Abstract
Silymarin has been used in the treatment of a number of liver diseases for a long time, but its efficacy in preventing triptolide induced acute hepatotoxicity has not been reported previously. The present study aimed to assess the protective effect of silymarin against triptolide (TP)-induced hepatotoxicity in rats. Rats were orally administrated with silymarin (50, 100 and 200 mg/kg) for 7 days and received intraperitoneal TP (2 mg/kg) on the day 8. Hepatic injuries were comprehensively evaluated in terms of serum parameters, morphological changes, oxidative damage, inflammation and apoptosis. The results demonstrated that TP-induced increases in serum parameters, including alanine transaminase, aspartate aminotransferase, alkaline phosphatase, total cholesterol and γ-glutamyl transpeptidase, which were determined using a biochemical analyzer, and histopathological alterations and hepatocyte apoptosis as determined by hematoxylin and eosin and TUNEL staining, respectively, were prevented by silymarin pretreatment in a dose-dependent manner. TP-induced depletions in the activity of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, glutathione S-transferase and catalase, and glutathione levels, were also significantly reversed by silymarin, as determined using specific kits. Additionally, silymarin dose-dependently exhibited inhibitory effects on malonaldehyde content in the liver. The production of proinflammatory cytokines was investigated using ELISA kits, and the results demonstrated that silymarin dose-dependently inhibited the production of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10 and IL-1β in the liver. To determine the mechanism of silymarin, western blot analysis was performed to investigate the protein expression of phosphorylated (p)-p38 and p-c-Jun N-terminal kinase (JNK) of the TNF-α induced inflammatory response and apoptotic pathways. Silymarin significantly blocked p38 and JNK phosphorylation and activation. Additionally, the expression of the proapoptotic proteins cytochrome c, cleaved caspase-3 and Bcl-2-associated X was also reduced following treatment with silymarin, as determined by ELISA, western blotting and immunohistochemistry, respectively. In conclusion, silymarin was demonstrated to dose-dependently protect rat liver from TP-induced acute hepatotoxicity, with the high dose (200 mg/kg) achieving a superior effect. This protective effect may be associated with the improvement of antioxidant and anti-inflammatory status, as well as the prevention of hepatocyte apoptosis. Therefore, silymarin may have the potential to be applied clinically to prevent TP-induced acute hepatotoxicity.
Keywords: silymarin, triptolide, hepatoprotective, antioxidant, anti-inflammatory, anti-apoptosis
Introduction
Triptolide (TP; C20H24O6; Fig. 1A), a diterpene triepoxide, is derived from the traditional Chinese medicinal herb Tripterygium wilfordii Hook F (TWHF) (1), and both are commonly prescribed to treat autoimmune and inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus and nephritis in Chinese clinics. However, in recent decades, TWHF, TP and their preparations have attracted increased attention regarding their potential toxicity, particularly liver and kidney injury, which have limited their clinical application to a certain degree (2–4). Reports have indicated that the adverse reactions observed were primarily caused by TP, its major active component and toxic ingredient. Currently, research efforts are focused on reducing these adverse effects.
Figure 1.
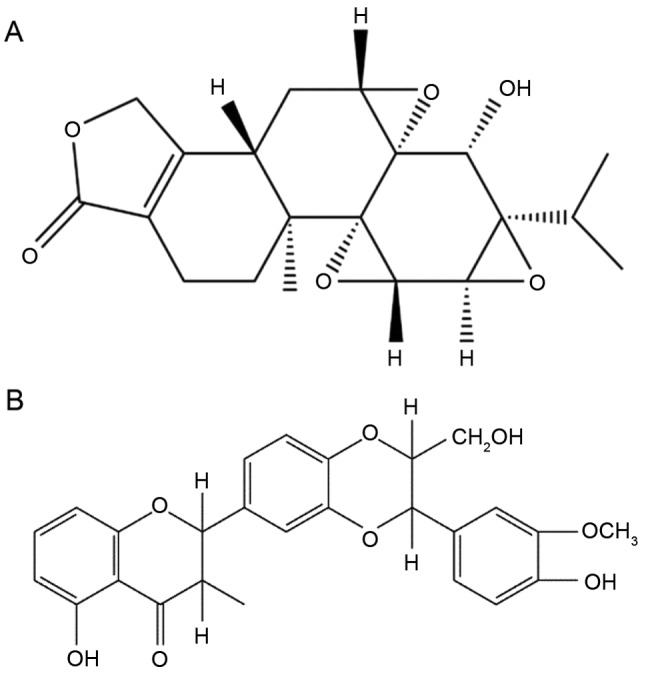
Chemical structure of (A) triptolide and (B) silibinin.
