Abstract
The interleukin (IL)-12 family cytokines have been examined as therapeutic targets in the treatment of several autoimmune diseases. Our previous study showed that a novel IL-12 family cytokine, IL-39 (IL-23p19/Ebi3) mediates inflammation in lupus-like mice. In the present study, the effect of anti-mouse IL-39 polyclonal antibodies on autoimmune symptoms in lupus-like mice was investigated. Rabbit anti-mouse IL-39 polyclonal antibodies were produced by immunization with recombinant mouse IL-39, and purified using protein A chromatography. These antibodies were subsequently used to treat lupus-like mice. Flow cytometry, captured images, ELISA and H&E staining were used to determine the effect of anti-IL-39 polyclonal antibodies on inflammatory cells, autoantibody titers, proteinuria, infiltrating inflammatory cells and the structure of the glomerular region. The anti-IL-39 polyclonal antibodies effectively reduced the numbers of inflammatory cells, splenomegaly, autoantibody titers, proteinuria, infiltrating inflammatory cells, and restored the structure of the glomerular region in MRL/lpr mice. Taken together, these results suggested that anti-IL-39 polyclonal antibodies ameliorated autoimmune symptoms in lupus-like mice. Therefore, IL-39 may be used as a possible target for the treatment of systemic lupus erythematosus.
Keywords: interleukin-39, interleukin-23p19, Ebi3, autoimmunity, systemic lupus erythematosus, lupus-like mice
Introduction
As interleukin (IL)-12 family members (IL-12, IL-23, IL-27 and IL-35) are important in several autoimmune diseases (1–4), they have been examined as therapeutic targets in the treatment of a number of autoimmune diseases. Ustekinumab, a therapeutic agent targeting IL-12/IL-23p40, has been approved to treat psoriasis and psoriatic arthritis (5). In addition, at least 10 therapeutic agents targeting IL-12, IL-23, or the IL-23-related signaling pathway are being clinically examined in >17 human immune-mediated diseases (5). Therefore, therapeutically targeting IL-12 family members appears to be an effective approach in treating several autoimmune diseases.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by increased autoantibody production, B cell hypersensitivity and ultimate end organ damage (6). Immunosuppressants, including corticosteroids are the most commonly used drugs to treat SLE (7,8). However, the immunosuppressants are unspecific, exhibit high toxicity and they have several negative side effects. Therefore, it is necessary to identify alternative and more specific drugs for SLE (9). Autoantibody-producing B cells appear to be central to the pathogenesis of SLE (6,7,10). In 2011, the US Food and Drug Administration approved the novel B cell-targeting reagent, belimumab, a fully human anti-B-cell activation factor (BAFF) monoclonal antibody, for the treatment of SLE.
MRL/lpr mice develop autoimmunity as they carry Fas/Fas ligand mutant genes, which result in lymphoproliferative disease similar to human SLE (11,12). Lymphoproliferation can significantly enhance the size of the spleen in MRL/lpr mice (13). The overactivation of B cells is considered to be an important hallmark feature of SLE (14,15). In addition, the numbers of activated GL7+B220+ B cells (16,17) and IgG+ class-switched memory B cells (15) are significantly increased in lupus-like mice. SLE and the lupus-like mouse model are characterized by a high level of autoantibodies due to the overactivation of autoreactive B cells (15,18,19). Autoantibodies from MRL/lpr mice induce increased proteinuria and lupus nephritis in normal mice (20).
Our previous study showed that the IL-12 family cytokine subunits, p19 and Ebi3, form a novel p19/Ebi3 heterodimer, termed IL-39, which mediates inflammation in lupus-like MRL/lpr mice (13). The present study investigated whether IL-39 is a potential therapeutic target for SLE. Rabbit anti-mouse IL-39 polyclonal antibodies were produced, and these antibodies were used to treat lupus-like MRL/lpr mice. The results suggested that anti-IL-39 polyclonal antibodies ameliorated autoimmune symptoms in the lupus-like MRL/lpr mice.
Materials and methods
Ethics Committee approval
The care, use and treatment of the mice used in the present study were in strict agreement with the international guidelines for the Care and Use of Laboratory Animals (21). The present study was approved by the Animal Ethics Committee of Beijing Institute of Basic Medical Sciences (Beijing, China).
Mice
A total of 36 female 6-month-old (35–40 mg) lupus-like MRL/MpJ/lpr/lpr (MRL/lpr) mice (Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China) have been described in detail previously (17,19) and were bred in our animal facilities under specific pathogen-free conditions in a room maintained at 22°C, 60% humidity with a 12/12-h light/dark cycle and with free access to food and water.
Production of anti-mouse IL-39 polyclonal antibodies
The production of anti-mouse polyclonal antibodies has been described in detail previously (22). The production of purified mouse IL-39 (p19/Ebi3) has also been described in detail previously (13), and these were used for the immunization of rabbits and the production of anti-mouse IL-39 antibodies. The polyclonal antibodies were purified using protein A chromatography, and its reactivity with recombinant mouse IL-39 was confirmed using ELISA.
