Abstract
The present study investigated the influence of cigarette smoke extract (CSE) and nicotine on the expression of thrombomodulin (TM) and endothelial protein C receptor (EPCR) in human umbilical vein endothelial cells (HUVECs). Smoking is associated with intravascular thrombosis. As a vital anticoagulation cofactor, TM is located on the endothelial cell surface and regulates intravascular coagulation by binding to thrombin, hence activating protein C. Activated protein C is a natural anticoagulant that interacts with EPCR to enhance the function of anticoagulation system. The effects of CSE (0.5–5%) and nicotine (10-3-10-9 mol/l) on the expression of TM and EPCR in HUVECs were observed. Reverse transcription-quantitative polymerase chain reaction and flow cytometric analysis techniques were used for detecting TM and EPCR mRNA and protein expression levels, respectively. After 6-h exposure, TM protein and mRNA expression levels decreased in a dose-dependent manner. Stimulation with 5% CSE for 0, 6, 10, 12 and 24 h led to a decrease in the levels of TM mRNA and protein over time, which reached a peak at 12 h. The levels were significantly reduced compared with the control group (P<0.001). However, CSE had no effect on EPCR. Furthermore, nicotine had no influence on TM and EPCR. In conclusion, the present study supports a novel molecular mechanism of cigarette smoking-associated thrombosis by the decreased expression of TM. Further studies are required to identify specific components in CSE responsible for decreasing TM expression and its associated consequences.
Keywords: cigarette smoke extract, endothelial protein C receptor, human umbilical vein endothelial cells, nicotine, thrombomodulin
Introduction
Cigarette smoking is an independent risk factor for cardiovascular disease that can lead to intravascular thrombosis. It is associated with significantly increased rates of acute coronary syndrome and sudden cardiac death (1). Autopsy and clinical studies also demonstrated that smoking is critical to arterial thrombosis via a direct toxic effect on the injured endothelial cells, resulting in alterations of the hemostatic system and an increase in platelet reactivity that is relevant for the pathogenic effect of atherothrombosis (2,3). Thrombosis is affected by the anticoagulation and hemostatic systems. However, the mechanism by which cigarette smoking causes thrombosis via the anticoagulant system is not clear.
Thrombomodulin (TM) expressed on the surface of the endothelial cells is critical for anticoagulation (4). Its major function is to form a complex with thrombin at a 1:1 high affinity, which activates protein C. Activated protein C is a natural anticoagulant that interacts with the endothelial protein C receptor (EPCR) to enhance the function of anticoagulation system. Functional deficiency of the anticoagulation system could enhance thrombosis formation. As observed, the endothelium-specific loss of TM in mice led to spontaneous and fatal thrombosis in their arterial and venous circulation (5). For human beings, the downregulation of endothelial TM or EPCR expression in certain pathologic conditions, including meningococcal sepsis and graft rejection, may result in thrombotic complications (6,7). Thus, it was hypothesized that cigarette smoking could reduce the essential antithrombotic functions of endothelial cells by inhibiting TM or EPCR expression.
Cigarette smoke comprises >5,600 distinct components (8). Hence, it is important to identify which specific components might be responsible for the observed effects. Nicotine is an indispensable constituent of cigarette smoke. Studies have demonstrated that nicotine serves as a catalyst for endothelial and smooth muscle cell proliferation, and angiogenesis, vascular inflammation and enhancement of atherogenesis (9–12). Therefore, it was hypothesized that nicotine may also contribute to the development of thrombosis by inhibiting TM or EPCR expression.
To evaluate these hypotheses, a novel study was performed to examine the effects of cigarette smoke extract (CSE) and nicotine on TM and EPCR expression in human umbilical vein endothelial cells (HUVECs) in vitro.