Attempts to identify the underlying mechanisms of TP in disease treatment have led to promising results. A previous study demonstrated that oral administration of TP in rats resulted in acute hepatic injury and markedly elevated levels of alanine transaminase (ALT) and aspartate aminotransferase (AST) in the serum (5). Further studies have revealed that TP-induced liver injury may be associated with the excessive release of peroxides, which subsequently induces oxidative stress and lipid peroxidation (6), the production of inflammatory mediators leading to immunological injury, the suppression of cell activities, the stimulation of apoptotic-associated proteins and damage of the mitochondrial respiratory chain leading to apoptosis (7–9).
Natural medicines from plants have been considered as potentially effective and safe alternative treatments for liver diseases, and are increasingly employed worldwide. Silybum marianum (Asteraceae), a typical component in traditional Chinese medicine, has been broadly utilized with a long history of use as a remedy for various hepatic disorders, including hepatitis and cirrhosis, and to prevent liver damage induced by chemicals and environmental toxins (10,11).
Silymarin is an extract of Silybum marianum, and is a lipophilic complex of two flavonoids (taxifolin and quercetin) and three flavonolignane diastereomers: Silibinin, the primary and active component (C24H19O10; Fig. 1B), silydianin and silychristin (12). Silymarin has been used clinically either alone or as a major component of various pharmaceutical preparations for centuries as a hepatoprotective agent, and has exhibited protective effects against inflammation, oxidation and apoptosis (11,13–15). The anti-hepatotoxic mechanism of silymarin is associated with its stabilizing effect on cytoplasmic membranes (16). Therefore, silymarin has been investigated in numerous animal models and exerts marked therapeutic effects on liver injury of different etiology (17), including alcoholic-induced liver damage (18), diethylnitrosamine-induced liver disease (19), hepatitis C virus and carbon tetrachloride-induced oxidative disorders (20). Based on these studies, it may be concluded that silymarin may scavenge reactive oxidative species (ROS) and alleviate lipid peroxidation induced by various lipophilic hepatotoxins. However, the potential protective effect of silymarin against TP-induced hepatotoxicity remains to be investigated.
Therefore, to the best of our knowledge, the present study was the first to investigate the protective effect of silymarin on TP-induced oxidative damage, inflammation and apoptosis, and the potential underlying mechanisms. The results indicated that silymarin pretreatment, particularly at high dose (200 mg/kg), markedly reduced TP-induced hepatotoxicity.
Materials and methods
Materials
TP (purity >98% by high-performance liquid chromatography analysis) was supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Silymarin was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and consisted primarily of silibinin (>30%), isosilibinin, silicristin and silidianin. TP was dissolved in 0.05% dimethyl sulfoxide saline. Silymarin was suspended in 0.5% sodium carboxymethyl cellulose (CMC-Na) distilled water solution. Other chemicals and reagents used were of analytical grade.
Animals
A total of 60 male Wistar rats (weight, 220±20 g; 6-8 weeks) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). All the experiments were performed in a specific-pathogen free laboratory at a controlled temperature of 25±2°C and relative humidity of 35–75% on a 12 h light/dark cycle. Animals had free access to rat chow and tap water. All experimental protocols were in compliance with the guidelines of the Committee for Animal Care and Use of Guangzhou University of Chinese Medicine, and were approved by the ethical committee of Guangzhou University of Chinese Medicine.
Animal grouping
Rats were randomly divided (n=12/group) into five experimental groups. Normal control (NC) and triptolide (TP) groups received oral administration of normal saline (1 ml/100 g), whereas silymarin-treated groups were orally administrated with silymarin (50, 100 and 200 mg/kg) dissolved in 0.5% CMC-Na distilled water. The doses of silymarin employed in the present study were based on our prior trial and previous study (21). Following treatment with normal saline or silymarin for 7 consecutive days, all groups, excluding the NC group, were given TP (2 mg/kg) by intraperitoneal injection to establish the liver injury animal model. At the end of the experimental period (day 8), all rats were fasted for 12 h. On the following day, the animals were anesthetized with an intraperitoneal injection of 10% chloral hydrate. Blood samples were collected from the abdominal aorta, and rats were subsequently sacrificed by spinal dislocation and liver tissue was collected from the left lobe.
