the number of apoptotic cells in PRED-treated rats (Fig. 3). These findings suggested that β-Ecd may inhibit the PRED-induced alterations in autophagy and apoptosis in osteoporotic rats.
Figure 3.
Immunofluorescence and TUNEL staining in rats from various experimental groups. The protein expression of Beclin-1 and LC3I/II was detected using immunofluorescence staining. Apoptosis was assessed using TUNEL staining. Magnification, ×200. Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling; LC3I/II, microtubule-associated protein 1A/1B-light chain 3I/II; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone.
Effects of β-Ecd on PRED-induced alterations in RUNX2, RANKL and BMP2 expression
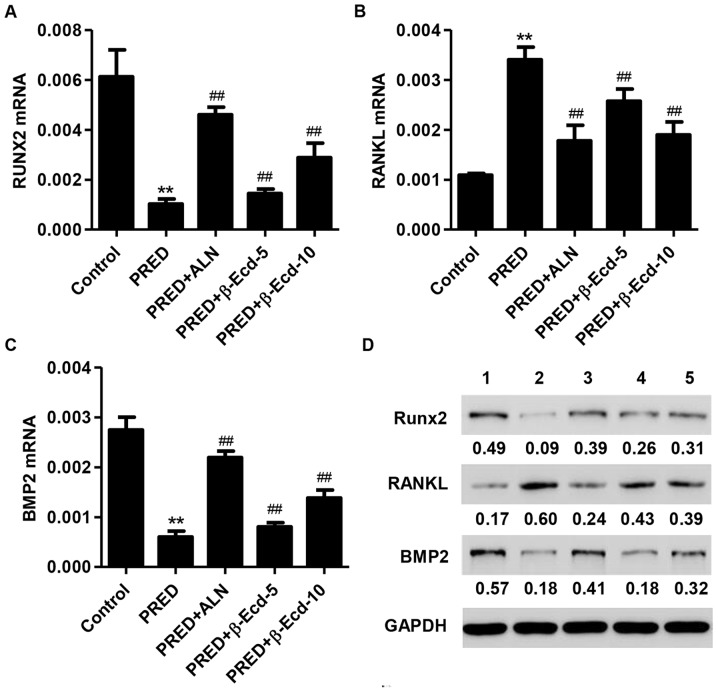
In order to investigate the effects of PRED and β-Ecd on osteoblast differentiation, the expression of differentiation-associated markers, including BMP2, RANKL and RUNX2, was assessed. Treatment with PRED was revealed to significantly suppress the mRNA expression of RUNX2 and BMP2, whereas it upregulated RANKL mRNA expression (Fig. 4A-C). Notably, treatment with ALN or β-Ecd significantly prevented the PRED-induced downregulation in RUNX2 and BMP2, and the upregulation in RANKL mRNA expression in osteoporotic rats (Fig. 4A-C). Similar results regarding protein expression were obtained following western blot analysis (Fig. 4D).
Figure 4.
Expression of RUNX2, RANKL and BMP2 in rats from the various experimental groups. The mRNA expression levels of (A) RUNX2, (B) RANKL and (C) BMP2 were measured using the reverse transcription-quantitative polymerase chain reaction. (D) The protein expression levels of RUNX2, RANKL and BMP2 were detected using western blot analysis. Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. Data are expressed as the mean ± standard deviation. **P<0.01 vs. control; ##P<0.01 vs. PRED. RUNX, Runt-related transcription factor; RANKL, receptor activator of nuclear factor-κB ligand; BMP, bone morphogenetic protein; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone.