Materials and methods
Materials
Nicotine was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The monoclonal antibodies for TM (cat no. ab6980) and EPCR (cat no. ab56689) were obtained from Abcam (Shanghai, China). Goat polyclonal anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) (cat no. ab6785) was purchased from Abcam. A fluorescent quantitation kit was purchased from Takara Biotechnology Co., Ltd. (Dalian, China). Tumor necrosis factor (TNF)-α was purchased from ProSpec (East Brunswick, NJ, USA). The reagents collagenase I, medium 200 (M200) and low serum growth supplement (LSGS) were bought from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum was purchased from Hyclone; GE Healthcare (Chicago, IL, USA). TRIzol reagent was purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Factor VIII-related antibody (cat no. ZA-0111) was purchased from Zhongshan Goldenbridge Biotechnology Co., Ltd. (Beijing, China). Goat polyclonal anti-rabbit immunoglobulin G (IgG)-streptavidin horseradish peroxidase (HRP; cat no. 12–348) was purchased from EMD Millipore (Billerica, MA, USA). Other reagents used in the experiments were all of analytical grade.
CSE preparation
CSE was prepared using a previously reported method with slight modification (13,14). Two cigarettes [each containing 1.1 mg nicotine, 13 mg carbon monoxide and 12 mg tar (without filters; Hongmei brand, Hongta Tobacco Group, Yuxi, China)] were smoked consecutively through a tea infusion using a 50-ml injection syringe with a constant airflow. The smoke was bubbled through 50 ml phosphate buffered-saline (PBS, 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 140 mM NaCl, pH=7.4) controlled by a three-way cock mimicking the gas fluid pathway during the smoking process in the human lung. The obtained CSE solution was filtered through a 0.22-µm filter to remove bacterial and large particles. The pH of the CSE-PBS buffer was adjusted to a final value of 7.4. The concentration of this obtained stock solution was defined as 100% CSE, and the solution was diluted using PBS to a final concentration required in the experiment. The CSE solution was prepared immediately prior to each experiment.
Cell culture
HUVECs were isolated using an enzymatic technique (15). Umbilical vein was obtained from the umbilical cord of a healthy baby born by caesarean section in the Department of Gynecology and Obstetrics, the General Hospital of Chinese People's Armed Police Forces (Beijing, China). Mothers agreed and provided signed consent forms. Approval was obtained from the hospital ethics committee. The veins were flushed with PBS, filled with 0.1% collagenase I, clamped at either end, and incubated in 95% air/5% CO2 at 37°C for 15–20 min. The collagenase solution was subsequently decanted into a sterile tube and centrifuged at 300 × g for 10 min at 37°C to produce an enteric-coated pellet. The pellet was then resuspended in the low serum endothelial cell medium (M200 + LSGS) in an atmosphere of 95% air/5% CO2 at 37°C. The medium was replaced after 24 h, and subsequently every 48 h; HUVECs were plated onto gelatin-coated 35-mm dishes (2×105 cells/ml) and incubated overnight to produce a confluent monolayer of cells. The cells were used for the experiment at passages 2–3. Factor VIII-related antibody was used to identify endothelial cells by immunocytochemistry staining (16). HUVECs were fixed in 4% paraformaldehyde solution for 30 min at 4°C, washed in 0.1 M glycine (2×5 min, 22°C), incubated with 3% hydrogen peroxide (5 min, 22°C), rinsed in 0.01 M PBS (6.8 mM Na2HPO4, 2.6 mM NaH2PO4, pH 7.2), and incubated with heat-inactivated goat serum (1:20 in 0.01 M PBS, 1 h, 22°C). Cells were incubated with Factor VIII-related antibody (1:100 dilution), overnight at 22°C in a humidified chamber. Cells were washed in 0.01 M PBS (6×5 min, 22°C), incubated with goat polyclonal anti-rabbit immunoglobulin G (IgG)-streptavidin HRP (1:200 in 0.01 M PBS; 1 h, 22°C). After a further 3×5 min washes in 0.01 M PBS, cells were incubated with 0.1 M acetate buffer (pH 5.3; 3 min, 22°C), and 3-amino-9-ethylcarbazole solution for 3 min at 22°C for detection of vWF. Cells were rinsed in distilled water. Coverslips were mounted onto microscope slides using glycerol. Photomicrographs were obtained using a Nikon DS-Fi2 camera connected to a Nikon Eclipse Ti microscope.