Blood biochemical studies
Blood samples were placed at room temperature for 1 h. Following centrifugation at 1,000 × g for 10 min at room temperature, the serum was separated for biochemical estimations. The serum levels of ALT, AST, alkaline phosphatase (ALP), total cholesterol (TC) and γ-glutamyl transferase (GGT) were measured using a Hitachi-7180 type biochemical analyzer following the standard methods.
Preparation of liver homogenate and sections
The liver tissues were chopped into 1 cm3 sections and divided into two groups. One was prepared to make 1:9 (w/v) homogenates with cold saline or phosphate buffer saline (PBS) using a tissue homogenizer (IKA T 18 Basic). The homogenate was centrifuged at 1,000 × g for 10 min at 4°C to obtain the supernatant samples for subsequent determinations. The other group of liver tissues were fixed in 10% formalin at room temperature for at least 24 h for further observation of tissue sections.
Measurement of antioxidant enzymatic activities and lipid peroxidation
The supernatant of normal saline homogenates was used to measure the activities of the important antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione S-transferase (GST) and catalase (CAT), as well as glutathione (GSH) and malonaldehyde (MDA) levels in the liver, according to the manufacturer's instructions [total SOD assay kit (hydroxylamine method), GSH-PX assay kit (colorimetric method), GST assay kit (colorimetric method), GSH assay kit (colorimetric method), CAT assay kit (ultraviolet) and MDA assay kit (TBA method); Nanjing Jiancheng Bioengineering Institute, Nanjing, China]
Hepatic cytokine and cytochrome assays
The levels of inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, IL-1β, and cytochrome c (Cyt C) levels were measured in supernatants using specific ELISA kits [E-30644 (IL-6), E-30418 (IL-1β), E-30633 (TNF-α), E-30649 (IL-10) and E-30273 (Cyt C); Beijing Chenglin Biotechnology, Beijing, China] according to the manufacturer's instructions.
Histopathological examination
After being fixed in 10% formalin for 24 h, the liver tissue was dehydrated in graded ethanol (50–100%), cleared in xylene and embedded in paraffin wax. The paraffin sections (5 µm thickness) were examined for pathological changes in the liver tissue using a light microscope (BK-DM320; Chongqing Optec Instrument Co., Ltd., Chongqing, China) and photographed at ×200 magnification following hematoxylin and eosin (H&E) staining for 5–10 min at room temperature.
Detection of apoptosis
A terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) apoptosis detection kit (Nanjing Lufei Biotechnology Co., Ltd., Nanjing, China) was used to assess the apoptosis rate of liver cells. Paraffin sections (5 µm) were incubated with the TUNEL reaction mixture at 37°C for 60 min, then incubated with converter-POD in a humidified chamber for 30 min at 37°C followed by incubation with DAB substrate (5 µl 20X DAB, 1 µl 30% H2O2 and 94 µ l PBS) for 10 min at 20°C. The sections were washed and mounted in PBS. A total of 15 fields (×200 magnification) for each section were randomly selected to quantify the positive cells and define the apoptosis indexes using ImageJ software (version 1.50; National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical staining and quantitative analysis
Immunohistochemical analyses were performed to determine the expression of Bcl-2-associated X (Bax) and Bcl-2 in the liver. Paraffin sections (5 µm thick) of the liver tissue were initially processed by dehydration. Following retrieval by citrate buffer (pH=6) microwave antigen retrieval for 15 min, 3% H2O2 was added to inactivate endogenous enzymes for another 15 min. Then, 5% normal goat serum (ab7481; Abcam, Cambridge, UK) diluted with PBS was used to block non-specific binding for 10 min at room temperature. Subsequently, sections were washed with 0.01 mol/l PBS following incubation of sections with 50 µl rabbit monoclonal antibodies for Bcl-2 (1:100, ab59348) and Bax (1:100; ab53154) (both from Abcam) at 4°C overnight. The secondary antibody was goat anti-rabbit IgG H&L (HRP) (1:1,000, ab6721; Abcam) for 30 min at 37°C. The slides were counterstained with hematoxylin (Modified Mayer's, ab220365; Abcam) for 2–3 min at room temperature. Finally, the sections were reacted with a staining solution containing 0.03% 3,3′-diaminobenzidine (Nanjing Lufei Biotechnology Co., Ltd.,) for 5–10 min at room temperature. The sections were observed with an Olympus IX71 fluorescence microscope (Olympus Corporation, Tokyo, Japan) at ×200 magnification. A total of 15 fields in each section were randomly selected to quantify positive cells by ImageJ software (version 1.50; National Institutes of Health).