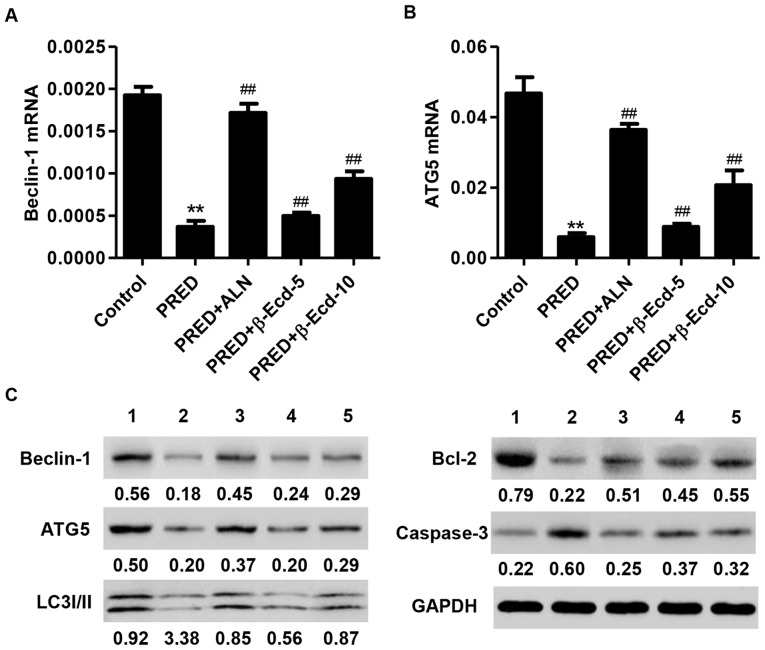
Effects of β-Ecd on PRED-induced alterations in Beclin-1, ATG5, LC3I/II, Bcl-2 and caspase-3 expression
The balance between autophagy and apoptosis is important for the maintenance of cellular homeostasis (24). ATG5 is required for LC3-I conjugation and LC3-II formation, and it is regarded as a key marker of autophagosome formation (25), while Beclin-1 has been reported to serve a role in autophagy (26). The present results demonstrated that PRED administration significantly downregulated the mRNA expression of Beclin-1 and ATG5 in rats, whereas treatment with ALN or β-Ecd significantly inhibited the PRED-induced decreases in the mRNA expression levels of Beclin-1 and ATG5 (Fig. 5A and B). In addition, western blot analysis revealed that PRED administration markedly suppressed the protein expression of Beclin-1, ATG5, LC3I/II and Bcl-2, whereas it upregulated the expression of caspase-3 (Fig. 5C). Notably, treatment with ALN or β-Ecd appeared to counteract the PRED-induced alterations in the expression of Beclin-1, ATG5, LC3I/II, Bcl-2 and caspase-3 in vivo (Fig. 5C).
Figure 5.
Expression of Beclin-1, ATG5, LC3I/II, Bcl-2 and caspase-3 in rats from various experimental groups. The mRNA expression levels of (A) Beclin-1 and (B) ATG5 were measured using reverse transcription-quantitative polymerase chain reaction. (C) The protein expression levels of Beclin-1, ATG5, LC3I/II, Bcl-2 and caspase-3 were detected using western blot analysis. Control, untreated rats; PRED, rats received PRED for 4 weeks; PRED + ALN, rats received PRED and ALN for 4 weeks; PRED + β-Ecd, rats received PRED and β-Ecd (5 or 10 mg/kg) for 4 weeks. Data are expressed as the mean ± standard deviation. **P<0.01 vs. control; ##P<0.01 vs. PRED. ATG, autophagy protein; LC3I/II, microtubule-associated protein 1A/1B-light chain 3I/II; Bcl, B-cell lymphoma; PRED, prednisolone; ALN, alendronate; β-Ecd, β-ecdysterone.
Discussion
In the present study, the decreases in BMD and the deterioration of trabecular microarchitecture were demonstrated to reflect bone fragility and bone mass loss in PRED-treated rats. In addition, the alterations in biochemical parameters, including ALP and TRAP activity, calcium and phosphorus levels, and in the expression of autophagy and apoptosis-associated factors, suggested that bone absorption was enhanced, whereas the activity of osteoblastic cells and the formation of new bone were suppressed in PRED-treated rats.