The HUVECs were seeded into 6-well plates (2×105 cells/ml). The cells were then grown in complete medium for 48 h to 90% confluence, with a change of the medium, and incubated with 0.5, 1, 3.5 or 5% CSE or 10-3 to 10-9 mol/l nicotine for 0, 6, 10, 12 or 24 h. Control cells received only the fresh medium. All experiments were performed in triplicate.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were washed with cold PBS twice, and total RNA was extracted using TRIzol reagent. Briefly, the cells were harvested and treated with 0.5 ml TRIzol reagent for 5 min at room temperature and then centrifuged at 12,000 × g for 15 min at 4°C. The aqueous portion containing RNA was transferred to another RNAase-free Eppendorf tube, and 0.5 ml isopropanol was added and incubated for 10 min at room temperature, followed by precipitating total RNA by centrifugation at 7,500 × g at 4°C for 10 min. The pellet was then washed with 75% ethanol twice and dried at room temperature on ice for 5 min; total RNA pellet was dissolved in 20 µl H2O. The RNA concentration was measured at A260/A280 using the NanoVue spectrophotometer. cDNA was obtained from reverse transcription of 1 µg total RNA in a 20-µl reaction volume using an iScrip cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The primers for TM and β-actin were designed using Beacon Designer 2.1 software (Premier Biosoft International, Palo Alto, CA, USA) and synthesized using Sangon Biotech, Co., Ltd. (Shanghai, China). The designed oligonucleotide sequences were as follows: TM: 5′-CCCAACACCCAGGCTAGCT-3′ (forward), 5′-CGTCGATGTCCGTGCAGAT-3′ (reverse); EPCR: 5′-ATTGCTGCCGATACTGCT-3′ (forward), 5′-AGAGGAAAGGCCAAGGTC-3′ (reverse); β-actin: 5′-TCACCAACTGGGACGACA-3′ (forward), 5′-ACAGCCTGGATAGCAACG-3′ (reverse). qPCR was performed using a fluorescent quantitation kit (Takara Biotechnology, Co., Ltd.). Briefly, 1 µl of the reaction product of the RT reaction was used in a 25-µl PCR reaction. The annealing temperature and primer concentration were adapted for each gene. The amplification product was initially incubated at 95°C for 10 sec at 20°C, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 45°C for 10 sec, extension at 72°C for 10 sec, and elongation at 80°C for 0.1 sec, using an PCR detection system. Data were analyzed using LightCycler 2.0 software (Applied Biosystems; Thermo Fisher Scientific, Inc.) and presented as the relative amount of mRNA using the formula 2−∆∆Cq, which stands for the difference in the quantitation cycle (Cq) between a gene of interest and the housekeeping gene β-actin (17). The Cq is the point at which sample fluorescence rises above the background level. Each sample was measured in triplicate. mRNA (no reverse transcription) or H2O (no DNA samples) were included as negative controls.