Treatment of MRL/lpr mice with anti-mouse IL-39 polyclonal antibodies
The MRL/lpr mice were divided into the following two groups: i) control mice; and ii) anti-mouse IL-39 polyclonal antibody-treated mice. In these groups, on day 0 and day 3, a single injection of 400 µg of preimmune IgG (control) or anti-IL-39 polyclonal antibodies was injected intravenously into 6-month-old female MRL/lpr mice (n=6 mice/group), for a total of two injections.
Flow cytometric analysis
Flow cytometric analysis has been described in detail previously (13,23). The spleens were removed and compressed firmly with the end of the plunger from a sterile 5 ml syringe to allow cells to pass through a 200-gauge stainless steel wire gauze. The cell suspension (1×106 cells/sample) was washed with fluorescence-activated cell sorting staining buffer, comprising phosphate-buffered saline, 2% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) or 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 0.1% sodium azide. All samples were incubated for 30 min at 4°C with 1:50 diluted anti-Fc receptor antibody (BD Biosciences, Franklin Lakes, NJ, USA). Subsequently, samples were incubated for 30 min at 4°C with the primary antibodies diluted 1:50 in fluorescence-activated cell sorting buffer supplemented with 2% anti-Fc receptor antibody. The following fluorescence-conjugated anti-mouse antibodies were used: CD3 (100204), B220 (103234), CD19 (115512), Gr-1 (108410) (all from BioLegend, Inc., San Diego, CA, USA), GL7 (12-5902-82), IgM (17-5790-82), IgG (11-4011-85), CD11c (12-0114-81) and CD11b (11-0112-85) (all from eBioscience; Thermo Fisher Scientific, Inc.). The samples were filtered immediately prior to analysis or cell sorting to remove any clumps. Data collection and analyses were performed on a FACSCalibur flow cytometer using CellQuest software (version 5.1; BD Biosciences).
Cytokine and autoantibody titer analysis using ELISA
The concentration of cytokines and the titer of anti-dsDNA autoantibody (IgM and IgG) were measured using ELISA kits, as described in detail previously (13,23). Anti-mouse IL-23p19, IL-27Ebi3, anti-dsDNA IgM and IgG ELISA kits were all purchased from eBioscience, Inc. Briefly, sera from mice were separated by centrifugation for 15 min at 900 × g at room temperature. Sera (1:250 dilution) or the purified p19/Ebi3 (4 µg/ml, 1:250 dilution) proteins were added in triplicate to the plate for 1 h at 37°C. The plate was subsequently washed with PBS + 0.05% Tween-20. The following antibodies were then diluted 1:250, added to the plate and incubated for 1 h at 37°C: Biotin rat anti-mouse IL-23p19 (88-7230-22), IL-27Ebi3 (14-7273-80), IgM (88-50470-88), and IgG (88-50400-22) antibodies (all from eBioscience; Thermo Fisher Scientific, Inc.). The unbound antibodies were then washed off, followed by the addition of avidin-HRP (1:1,000 dilution) and incubation of the plates for 1 h at 37°C. Finally, the color was developed by incubation with o-phenylenediamine. The optical density was read at 492 nm with an ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Standard curves were established to quantify the concentrations of the respective cytokines.
Assessment of proteinuria
The assessment of proteinuria has been described in detail previously (13). Urine (10 ml) was manually expressed into a sterile container from each mouse on days 0 and 7 following treatment, and assayed for the presence of protein, specifically albumin, using a colorimetric method with Albustix reagent strips (Bayer Corporation, Elkhart, IN, USA).
Hematoxylin and eosin (H&E) staining
H&E staining has been described in detail previously (19,24). Kidney specimens were fixed in formalin for 24 h, dehydrated by successive incubation in 70, 80 and 90% ethanol for 3 h, 100% ethanol I for 2 h and 100% ethanol II for 2 h, and then vitrified with xylene I and xylene II for 20 min. Following immersion in paraffin I and II for 40 min, the specimens were embedded and sectioned (4 µm). Staining was performed as follows: Hematoxylin staining for 15 min, followed by hydrochloric acid alcohol solution for 35 sec, eosin staining for 10 min followed by 90% ethanol for 40 sec. Neutral balsam was then used for mounting, following which the section was visualized and images were captured using a light microscope.