Western blotting for cleaved caspase-3 (c-caspase-3), phosphorylated (p)-p38 and p-c-Jun N-terminal kinase (JNK) protein expression
Western blot analysis was performed to examine the expression of c-caspase-3, which stimulates cell apoptosis, and TNF-α induced phosphorylation of p38 and JNK in the inflammatory pathway. Liver tissue was homogenized with proteinase inhibitor on ice to obtain total protein extract according to the protocol of the T-PER Tissue Protein Extraction reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein concentration was determined following the protocol of a BCA protein assay kit (G2026; Wuhan Goodbio Technology Co., Ltd., Wuhan, China). Extracts containing equal amounts of protein (50 µg) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes, which were blocked at room temperature for 1 h in 0.5% TBST (50 mmol/l Tris-HCl, pH 7.6; 150 mmol/l NaCl; 0.1% Tween-20) containing 5% non-fat milk and subsequently incubated with primary rabbit anti-rat polyclonal antibodies against c-caspase-3 (1:1,000, 9661; Cell Signaling Technology, Inc., Danvers, MA, USA) and β-actin (1:1,000, GB13001-1; Wuhan Goodbio Technology Co., Ltd.), and rabbit anti-rat monoclonal antibodies against p-p38 (1:1,000, 4511) and p-JNK (1:1,000, 4671) (both from Cell Signaling Technology, Inc.) overnight at 4°C. β-actin was used as an internal control. All primary antibodies were purchased from Cell Signaling Technology, Inc. Subsequently, the membranes were washed with TBST for 15 min and incubated with secondary antibodies (anti-rabbit IgG, HRP-linked antibody, 1:3,000; Cell Signaling Technology, Inc.) labeled with horseradish peroxidase for 30 min at room temperature, followed by washing with TBST for 15 min. The membranes were then developed using an electrochemiluminescence kit (G2014; Wuhan Goodbio Technology Co., Ltd.) and the densities of the immunoreactive bands were analyzed using ImageJ software (version 1.50; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard deviation. One-way analysis of variance followed by Fisher's least significant difference tests was performed to statistically analyze the data. SPSS software (version 16; SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Silymarin reduces the serum levels of biochemical parameters
The levels of ALT, AST, ALP, TC and GGT were estimated in serum samples as sensitive biomarkers of liver function. These enzymes (ALT, AST, ALP and GGT) were enzymes derived from the injured liver tissue cells. As presented in Table I, the levels of these serum biochemical parameters in the TP group were significantly higher compared with the NC group (P<0.01), which was indicative of liver dysfunction. However, the levels of these indicators in the three silymarin-pretreated groups markedly reduced to normal levels in a dose-dependent manner, indicating that silymarin may effectively ameliorate TP-induced liver injury.
Table I.
Levels of ALT, AST, ALP, TC and GGT in the serum of different treatment groups.
| Group | ALT, U/l | AST, U/l | ALP, U/l | TC, mmol/l | GGT, U/l |
|---|---|---|---|---|---|
| NC | 31.1±4.3 | 65.4±10.0 | 128.7±13.9 | 1.5±0.1 | 1.0±0.0 |
| TP | 78.3±13.7a | 300.3±118.2a | 172.0±32.5a | 2.6±0.8a | 1.8±0.9a |
| SLT | 46.0±7.5c | 138.5±24.3c | 164.5±35.2 | 1.9±0.3c | 1.2±0.6 |
| SMT | 42.4±7.0c | 130.9±31.0c | 139.9±31.3b | 1.8±0.2c | 1.1±0.3b |
| SHT | 40.8±7.8c | 105.9±17.6c | 136.0±20.8b | 1.7±0.6c | 1.0±0.3b |
Doses of 50, 100 and 200 mg/kg silymarin were employed in SLT, SMT and SHT groups, respectively. Data are presented as the mean ± standard deviation, n=12.