Flow cytometry
The cells were harvested and isolated by papain and centrifuged at 500 × g for 5 min at 10°C twice. They were then incubated with anti-TM and monoclonal anti-EPCR antibodies (1:100 dilution) for 30 min at 4°C, washed twice with the PBS buffer, and incubated with FITC-conjugated goat anti-mouse IgG (1:100 dilution) in the PBS buffer at 4°C for 30 min. The cells were washed again with the PBS buffer, and cell-bound fluorescence was determined by flow cytometry, with 30,000 events/sample counted using a FACS calibur cytofluorometer (BD Biosciences, Franklin Lakes, NJ, USA). The data were analyzed using WinMDI 2.9 software (Scripps Research Institute, La Jolla, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation and statistically analyzed using one-way or two-way analysis of variance as appropriate, followed by the Student-Newman-Keuls method. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of CSE and nicotine on TM mRNA expression in HUVECs
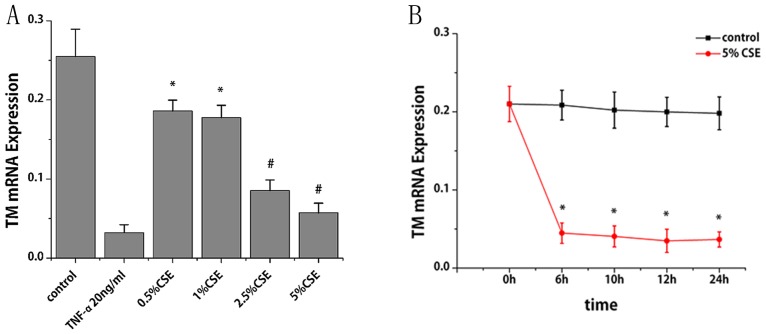
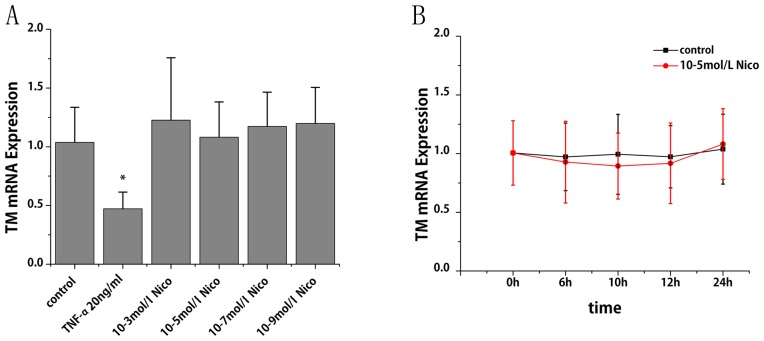
More than 95% of the endothelial cell cytoplasm contained brown granules, confirming that the cultured cells were HUVECs (data not shown). RT-qPCR was performed to study the effect of CSE and nicotine on TM mRNA expression. CSE treatment for 6 h led to a reduction TM mRNA expression in a dose-dependent manner. Compared with the control group, TM mRNA levels decreased by 27, 30, 67 and 78% following treatment of CSE at 0.5, 1, 2.5 and 5%, respectively (P<0.01; Fig. 1A). With the treatment of CSE (5%), TM mRNA decreased by 79, 80, 83 and 82% at 6, 10, 12 and 24 h, respectively (P<0.001; Fig. 1B). Notably, TM mRNA expression decreased progressively within 12 h; however, it slightly increased at 24 h compared with at 12 h. The specific constituent of cigarette smoke that might be responsible for the observed inhibition was subsequently investigated. As presented in Fig. 2A and B, nicotine at a dose ranging from 10-3 to 10-9 mol/l did not cause any significant alterations in TM mRNA expression.
Figure 1.
Effects of CSE on TM mRNA expression in human umbilical vein endothelial cells. TM mRNA expression levels were assessed by reverse transcription-quantitative polymerase chain reaction (A) following various doses of CSE and (B) across various time points. β-actin served as an internal control. Data are expressed as the mean ± standard deviation. *P<0.01 and #P<0.001 vs. control group. CSE, cigarette smoke extract; TM, thrombomodulin; TNF-α, tumor necrosis factor-α (positive control).
Figure 2.
Effects of nicotine on TM mRNA expression in human umbilical vein endothelial cells. TM mRNA expression levels were assessed by reverse transcription-quantitative polymerase chain reaction (A) following various doses of nicotine and (B) across various time points. Data are expressed as the mean ± standard deviation. *P<0.01 vs. control group. Nico, nicotine; TM, thrombomodulin; TNF-α, tumor necrosis factor-α (positive control).