The PRED-induced osteoporosis model has been used in a number of previous studies: Huang et al (4) reported a significant decrease in BMD and in the serum levels of calcium, phosphorus and osteocalcin in PRED-induced osteoporotic rats. PRED treatment has additionally been reported to decrease cortical bone mineral contents, cortical thickness, the stress/strain index and mandibular volume, whereas it did not produce marked alterations in trabecular structural parameters (27). Similarly, the findings of the present study demonstrated that PRED induced a significant decrease in BMD, in the serum levels of calcium and phosphorus, and in the serum ALP and TRAP activity in rats, thus indicating high rates of bone turnover following treatment with PRED. To the best of our knowledge, this is the first time that a decrease in volumetric BMD was revealed to be accompanied by the deterioration of trabecular microarchitecture, as measured by micro-CT in rats in vivo. Previous studies have reported that the maintenance of trabecular structure is a critical factor for the strength of the lumbar vertebra (28,29). In accordance with the present results, previous reports have demonstrated that the BMD was correlated with bone microarchitectural parameters (30,31). In the present study, treatment with PRED was revealed to decrease BS/TV, BV/TV and Tb.N, whereas it increased Tb.Sp values in the trabecular bones of rats in vivo; Tb.Th and SMI values remained unaltered in PRED-treated rats. The present findings suggested that BS/TV, BV/TV, Tb.N and Tb.Sp may have potential as sensitive biomarkers for the early detection of alterations in trabecular structure, indicative of osteoporosis development.
A previous study used a combination of linkage and association analysis, and linked BMP2 expression to a phenotype of low BMD combined with a high fracture risk (32). BMP2 is produced and secreted by osteoblasts, and has been reported to exert independent effects on the regulation of osteoblast proliferation and mineralization (33). Osteoblasts are involved in bone formation and, through the production of RANKL, are able to modulate the formation and differentiation of osteoclasts; RANKL provides a signal to osteoclast progenitors through RANK, leading to the activation of osteoclast differentiation and function (34). Overexpression of RUNX2 in transgenic mice resulted in an osteoporotic phenotype, thus suggesting that RUNX2 may be involved in genetic processes associated with osteoporosis (35). In the present study, PRED treatment was revealed to suppress the expression of RUNX2 and BMP2, whereas it potentiated the expression of RANKL. In accordance with the present findings, previous studies have reported decreased BMP2 and increased RANKL expression in glucocorticoid-induced osteoporosis models in vivo (36) and in vitro (37).
A number of mechanisms have been suggested to underlie glucocorticoid-induced osteoblast apoptosis: PRED administration (2.1 mg/kg) has been reported to enhance osteoblast apoptosis in mouse vertebrae, and increase the frequency of osteocyte apoptosis in metaphysical cortical bone, thus resulting in a decrease in vertebral cancellous bone due to reduced bone formation (38). In accordance with this previous study, the present results demonstrated that PRED induced cell apoptosis, via suppressing the expression of the antiapoptotic factor Bcl-2 and promoting the activation of the proapoptotic caspase-3. In addition, PRED administration was revealed to inhibit the expression of autophagosome-regulatory proteins, including Beclin-1, ATG5 and LC3I/II in tibia tissue samples of rats (39); in agreement with the present results, transfection of osteoblasts with Beclin-1-targeting small interfering-RNA effectively inhibited autophagosome formation in vitro (39). However, a study by Piemontese et al (40) reported contradictory results, as PRED was demonstrated to increase the autophagic flux in osteocyte-enriched mouse bones, as measured using LC3 conversion. The variations in the experimental animals and drug dosages that were used may explain the contradictory findings in the study by Piemontese et al (40). A previous study revealed that dexamethasone, in contrast to PRED, induced osteocyte apoptosis, resulted in an increase in autophagy markers and promoted the accumulation of autophagosome vacuoles in vitro and in vivo, thus promoting the onset of osteocyte autophagy (10).
The present results suggested that β-Ecd may inhibit PRED-induced bone loss and apoptosis, and enhance autophagy in rats in vivo. These effects were similar to ALN, which is a bisphosphonate used in the treatment of osteoporosis as it inhibits bone resorption by interfering with the activity of osteoclasts; ALN was used as a positive control in the present study (41). In accordance with the present results, a previous study reported that β-Ecd induced osteogenic differentiation in mouse mesenchymal stem cells and attenuated the development of osteoporosis through an endoplasmic reticulum-associated signaling pathway (16). Additionally, β-Ecd has been demonstrated to prevent glucocorticoid-induced alterations in bone formation, bone cell viability and bone mass, and to prevent the glucocorticoid-induced increase in autophagy in bone marrow stromal cells and whole bone tissue (9). These data generated from an in vitro system may not be necessarily and comprehensively consistent with the effects of β-Ecd on autophagy in vivo.