Histological analysis
The complete kidney sections were analyzed under a light microscope (Carl Zeiss AG, Oberkochen, Germany) using a ×20 magnification lens by two investigators blinded to the groups (anti-IL-39-treatment vs. control). The degrees of glomerular lesions were evaluated using a semiquantitative score methodology, as described previously (25). The severity of the lesion was graded between 0 and 4+ according to the percentage of glomerular involvement. A 1+ lesion represented an involvement of 25% of the glomerulus, wheras a 4+ lesion indicated that 100% of the glomerulus was involved.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA USA). The data are shown as the mean ± standard error of the mean. Student's t-test was used to determine significance between two groups (paired or unpaired) and two-way analysis of variance was used to determine significance among several groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Production of anti-mouse IL-39 polyclonal antibodies
To determine whether IL-39 can be used as a possible target for the treatment of autoimmune diseases, including SLE, the initial production of anti-mouse IL-39 polyclonal antibodies was required. Anti-mouse IL-39 polyclonal antibodies from the serum of IL-39-immunized rabbits were purified using protein A chromatography (Fig. 1A). The results of the ELISA assay demonstrated that anti-IL-39 antibodies dose-dependently bound with recombinant mouse IL-39 (p19/Ebi3), whereas the commercial anti-mouse p19 antibody, used as a control, showed no binding capacity (Fig. 1B). These results suggested that anti-mouse IL-39 polyclonal antibodies were able to effectively bind to its molecular target.
Figure 1.
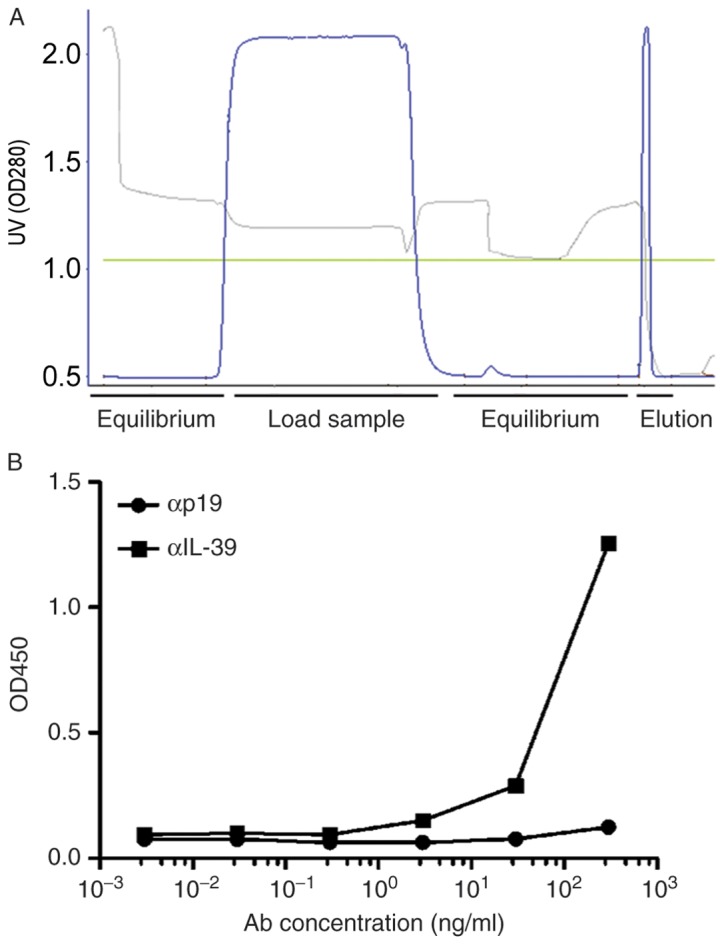
Production of αIL-39. Purified mouse IL-39 was used for immunization of rabbits and production of αIL-39. (A) Purification of αIL-39 was performed by protein A chromatography. Blue, green and grey lines represent the UV absorption value, column pressure and pH values, respectively. (B) The binding of αIL-39 with recombinant mouse IL-39 (p19/Ebi3) was confirmed by ELISA. Commercial anti-mouse p19 antibody was used as a control. αIL-39, anti-mouse interleukin-39 polyclonal antibodies; OD, optical densisty; Ab, antibody.
Anti-IL-39 polyclonal antibodies reduce the numbers of inflammatory cells in MRL/lpr mice
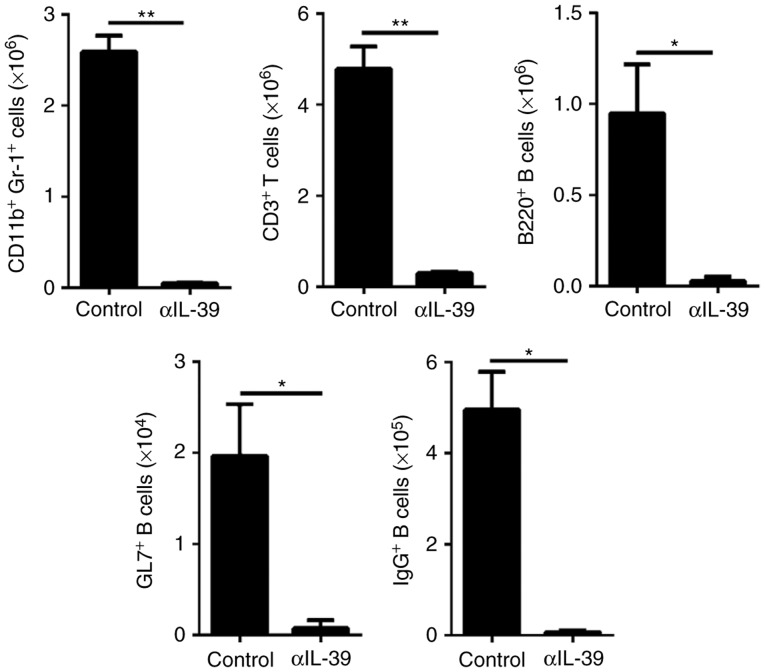
To examine the effect of targeting IL-39 on autoimmune diseases, anti-IL-39 polyclonal antibodies were intravenously injected into 6-month-old female MRL/lpr mice. The effect of anti-IL-39 polyclonal antibodies on lymphoproliferation was first examined. As expected, it was found that the absolute numbers of innate lymphocytes, including CD11b+Gr-1+ neutrophils, and adaptive lymphocytes, including CD3+ T and B220+ B, were significantly decreased in the spleen (Fig. 2) and mesenteric lymph nodes (data not shown) in the IL-39 antibody-treated group. In addition, it was found that the levels of activated GL7+B220+ B cells and IgG+ class-switched memory B cells were significantly decreased in the IL-39 antibody-treated group (Fig. 2). These results suggested that anti-IL-39 polyclonal antibodies reduced the numbers of inflammatory cells in the MRL/lpr mice.