Effects of CSE and nicotine on TM protein expression levels in HUVECs
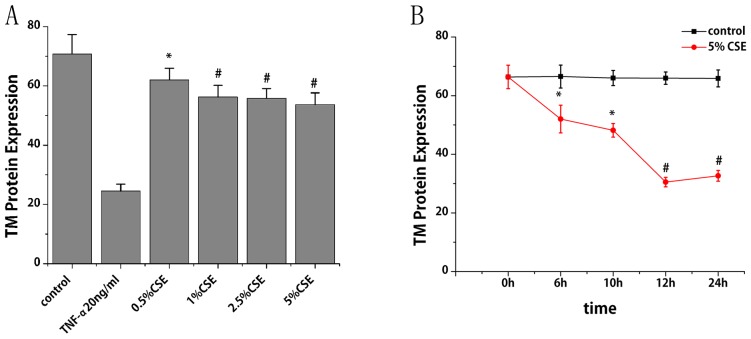
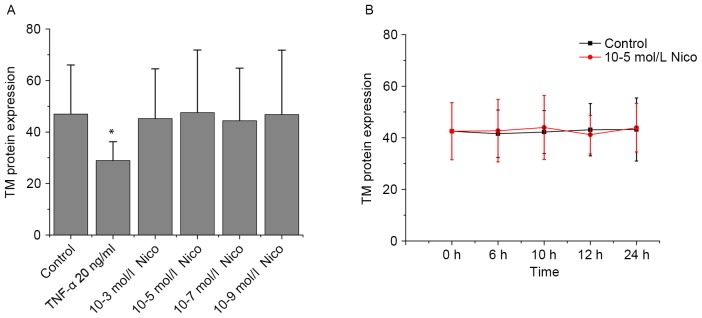
Flow cytometry was performed to study the effect of CSE on TM protein expression. With the treatment of CSE (0.5, 1, 2.5 and 5%) for 6 h, TM protein expression levels reduced by 12, 21, 21 and 24%, respectively, compared with the control group (P<0.05; Fig. 3A). With the treatment of CSE (5%) for different times, TM protein expression levels decreased by 22, 27, 54 and 50% at 6, 10, 12 and 24 h, respectively, compared with the control group (P<0.05; Fig. 3B). TM protein expression levels were also observed to decrease progressively within 12 h, and then increase slightly at 24 h. As presented in Fig. 4A and B, nicotine did not induce any significant alterations in TM protein expression.
Figure 3.
Effects of CSE on TM protein expression in HUVECs. TM protein expression levels in HUVECs were measured by flow cytometry (A) following various doses of CSE and (B) across various time points. Data are expressed as the mean ± standard deviation. *P<0.05 and #P<0.01 vs. control group. TM, thrombomodulin; TNF-α, tumor necrosis factor-α (positive control); HUVECs, human umbilical vein endothelial cells; CSE, cigarette smoke extract.
Figure 4.
Effects of nicotine on TM protein expression in human umbilical vein endothelial cells. TM protein expression levels were assessed by flow cytometry (A) following various doses of nicotine and (B) across various time points. Data are expressed as the mean ± standard deviation. *P<0.01 vs. control group. Nico, nicotine; TM, thrombomodulin; TNF-α, tumor necrosis factor-α (positive control).
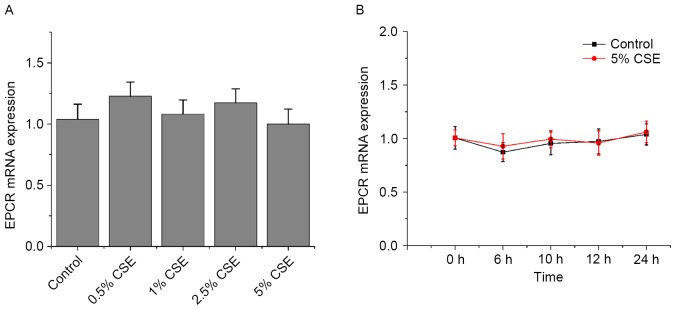
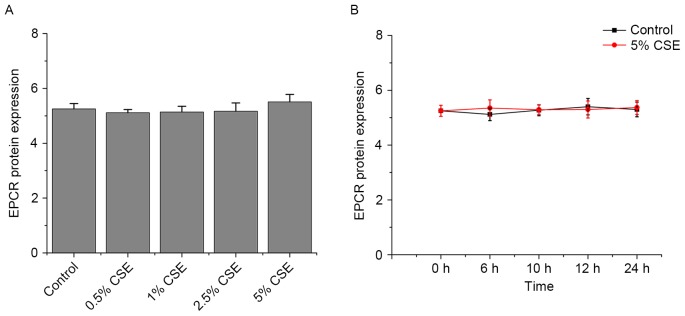
Effects of CSE and nicotine on EPCR expression in HUVECs
As presented in Figs. 5 and 6, CSE and nicotine had no effect on EPCR expression.