In conclusion, the results of the present study suggested that β-Ecd may prevent PRED-induced osteoporosis in rats in vivo through the inhibition of bone loss and apoptosis and through the induction of autophagy. Therefore, β-Ecd may in the future be used for the development of treatment strategies for osteoporosis in humans.
Acknowledgements
The present study was supported by the Science and Technology Project from Traditional Chinese Medicine of Zhejiang Province (grant no. 2015ZA174), and the Social Development Major Scientific and Technological Projects in Xiaoshan District of Hangzhou City (grant no. 2014207).
References
- 1.Emkey GR, Epstein S. Secondary osteoporosis: Pathophysiology & diagnosis. Best Pract Res Clin Endocrinol Metab. 2014;28:911–935. doi: 10.1016/j.beem.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Shiraishi A, Higashi S, Masaki T, Saito M, Ito M, Ikeda S, Nakamura T. A comparison of alfacalcidol and menatetrenone for the treatment of bone loss in an ovariectomized rat model of osteoporosis. Calcif Tissue Int. 2002;71:69–79. doi: 10.1007/s00223-001-2090-y. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson F, Mattsson P, Larsson S. The effect of augmentation with resorbable or conventional bone cement on the holding strength for femoral neck fracture devices. J Orthop Trauma. 2002;16:302–310. doi: 10.1097/00005131-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Bo Y, Wu X, Wang Q, Qin F, Zhao L, Xiong Z. An intergated serum and urinary metabonomic research based on UPLC-MS and therapeutic effects of Gushudan on prednisolone-induced osteoporosis rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1027:119–130. doi: 10.1016/j.jchromb.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Siu WS, Qin L, Cheung WH, Leung KS. A study of trabecular bones in ovariectomized goats with micro-computed tomography and peripheral quantitative computed tomography. Bone. 2004;35:21–26. doi: 10.1016/j.bone.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Lill CA, Fluegel AK, Schneider E. Sheep model for fracture treatment in osteoporotic bone: A pilot study about different induction regimens. J Oorthop Trauma. 2000;14:559–566. doi: 10.1097/00005131-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Zhao J. Research progress in osteoporotic models. Chin J Osteoporos. 2012 [Google Scholar]
- 8.Silverman SL, Lane NE. Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2009;7:23–26. doi: 10.1007/s11914-009-0005-4. [DOI] [PubMed] [Google Scholar]
- 9.Dai W, Jiang L, Lay YA, Chen H, Jin G, Zhang H, Kot A, Ritchie RO, Lane NE, Yao W. Prevention of glucocorticoid induced bone changes with beta-ecdysone. Bone. 2015;74:48–57. doi: 10.1016/j.bone.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia X, Kar R, Gluhak-Heinrich J, Yao W, Lane NE, Bonewald LF, Biswas SK, Lo WK, Jiang JX. Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res. 2010;25:2479–2488. doi: 10.1002/jbmr.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 12.Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Schober A, Parlato R, Huber K, Kinscherf R, Hartleben B, Huber TB, Schütz G, Unsicker K. Cell loss and autophagy in the extra-adrenal chromaffin organ of Zuckerkandl are regulated by glucocorticoid signalling. J Neuroendocrinol. 2013;25:34–47. doi: 10.1111/j.1365-2826.2012.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258:148–156. doi: 10.1016/j.tox.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, Bonewald L, Jiang JX, Lane NE. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–3376. doi: 10.1096/fj.11-182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L, Cai G, Shi X. Beta-ecdysterone induces osteogenic differentiation in mouse mesenchymal stem cells and relieves osteoporosis. Biol Pharm Bull. 2008;31:2245–2249. doi: 10.1248/bpb.31.2245. [DOI] [PubMed] [Google Scholar]