P<0.01 vs. NC group
P<0.05
P<0.01 vs. TP group. ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TC, total cholesterol; GGT, γ-glutamyl transferase; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Silymarin alleviates alteration of histological structure
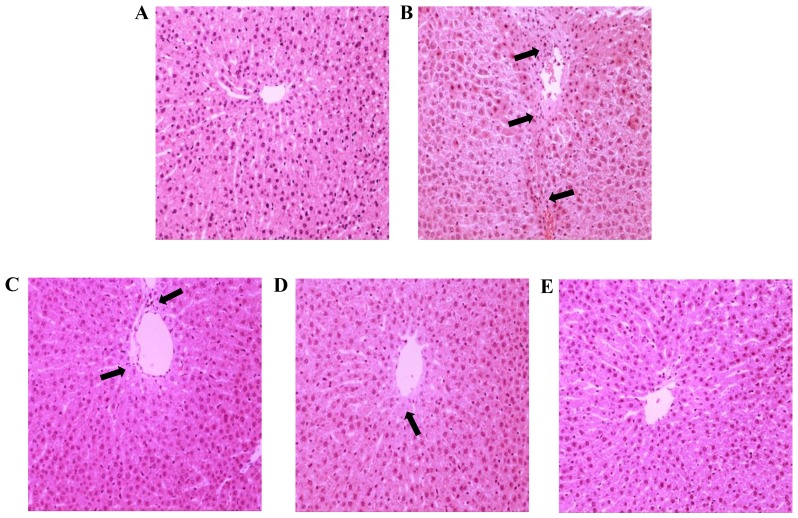
Hepatic histological changes directly depict the degree of liver injury. To morphologically characterize the potential pathological alternations in hepatocytes, routine H&E staining was performed. As demonstrated in Fig. 2A, a clear and regular lobular structure was observed, where hepatic cords remained legible and radiated from the central vein in the NC group. Compared with the NC group, hepatic parenchymal necrosis and inflammatory cell infiltration was apparent, and disordered hepatic cords accompanied by extensive vacuolation congestion was also observed in the TP group (Fig. 2B), which was consistent with the assay results for biochemical parameters in the serum, indicating the successful establishment of the hepatic injury model. Furthermore, hepatocytes were markedly swollen, with condensed karyons and loosened cytoplasts. However, silymarin pretreatment of three tested doses appeared to alleviate TP-induced liver injury in a dose-dependent manner, as evidenced by reduced inflammation infiltration, intact tissue structure, and reduced necrotic and apoptotic mass (Fig. 2C-E), compared with the TP group. Cell apoptosis and inflammatory infiltration was particularly limited when subjected to silymarin pretreatment at the doses of 100 and 200 mg/kg.
Figure 2.
Histological assessments were performed by hematoxylin and eosin staining of liver tissues from different treatment groups. (A) NC group sections exhibited normal histological structure and the central vein was obvious. (B) TP group sections appeared to exhibit fibrosis, focal necrosis and congestion of the hepatoportal blood vessel, which was covered by inflammatory cell infiltration (arrows). (C) SLT and (D) SMT groups exhibited sporadic necrosis of hepatocytes, with improvements compared with the TP group (arrows). (E) SHT group sections exhibited normal morphology and a marked improvement in hepatocyte structure, which was similar to that in the NC group, compared with the TP group. Magnification, ×200. NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Silymarin upregulates antioxidant enzymatic activities
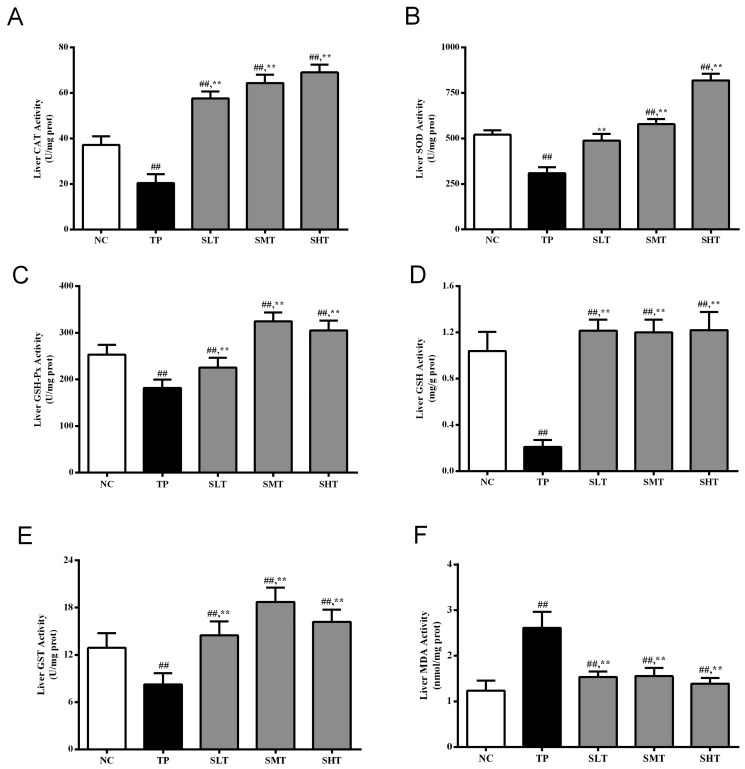