Figure 5.
Effects of CSE on TM mRNA expression in human umbilical vein endothelial cells. TM mRNA expression levels were assessed by reverse transcription-quantitative polymerase chain reaction (A) following various doses of CSE and (B) across various time points. β-actin served as an internal control. Data are expressed as the mean ± standard deviation. CSE, cigarette smoke extract; TM, thrombomodulin; EPCR, endothelial protein C receptor.
Figure 6.
Effects of CSE on TM protein expression in human umbilical vein endothelial cells. TM protein expression levels were assessed by flow cytometry (A) following various doses of CSE and (B) across various time points. β-actin served as an internal control. Data are expressed as the mean ± standard deviation. CSE, cigarette smoke extract; TM, thrombomodulin; EPCR, endothelial protein C receptor.
Discussion
The present study demonstrated that CSE significantly decreased TM expression in a dose- and time-dependent manner in HUVECs. Both mRNA and protein expression levels were significantly reduced in CSE-treated HUVECs. The data from this study suggested a novel molecular mechanism of cigarette smoking-associated thrombosis by the decreased expression of TM. However, CSE had no effect on EPCR.
Previous studies on the control of TM expression have been performed in HUVECs, but studies on the influence of cigarette smoke on TM and EPCR expression are lacking. Previous studies have demonstrated that the expression of TM is inhibited by inflammatory and procoagulant molecules and environmental factors, including interleukin (IL)-1β, lipopolysaccharide, TNF-α, C-reactive protein, oxidized low-density lipoprotein, transforming growth factor (TGF)-β1, TGF-β2, irradiation and shear (18–20). TM expression was increased by anti-inflammatory and anticoagulant molecules and environmental factors, including retinoic acid, cyclic adenosine monophosphate, IL-4, prostacyclin, okadaic acid, curcumin, vascular endothelial growth factor, prostaglandin E1, statins, histamine and heat shock (21–23). Cigarette smoking is associated with oxidative stress, increased blood thrombogenicity and an inflammatory response. In humans, cigarette smoking is associated with increased levels of multiple inflammatory and procoagulant molecules, such as TNF-α, peripheral leukocytes, C-reactive protein, IL-6 and homocysteine (24–26). Furthermore, cigarette smoke can directly provide free radicals to induce oxidative stress. The present study examined the damaging effect of cigarette smoke on TM expression. It was earlier observed that CSE could notably reduce the binding probability of TM and thrombin (27). EPCR can be downregulated at the transcriptional level by shear stress and inflammation to kinases, including IL-1α and TNF-α (28,29). However, CSE had no effect on EPCR.
Cigarette smoke comprises >5,600 distinct components, of which >200 have been identified as carcinogens and respiratory toxins. The present study investigated whether nicotine was responsible for inhibiting TM and EPCR expression. Although nicotine is the well-recognized component of cigarette smoke, it had no effect on TM and EPCR expression in HUVECs. Cornel et al (30) observed that Korean teenage and adult smokers had 0.27 and 0.5 µM plasma nicotine concentrations, respectively. Al Mutairi et al (31) reported a nicotine concentration of up to 13 µM in Kuwaiti smokers. However, the highest concentration tested in this study was ~three times greater than the reported blood levels in smokers. Nicotine still did not induce any change in TM and EPCR expression in HUVECs. Previous studies have demonstrated that the effect of nicotine on thrombohemostatic factors, including platelets, fibrinogen, tissue plasminogen activator and plasminogen activator inhibitor-1, is small and probably serves only a minor role in atherothrombotic events directly (32–34).