Figure 2.
Treatment with αIL-39 reduces inflammatory cells in MRL/lpr mice. On day 7 following intravenous injection of 400 µg preimmune IgG (control) or αIL-39 into 6-month-old female MRL/lpr mice (6 mice/group), lymphocytes were collected from the spleen. The cells were stained with fluorescence-conjugated anti-mouse CD3, B220, GL7, IgG, CD11b and Gr-1 antibodies, and then analyzed by flow cytometry. The absolute numbers of CD11b+Gr-1+ neutrophils, CD3+ T cells, B220+ B cells, GL7+ B cells, 220+ B cells, and IgG class-switch B cells per mouse are shown. The data are presented as the mean ± standard error of the mean of three independent experiments (n=18). *P<0.05; **P<0.01 (two-tailed Student's t-test). αIL-39, anti-mouse interleukin-39 polyclonal antibodies.
Anti-IL-39 polyclonal antibodies reduce splenomegaly in MRL/lpr mice
To examine the effect of anti-IL-39 polyclonal antibodies on splenomegaly in the MRL/lpr mice, the spleens were harvested and images were captured 7 days following intravenous injection of anti-IL-39 polyclonal antibodies into the 6-month-old female MRL/lpr mice. The results revealed that treatment with anti-IL-39 polyclonal antibodies reduced splenomegaly in the MRL/lpr mice (Fig. 3A). Statistical analysis showed that anti-IL-39 polyclonal antibodies significantly reduced the size of the spleen (Fig. 3B). These results suggested that anti-IL-39 polyclonal antibodies effectively reduced splenomegaly in the MRL/lpr mice by reducing the number of inflammatory cells.
Figure 3.
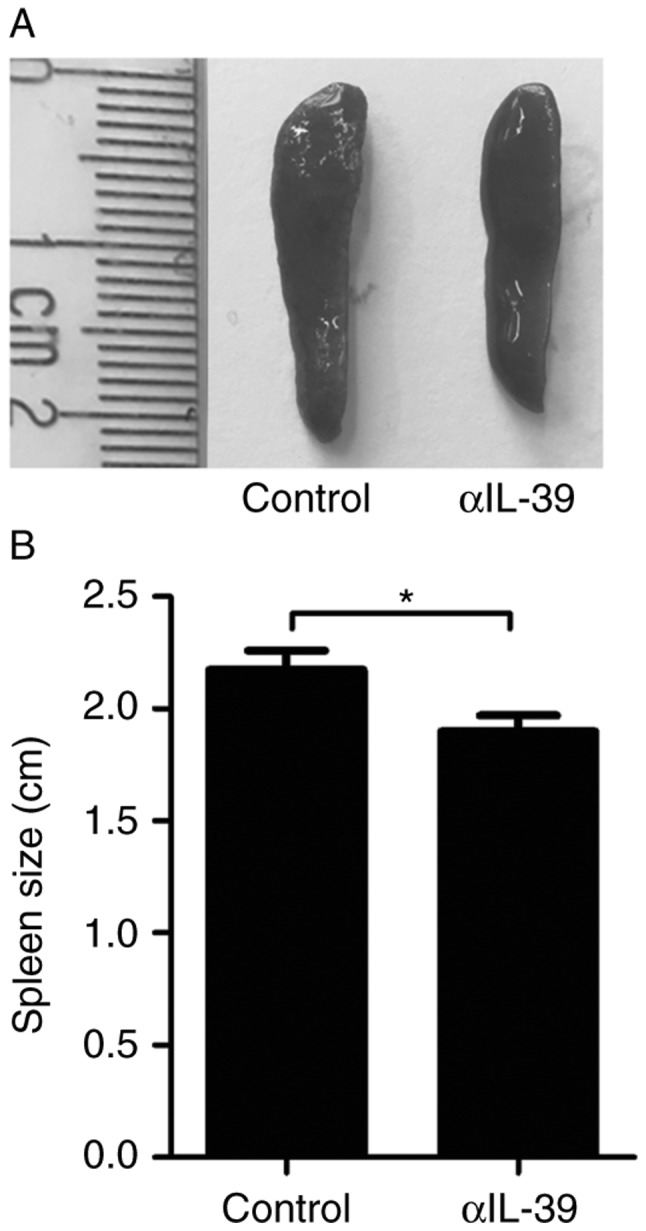
Treatment with αIL-39 reduces splenomegaly in MRL/lpr mice. On day 7 following intravenous injection of 400 µg preimmune IgG (control) or αIL-39 into 6-month-old female MRL/lpr mice (6 mice/group), spleens were harvested, (A) images were captured and (B) spleen length was analyzed. The data are presented as the mean ± standard error of the mean of three independent experiments (n=12). *P<0.05 (two-tailed Student's t-test). αIL-39, anti-mouse interleukin-39 polyclonal antibodies.