- 17.Omanakuttan A, Bose C, Pandurangan N, Kumar GB, Banerji A, Nair BG. Nitric Oxide and ERK mediates regulation of cellular processes by Ecdysterone. Exp Cell Res. 2016;346:167–175. doi: 10.1016/j.yexcr.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Xu X, Xu T, Qin S. β-Ecdysterone suppresses interleukin-1beta-induced apoptosis and inflammation in rat chondrocytes via inhibition of NF-kappaB signaling pathway. Drug Dev Res. 2014;75:195–201. doi: 10.1002/ddr.21170. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Wang R, Guo H, Dong M. Phytoestrogen β-ecdysterone protects PC12 cells against MPP+−induced neurotoxicity in vitro: Involvement of PI3K-Nrf2-regulated pathway. Toxicol Sci. 2015;147:28–38. doi: 10.1093/toxsci/kfv111. [DOI] [PubMed] [Google Scholar]
- 20.Pan Z, Niu Y, Liang Y, Zhang X, Dong M. β-Ecdysterone protects SH-SY5Y cells against 6-hydroxydopamine-induced apoptosis via mitochondria-dependent mechanism: Involvement of p38(MAPK)-p53 signaling pathway. Neurotox Res. 2016;30:453–466. doi: 10.1007/s12640-016-9631-7. [DOI] [PubMed] [Google Scholar]
- 21.Ominsky MS, Li X, Asuncion FJ, Barrero M, Warmington KS, Dwyer D, Stolina M, Geng Z, Grisanti M, Tan HL, et al. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res. 2008;23:672–682. doi: 10.1359/jbmr.080109. [DOI] [PubMed] [Google Scholar]
- 22.Houlihan DD, Mabuchi Y, Morikawa S, Niibe K, Araki D, Suzuki S, Okano H, Matsuzaki Y. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat Protoc. 2012;7:2103–2111. doi: 10.1038/nprot.2012.125. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Walsh CM, Edinger AL. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol Rev. 2010;236:95–109. doi: 10.1111/j.1600-065X.2010.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 26.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonnell P, McHugh PE, O'Mahoney D. Vertebral osteoporosis and trabecular bone quality. Ann Biomed Eng. 2007;35:170–189. doi: 10.1007/s10439-006-9239-9. [DOI] [PubMed] [Google Scholar]
- 29.Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R. The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: An ex vivo study. Osteoporos Int. 2013;24:2455–2460. doi: 10.1007/s00198-013-2316-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Miller PD, Recker R, Resch H, Rana A, Pavo I, Sipos AA. Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. J Bone Miner Res. 2007;22:1173–1180. doi: 10.1359/jbmr.070413. [DOI] [PubMed] [Google Scholar]
- 31.Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: A randomized controlled trial. J Clin Endocrinol Metab. 2013;98:207–217. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 32.Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mundy C, Gannon M, Popoff SN. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J Cell Physiol. 2014;229:672–681. doi: 10.1002/jcp.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Sakai E, Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K, Tsukuba T. Inhibitory effects of tert-butylhydroquinone on osteoclast differentiation via up-regulation of heme oxygenase-1 and down-regulation of HMGB1 release and NFATc1 expression. J Appl Toxicol. 2014;34:49–56. doi: 10.1002/jat.2827. [DOI] [PubMed] [Google Scholar]
- 35.Bustamante M, Nogués X, Agueda L, Jurado S, Wesselius A, Cáceres E, Carreras R, Ciria M, Mellibovsky L, Balcells S, et al. Promoter 2 −1025 T/C polymorphism in the RUNX2 gene is associated with femoral neck bmd in Spanish postmenopausal women. Calcif Tissue Int. 2007;81:327–332. doi: 10.1007/s00223-007-9069-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhu FB, Wang JY, Zhang YL, Quan RF, Yue ZS, Zeng LR, Zheng WJ, Hou Q, Yan SG, Hu YG. Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts. Int J Clin Exp Med. 2015;8:12337–12346. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ, Wu SL. Inhibition of glycogen synthase kinase-3beta attenuates glucocorticoid-induced bone loss. Life Sci. 2009;85:685–692. doi: 10.1016/j.lfs.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Dai N, Wang Y, Xu C, Zhao H, Xia P, Gu J, Liu X, Bian J, Yuan Y, et al. Role of autophagy in cadmium-induced apoptosis of primary rat osteoblasts. Sci Rep. 2016;6:20404. doi: 10.1038/srep20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piemontese M, Onal M, Xiong J, Wang Y, Almeida M, Thostenson JD, Weinstein RS, Manolagas SC, O'Brien CA. Suppression of autophagy in osteocytes does not modify the adverse effects of glucocorticoids on cortical bone. Bone. 2015;75:18–26. doi: 10.1016/j.bone.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;1:CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]