The present study demonstrated following CSE treatment, TM expression decreased progressively within 12 h; however, it increased slightly at 24 h. It is possible that the effect of CSE on TM expression reached saturation after 12 h, or a part of soluble substances in CSE volatilized over time, weakening the effect of CSE on TM expression. In addition, the decrease in TM mRNA expression was more obvious than the decrease in TM protein expression at 6 h. It is possible that CSE acts at the transcriptional level, leading to the downregulation of TM protein. The present study had some limitations. The insoluble and volatile harmful ingredients in tobacco were not included in the study, and HUVECs were the only cell model used.
In conclusion, the present study demonstrated that exposure to CSE (except nicotine) significantly decreased the expression of TM in HUVECs in a concentration-dependent manner. The results of the present study support a noevl molecular mechanism of cigarette smoking-associated thrombosis by the decreased expression of TM. Further studies are required to investigate the distinct components in CSE responsible for decreasing TM expression and its associated consequences.
Acknowledgements
The present study was supported by the Nature Science Foundation of China (grant no. 81370315).
References
- 1.Dunbar A, Gotsis W, Frishman W. Second-hand tobacco smoke and cardiovascular disease risk: An epidemiological review. Cardiol Rev. 2014;21:94–100. doi: 10.1097/CRD.0b013e31827362e4. [DOI] [PubMed] [Google Scholar]
- 2.Szpak D, Grochowalski A, Chrzaszcz R, Florek E, Jawień W, Undas A. Tobacco smoke exposure and endothelial dysfunction in patients with advanced coronary artery disease. Pol Arch Med Wewn. 2013;123:474–481. doi: 10.20452/pamw.1889. [DOI] [PubMed] [Google Scholar]
- 3.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol. 2013;33:1460–1467. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Maruyama I. Thrombomodulin: Protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011;9(Suppl 1):S168–S173. doi: 10.1111/j.1538-7836.2011.04319.x. [DOI] [PubMed] [Google Scholar]
- 5.Isermann B, Hendrickson SB, Zogg M, Wing M, Cummiskey M, Kisanuki YY, Yanagisawa M, Weiler H. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest. 2001;108:537–546. doi: 10.1172/JCI200113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, Esmon CT, Heyderman RS. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408–416. doi: 10.1056/NEJM200108093450603. [DOI] [PubMed] [Google Scholar]
- 7.Iino S, Abeyama K, Kawahara KI, Aikou T, Maruyama I. Thrombomodulin expression on Langerhans' islet: Can endogenous ‘anticoagulant on demand’ overcome detrimental thrombotic complications in clinical islet transplantation. J Thromb Haemost. 2011;2(833–844):2004. doi: 10.1111/j.1538-7836.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 8.Perfett TA, Rodgman A. The complexity of tobacco and tobacco smoke. Beitr Tabakforsch Int. 2011;24:215–232. [Google Scholar]
- 9.Li JM, Cui TX, Shiuchi T, Liu HW, Min LJ, Okumura M, Jinno T, Wu L, Iwai M, Horiuchi M. Nicotine enhances angiotensin II-induced mitogenic response in vascular smooth muscle cells and fibroblasts. Arterioscler Thromb Vasc Biol. 2004;24:80–84. doi: 10.1161/01.ATV.0000104007.17365.1c. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Cooke JP. Nicotine and pathological angiogenesis. Life Sci. 2012;91:1058–1064. doi: 10.1016/j.lfs.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–496. doi: 10.1016/S0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 12.Lau PP, Li L, Merched AJ, Zhang AL, Ko KW, Chan L. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor(−/-) mice. Arterioscler Thromb Vasc Biol. 2006;26:143–149. doi: 10.1161/01.ATV.0000193510.19000.10. [DOI] [PubMed] [Google Scholar]