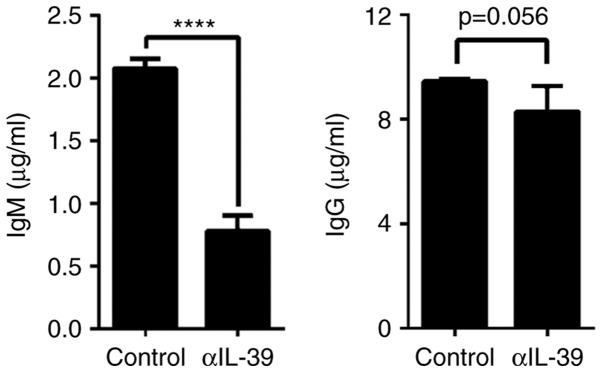
Anti-IL-39 polyclonal antibodies reduce autoantibody titers in MRL/lpr mice
To examine the effect of anti-IL-39 polyclonal antibodies on the production of autoantibody in MRL/lpr mice, sera were obtained from the preimmune IgG-(control) or anti-IL-39 polyclonal antibody-treated MRL/lpr mice. The ELISA assay showed that anti-IL-39 polyclonal antibodies significantly reduced anti-dsDNA IgM autoantibody titers in the MRL/lpr mice (Fig. 4, left panel). The anti-IL-39 polyclonal antibodies also reduced the anti-dsDNA IgG autoantibody titers, although the reduction was not significant (Fig. 4, right panel). Taken together, these results suggested that anti-IL-39 polyclonal antibodies reduced autoantibody titers in the MRL/lpr mice.
Figure 4.
Treatment with αIL-39 reduces autoantibody titers in MRL/lpr mice. Either 400 µg of preimmune IgG (control) or αIL-39 were intravenously injected into 6-month-old female MRL/lpr mice (6 mice/group). On day 7 post-treatment, sera were separated from the peripheral blood of the control- and αIL-39-treated mice. The concentrations of anti-dsDNA IgM and IgG antibody in the sera were measured using an ELISA assay. The data are presented as the mean ± standard error of the mean of three independent experiments (n=18). ****P<0.0001 (two-tailed Student's t-test). αIL-39, anti-mouse interleukin-39 polyclonal antibodies.
Anti-IL-39 polyclonal antibodies reduce proteinuria in MRL/lpr mice
The present study also examined the effect of anti-IL-39 polyclonal antibodies on the production of proteinuria in MRL/lpr mice. Proteinuria was measured on days 0 and 7 following injection with anti-IL-39 polyclonal antibodies in the MRL/lpr mice. Proteinuria increased in a time-dependent manner in the control-treated MRL/lpr mice (Fig. 5). Anti-IL-39 polyclonal antibodies effectively inhibited the upregulation of proteinuria in the MRL/lpr mice (Fig. 5). The results suggested that anti-IL-39 polyclonal antibodies reduced proteinuria in the MRL/lpr mice.
Figure 5.
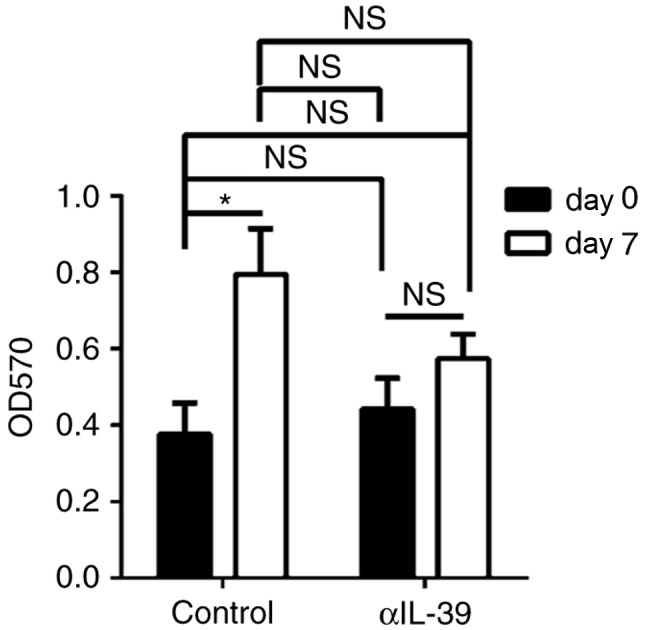
Treatment with αIL-39 reduces proteinuria in MRL/lpr mice. Proteinuria was measured on days 0 and 7 following intravenous injection of 400 µg of preimmune IgG (control) or αIL-39 into 6-month-old female MRL/lpr mice (6 mice/group). The data are presented as the mean ± standard error of the mean of three independent experiments (n=18). *P<0.05 (two-way analysis of variance). αIL-39, anti-mouse interleukin-39 polyclonal antibodies; OD, optical density; d, day; NS, not significant.