As demonstrated in Fig. 3A-E, TP administration markedly reduced the activities of the antioxidant enzymes SOD, GSH-Px, CAT and GST and GSH levels by 40.61, 28.18, 45.10, 35.97 and 79.81%, respectively, compared with the NC group (P<0.05). However, pretreatment with silymarin, particularly at middle and high doses, significantly upregulated the activities compared with the TP group (P<0.05). Furthermore, silymarin significantly elevated the CAT and GST activities to levels that surpassed those in the NC group (Fig. 3A and E). Therefore, silymarin exhibited excellent effects in mitigating TP-induced systemic antioxidant enzyme exhaustion.
Figure 3.
Effect of silymarin on the activity of antioxidant enzymes and MDA levels in the liver. The activities/levels of (A) CAT, (B) SOD, (C) GSH-Px, (D) GSH, (E) GST and (F) MDA in the liver of different treatment groups were determined using respective kits. Data are presented as the mean ± standard deviation, n=12. ##P<0.01 vs. NC group; **P<0.01 vs. TP group. MDA, malonaldehyde; CAT, catalase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; GSH, glutathione; GST, glutathione S-transferase; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Silymarin suppresses TP-induced lipid peroxidation
The hepatic MDA level was determined to evaluate the degree of lipid peroxidation (Fig. 3F). MDA content increased significantly in the liver of the rats in the TP group compared with the NC group (P<0.01). However, silymarin significantly suppressed this excessive accumulation of MDA compared with the TP group (P<0.01), and no significant difference was observed between the NC and high-dose silymarin group. Therefore, silymarin markedly suppressed TP-induced lipid peroxidation, which was consistent with the improvement in antioxidant enzymatic activities.
Silymarin decreases the production of inflammatory cytokines
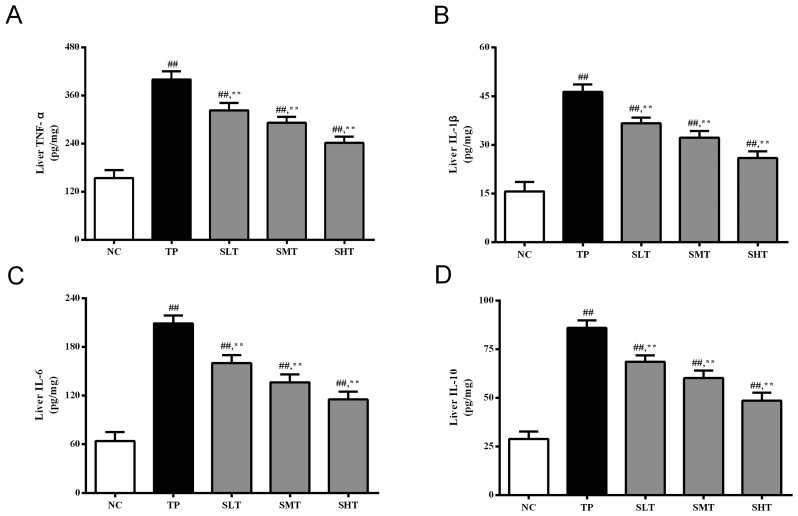
An excessive inflammatory response is associated with oxidative stress (22). In the present study, the levels of important inflammatory cytokines, TNF-α, IL-1β, IL-6 and IL-10, in the liver were determined. The levels of these inflammatory mediators were significantly higher in the TP group compared with the NC group (Fig. 4), indicating that TP induced severe inflammatory response in the liver. Promisingly, pretreatment with silymarin significantly inhibited the production of these proinflammatory factors in a dose-dependent manner compared with the TP group (P<0.01). These results indicate that silymarin may attenuate TP-induced liver injury by suppressing the inflammatory response.