- 13.Chen HW, Chien ML, Chaung YH, Lii CK, Wang TS. Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways. Chem Biol Interact. 2004;150:233–241. doi: 10.1016/j.cbi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1998;19:819–825. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Shi XL, Liu HL, Yi SQ, Zhang XJ, Fang XH. Study of the effect of atorvastatin on the interaction between ICAM-1 and CD11b by live-cell single-molecule force spectroscopy. Science China (Chemistry) 2010;53:752–758. doi: 10.1007/s11426-010-0111-2. [DOI] [Google Scholar]
- 16.Bürgin-Maunder CS, Brooks PR, Russell FD. Omega-3 fatty acids modulate weibel-palade body degranulation and actin cytoskeleton rearrangement in PMA-stimulated human umbilical vein endothelial cells. Mar Drugs. 2013;11:4435–4450. doi: 10.3390/md11114435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Nan B, Lin P, Lumsden AB, Yao Q, Chen C. Effects of TNF-alpha and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res. 2005;115:417–426. doi: 10.1016/j.thromres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Nan B, Yang H, Yan S, Lin PH, Lumsden AB, Yao Q, Chen C. C-reactive protein decreases expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Surgery. 2005;138:212–222. doi: 10.1016/j.surg.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 21.Masamura K, Oida K, Kanehara H, Suzuki J, Horie S, Ishii H, Miyamori I. Pitavastatin-induced thrombomodulin expression by endothelial cells acts via inhibition of small G proteins of the Rho family. Arterioscler Thromb Vasc Biol. 2003;23:512–517. doi: 10.1161/01.ATV.0000060461.64771.F0. [DOI] [PubMed] [Google Scholar]
- 22.Weiler-Guettler H, Yu K, Soff G, Gudas LJ, Rosenberg RD. Thrombomodulin gene regulation by cAMP and retinoic acid in F9 embryonal carcinoma cells. Proc Natl Acad Sci USA. 1992;89:2155–2159. doi: 10.1073/pnas.89.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets. 2012;13:421–431. doi: 10.2174/138945012799424606. [DOI] [PubMed] [Google Scholar]
- 24.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, Kuller LH. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17:2167–2176. doi: 10.1161/01.ATV.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 25.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–1119. doi: 10.1016/S0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 26.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Zhang X, Xu L, Yi S, Li Y, Fang X, Liu H. The effect of cigarette smoke extract on thrombomodulin-thrombin binding: An atomic force microscopy study. Sci China Life Sci. 2012;55:891–897. doi: 10.1007/s11427-012-4383-y. [DOI] [PubMed] [Google Scholar]
- 28.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 29.Jun P, Huangqing C, Xiaoheng L, Ruheng L, Xiaohong Z. Effects of shear stress on protein C activation, EPCR expression and TM expression in endothelial cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009;26:303–309. [PubMed] [Google Scholar]
- 30.Cornel JH, Becker RC, Goodman SG, Husted S, Katus H, Santoso A, Steg G, Storey RF, Vintila M, Sun JL, et al. Prior smoking status, clinical outcomes, and the comparison of ticagrelor with clopidogrel in acute coronary syndromes-insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2012;164:334–342.e1. doi: 10.1016/j.ahj.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Al Mutairi SS, Shihab-Eldeen AA, Mojiminiyi OA, Anwar AA. Comparative analysis of the effects of hubble-bubble (Sheesha) and cigarette smoking on respiratory and metabolic parameters in hubble-bubble and cigarette smokers. Respirology. 2006;11:449–455. doi: 10.1111/j.1440-1843.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 32.Ambrose JA, Barua R. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 33.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2011;158:257–267. doi: 10.1016/S0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 34.Yang YM, Liu GT. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomed Environ Sci. 2004;17:121–134. [PubMed] [Google Scholar]