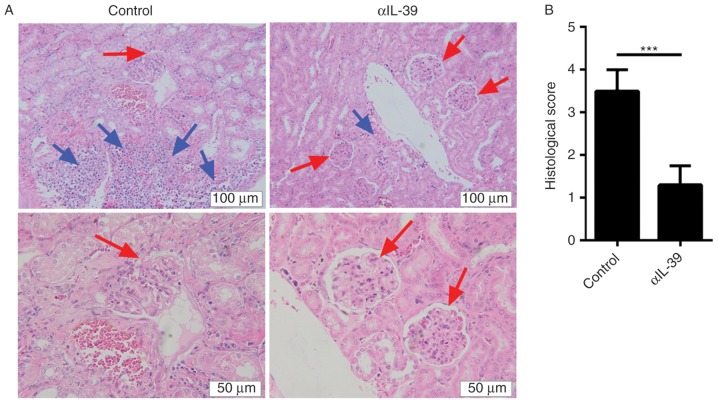
Anti-IL-39 polyclonal antibodies reduce infiltrating inflammatory cells and restore the structure of the glomerular region in MRL/lpr mice
Autoantibody production and immune-complex-mediated glomerulonephritis are important features of systemic autoimmunity in human SLE. As the anti-IL-39 polyclonal antibodies were shown to reduce the levels of autoantibody and proteinuria in the MRL/lpr mice in the present study, it was hypothesized that anti-IL-39 polyclonal antibodies can reduce infiltrating inflammatory cells and glomerulonephritis. To examine this, kidney sections were stained with H&E and analyzed 7 days following treatment with anti-IL-39 polyclonal antibodies. As expected, the untreated MRL/lpr mice developed severe renal lesions, consisting predominantly of glomerulosclerosis, and multifocal mononuclear cell infiltration (Fig. 6A). However, the frequency and severity of renal lesions were markedly reduced in the anti-IL-39-treated MRL/lpr mice (Fig. 6A). Quantification showed that the anti-IL-39-treated MRL/lpr mice had significantly fewer glomerular lesions, compared with the untreated MRL/lpr mice (Fig. 6B). These results indicated that anti-IL-39 polyclonal antibodies reduced infiltrating inflammatory cells and restored the structure of the glomerular region in MRL/lpr mice.
Figure 6.
Treatment with αIL-39 reduces infiltrating inflammatory cells and restores the structure of the glomerular region in MRL/lpr mice. (A) Kidney sections were stained with hematoxylin and eosin on day 7 following intravenous injection of 400 µg preimmune IgG (control) or αIL-39 into 6-month-old female MRL/lpr mice (6 mice/group). Red arrows indicate glomeruli and blue arrows indicate infiltrating inflammatory cells. Scale bar, 100 µM (upper panel) and 50 µM (lower panel). (B) Glomerular lesion scoring of kidneys was assessed in a blinded-manner using a four-point scale, and the results are expressed as the mean ± standard error of the mean from 10 glomeruli per kidney from both kidneys of 10 mice per group. ***P<0.001 (two-tailed Student's t-test). αIL-39, anti-mouse interleukin-39 polyclonal antibodies.
Discussion
IL-12 family members include important pro- and anti-inflammatory cytokines, which are important in autoimmune diseases (1–3). Our previous study showed that IL-12 family cytokine subunits IL-23p19 and Ebi3 formed a novel heterodimer (IL-23p19/Ebi3) cytokine in lupus-like mice (13). In the present study, it was demonstrated that the inhibition of IL-39 with anti-mouse IL-39 polyclonal antibodies ameliorated autoimmune symptoms in lupus-like mice. Together, the results suggested that this novel IL-12 family member, IL-39, is an important pro-inflammatory cytokine, which appears to be important in autoimmune diseases, including SLE.
Compared with the anti-mouse p19 antibody, the anti-mouse IL-39 polyclonal antibody had a higher capacity to bind with the recombinant mouse IL-39 (p19/Ebi3) heterodimer (Fig. 1B). Of note, the binding of anti-mouse IL-39 polyclonal antibodies with recombinant mouse IL-39 subunits p19 or Ebi3 was marginal (data not shown). Taken together, these results suggested that anti-mouse IL-39 polyclonal antibodies were able to effectively and specifically bind with its target, molecular IL-39 (p19/Ebi3 heterodimer).