Figure 4.
Effect of silymarin on inflammatory cytokines in the liver. ELISAs were performed to determine the levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10 in the liver of rats in different treatment groups. Data are presented as the mean ± standard deviation, n=12. ##P<0.01 vs. NC group; **P<0.01 vs. TP group. TNF, tumor necrosis factor; IL, interleukin; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Silymarin inhibits transduction of the inflammatory pathway and reduces c-caspase-3 protein expression
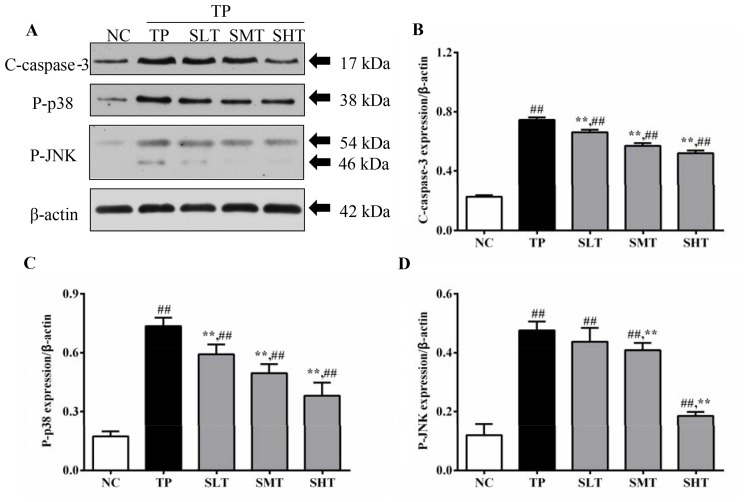
To illustrate the underlying mechanism of silymarin inhibiting inflammatory responses, the protein expression of key downstream proteins in the TNF-α pathway, and the apoptosis-associated protein c-caspase-3, was measured (Fig. 5A). Expression levels of p-p38 in the TP group were 3.5 times higher compared with the NC group (P<0.01). However, the overexpression of p-p38 induced by TP was reduced by 20.04, 33.11 and 48.44% in silymarin treated groups in a dose-dependent manner (Fig. 5B). A similar inhibitory effect of silymarin was also observed in the expression of p-JNK. The level of p-JNK in TP group was three times higher compared with the NC group (P<0.01). In silymarin pretreated groups, the level of p-JNK decreased by 8.82, 14.93 and 61.46% in low, middle and high-dose groups, respectively, compared with the TP group (Fig. 5C). Therefore, the results indicated that silymarin, particularly at 100 and 200 mg/kg, significantly inhibited the phosphorylation of inflammatory mediators that are induced by TNF-α. In addition, the protein expression of c-caspase-3, a protein associated with apoptosis, was also determined by western blot analysis. Following TP injection, the level of c-caspase-3 in the TP group was 2.24 times higher compared with the NC group (P<0.01). In silymarin-treated groups, the expression level of c-caspase-3 was significantly decreased by 10.55, 22.93 and 29.73% in silymarin low, middle and high-dose groups, respectively, compared with the TP group (P<0.01; Fig. 5D). These results indicate that silymarin may also exert antiapoptotic effects in the liver of TP-treated rats.
Figure 5.