SLE is an autoimmune disease with hyperactive immune responses, abnormal autoantibody production and B-cell overactivation. Belimumab, a monoclonal antibody inhibiting BAFF, is the only biotherapy to be approved for SLE in the last 50 years, and only modest and time-limited effects have been recorded in large, phase three clinical trials (10,26,27). Drugs directly targeting/depleting B cells, including rituximab, atumumab and epratuzumab, have also been used to treat patients with SLE and other autoimmune diseases, and they have all shown biological effects against SLE (18), although the identification of novel relevant targets is required. In the present study, anti-mouse IL-39 polyclonal antibodies were used to effectively ameliorate autoimmune symptoms in lupus-like mice. Therefore, targeting IL-39 may be an effective approach to treat autoimmune diseases, including SLE.
Increased quantities of inflammatory cytokines have been described in chronic immune activation in SLE, which actively contribute to local inflammation and tissue damage (27). Understanding the inflammatory pathways involved in SLE is of primary importance for the development of novel targeted biotherapies, which are currently lacking for the therapeutic treatment of SLE (28). In our previous study, it was found that IL-39 induced the differentiation and/or expansion of neutrophils, and IL-39-induced neutrophils secreted BAFF in lupus-prone mice (29). Accordingly, the present study showed that anti-mouse IL-39 polyclonal antibodies effectively reduced the number of neutrophils (Fig. 2). Neutrophils contribute to excess serum levels of BAFF, and promote CD4+ T cell and B cell activation and responses in lupus-prone mice (30). It is possible that anti-mouse IL-39 polyclonal antibodies effectively reduce activated B and T cells by reducing BAFF-secreting neutrophils. By reducing the number of inflammatory cells, the anti-mouse IL-39 polyclonal antibodies also reduced splenomegaly, autoantibody titers, proteinuria and the infiltration of inflammatory cells, and restored structure in the glomerular region in lupus-like mice. Therefore, anti-IL-39 may be an effective reagent in the treatment of autoimmune diseases, including SLE.
A previous study involving anti-BAFF treatment in mice demonstrated that memory B cells, long-lived plasma cells and secondary immune responses were transiently increased and maintained at high levels, during and following exposure (31,32). IgG-bearing memory B cells and natural antibody-secreting B cells were insensitive to anti-BAFF treatment, which suggests they were BAFF-independent and, therefore, may be manipulated separately (33). In the present study, anti-IL-39 polyclonal antibodies effectively reduced IgM titers (Fig. 4) and IgG-bearing memory B cells (Fig. 2). These results showed the potential advantages of anti-IL-39 polyclonal antibodies in the treatment of autoimmune diseases. However, the polyclonal antibodies reduced only anti-dsDNA IgM, but not anti-dsDNA IgG (Fig. 4). A previous study suggested that pathogenic IgG autoantibody production requires B-cell activation, leading to the production of activation-induced deaminase (AID), and class switching of IgM genes to IgG. The absence of AID in early developing B cells resulted in the increased production of self-reactive IgM in lupus-prone mice (34), which also suggests that the production of IgM and IgG are separate.
In the present study, a single dose (400 µg/mouse) of anti-mouse IL-39 polyclonal antibodies was used for injection. This was based on our previous experiments, in which a single dose (400 µg/mouse) of anti-mouse C5a polyclonal antibody neutralized the effect of C5a in mice with sepsis (22). However, the present study showed no statistically significant difference in proteinuria 7 days following treatment between the control- and anti-IL-39 antibody-treated MRL/lpr mice (Fig. 5). In addition, the polyclonal antibodies reduced anti-dsDNA IgM, but not anti-dsDNA IgG (Fig. 4). These results suggest that further investigations are required to examine the detailed effect of anti-IL-39 polyclonal antibodies by comparing a single dose, vs. multiple doses, and a 7 day interval, vs. longer intervals between injection and sacrifice, considering dose-dependency, the duration of effects of single administration, and the half-life of anti-IL-39 following administration into MLR/lpr mice. Future investigations aim to examine these and other parameters.
In conclusion, the anti-mouse IL-39 polyclonal antibodies produced in the present study effectively ameliorated autoimmune symptoms in lupus-like mice. IL-12 family members have been examined as the therapeutic targets for several autoimmune diseases. The results of the present study suggested that IL-39 may be used as a potential target for the treatment of SLE. Future investigations are required to understand the immunobiology of this novel IL-12 family member, which is likely to provide valuable knowledge for therapeutic exploitation.
Acknowledgements
This study was supported by the National Basic Research Program 973 Grants (grant nos. 2013CB530506 and 2015CB553704), the National Nature and Science Funds (grant nos. 81471529, 81401332, 81272320, 81471540 and 81472647), the Key Program of the Beijing Natural Science Foundation (grant no. 7141007) and the Service Industry Scientific Research of National Health and Family Planning Commission of China (grant no. 2015SQ00192).
Glossary
Abbreviations
- SLE
systemic lupus erythematosus
- IL
interleukin
- BAFF
B-cell activating factor
References
- 1.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 2.Vignali DA, Kuchroo VK. IL-12 family cytokines: Immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015;75:249–255. doi: 10.1016/j.cyto.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova J, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 6.De S, Barnes BJ. B cell transcription factors: Potential new therapeutic targets for SLE. Clin Immunol. 2014;152:140–151. doi: 10.1016/j.clim.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18:468–475. doi: 10.1097/01.bor.0000240356.45550.13. [DOI] [PubMed] [Google Scholar]
- 8.Isenberg D, Rahman A. Systemic lupus erythematosus-2005 annus mirabillis. Nat Clin Pract Rheumatol. 2006;2:145–152. doi: 10.1038/ncprheum0116. [DOI] [PubMed] [Google Scholar]
- 9.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa F. Animal models of cutaneous lupus erythematosus and lupus erythematosus photosensitivity. Lupus. 1997;6:193–202. doi: 10.1177/096120339700600215. [DOI] [PubMed] [Google Scholar]
- 12.Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Ma N, Shen B, et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in lupus-like mice. Eur J Immunol. 2016;46:1343–1350. doi: 10.1002/eji.201546095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365–373. doi: 10.1038/nrrheum.2014.33. [DOI] [PubMed] [Google Scholar]
- 15.Ma N, Xing C, Xiao H, He Y, Han G, Chen G, Hou C, Marrero B, Wang Y, Zhang S, et al. BAFF suppresses IL-15 expression in B cells. J Immunol. 2014;192:4192–4201. doi: 10.4049/jimmunol.1302132. [DOI] [PubMed] [Google Scholar]
- 16.Cervenak L, Magyar A, Boja R, László G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett. 2001;78:89–96. doi: 10.1016/S0165-2478(01)00239-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Zhang L, Ma N, et al. Pre-existing CD19-independent GL7(−) Breg cells are expanded during inflammation and in mice with lupus-like disease. Mol Immunol. 2016;71:54–63. doi: 10.1016/j.molimm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent FB, Morand EF, Mackay F. BAFF and innate immunity: New therapeutic targets for systemic lupus erythematosus. Immunol Cell Biol. 2012;90:293–303. doi: 10.1038/icb.2011.111. [DOI] [PubMed] [Google Scholar]
- 19.Ma N, Liu X, Xing C, Wang X, Wei Y, Han G, Chen G, Hou C, Shen B, Li Y, et al. Ligation of metabotropic glutamate receptor 3 (Grm3) ameliorates lupus-like disease by reducing B cells. Clin Immunol. 2015;160:142–154. doi: 10.1016/j.clim.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2:1345–1354. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 21.Avey MT, Fenwick N, Griffin G. The use of systematic reviews and reporting guidelines to advance the implementation of the 3Rs. J Am Assoc Lab Anim Sci. 2015;54:153–162. [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R, Wang R, Han G, Wang J, Chen G, Wang L, Li X, Guo R, Shen B, Li Y. Complement C5a regulates IL-17 by affecting the crosstalk between DC and gammadelta T cells in CLP-induced sepsis. Eur J Immunol. 2010;40:1079–1088. doi: 10.1002/eji.200940015. [DOI] [PubMed] [Google Scholar]
- 23.Zheng M, Xing C, Xiao H, Ma N, Wang X, Han G, Chen G, Hou C, Shen B, Li Y, Wang R. Interaction of CD5 and CD72 is involved in regulatory T and B cell homeostasis. Immunol Invest. 2014;43:705–716. doi: 10.3109/08820139.2014.917096. [DOI] [PubMed] [Google Scholar]
- 24.Xing C, Ma N, Xiao H, Wang X, Zheng M, Han G, Chen G, Hou C, Shen B, Li Y, Wang R. Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. J Leukoc Biol. 2015;97:547–556. doi: 10.1189/jlb.3A0414-213RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 26.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 27.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: Challenges to treatment. Lancet. 2013;382:809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, Chen G, Hou C, Wang T, Ma N, et al. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol. 2016;186:144–156. doi: 10.1111/cei.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coquery CM, Wade NS, Loo WM, Kinchen JM, Cox KM, Jiang C, Tung KS, Erickson LD. Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice. PLoS One. 2014;9:e102284. doi: 10.1371/journal.pone.0102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, Lappin PB, Riccobene T, Abramian D, Sekut L, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 32.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 33.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O'Neill PJ, Quinn WJ, III, Goenka R, Miller JP, Cho YH, Long V, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci USA. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umiker BR, McDonald G, Larbi A, Medina CO, Hobeika E, Reth M, Imanishi-Kari T. Production of IgG autoantibody requires expression of activation-induced deaminase in early-developing B cells in a mouse model of SLE. Eur J Immunol. 2014;44:3093–3108. doi: 10.1002/eji.201344282. [DOI] [PMC free article] [PubMed] [Google Scholar]