Effects of silymarin on the protein expression c-caspase-3, p-p38 and p-JNK. (A) Representative western blot analysis of c-caspase-3, p-p38 and p-JNK expression. Densitometric analysis was performed to quantify the expression levels of (B) p-p38, (C) p-JNK and (D) c-caspase-3 in different treatment groups. Data are presented as the mean ± standard deviation, n=6. ##P<0.01 vs. NC group; **P<0.01 vs. TP group. c-caspase, cleaved-caspase; p, phosphorylated; JNK, c-Jun N-terminal kinase; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Silymarin inhibits hepatocyte apoptosis
The apoptosis rates of different treatment groups were detected using TUNEL staining and quantified by ImageJ software, and representative photographs were taken under a light microscope. As demonstrated in Fig. 6, TP administration resulted in numerous TUNEL-positive hepatocytes and a significantly higher apoptotic index compared with the NC group (Table II), indicating the induction of severe hepatic apoptosis within 24 h of treatment with TP. However, fewer TUNEL-positive hepatocytes and significantly decreased apoptotic indexes (P<0.01) were observed in livers pretreated with silymarin compared with the TP group, indicating that silymarin pretreatment may protect the liver against TP-induced hepatic injury. Notably, high-dose silymarin pretreatment exhibited a superior effect in inhibiting the cell apoptosis. These results indicated that silymarin may effectively ameliorate the hepatocyte apoptosis induced by TP.
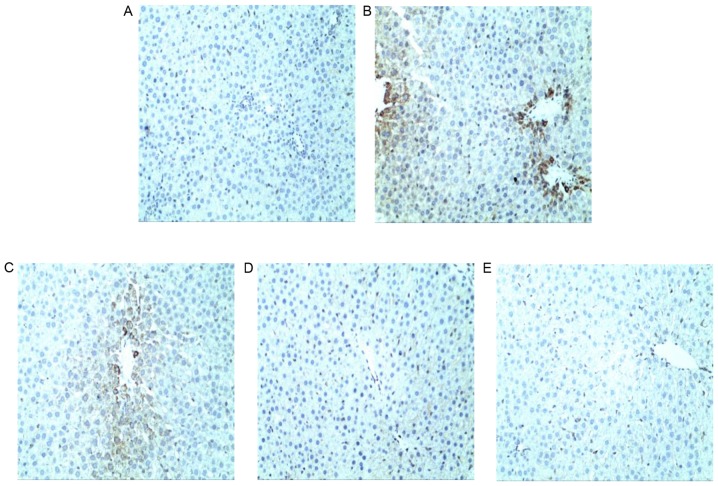
Figure 6.
Hepatocyte apoptosis was examined by TUNEL staining. (A) NC group sections exhibited normal hepatic lobular structure. (B) TP group sections appeared to exhibit apoptotic lesions and increased TUNEL positivity in the central lobular areas compared with the NC group. (C) SLT and (D) SMT groups exhibited moderate to mild TUNEL positivity and necrosis. (E) SHT group sections exhibited markedly reduced TUNEL positivity and apoptotic cells compared with the TP group, resembling that of the NC group. Magnification, ×200. TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Table II.
Positive rates for TUNEL analysis, Bcl-2 and Bax immunohistochemistry, Bax/Bcl-2 expression ratio and Cyt C content in the liver tissue of different treatment groups.
| Group | Cyt C (nmol/l) | TUNEL (%) | Bcl-2 (%) | Bax (%) | Bax/Bcl-2 ratio |
|---|---|---|---|---|---|
| NC | 105.8±9.2 | 8.5±0.3 | 28.5±1.1 | 7.2±0.7 | 0.27±0.1 |
| TP | 290.6±10.3a | 30.6±1.0a | 29.6±0.6 | 34.9±1.1a | 1.19±0.2a |
| SLT | 235.0±10.6b | 15.9±0.7b | 28.2±0.9 | 19.0±0.9b | 0.68±0.1b |
| SMT | 191.4±9.8b | 12.9±0.8b | 28.4±0.8 | 10.0±0.7b | 0.37±0.1b |
| SHT | 156.9±6.5b | 10.6±0.4b | 29.0±0.5 | 3.7±0.5b | 0.14±0.1b |
Doses of 50, 100 and 200 mg/kg silymarin were employed in SLT, SMT and SHT groups, respectively. Data are presented as the mean ± standard deviation, n=12.
P<0.01 vs. NC group
P<0.01 vs. TP group. Cyt C, cytochrome c; TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling; Bax, Bcl-2-associated X; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Effects of silymarin on the expression of apoptosis-associated proteins
Representative photographs of different treatment groups were taken under a light microscope. The positive rates were calculated by ImageJ software based on 15 images of each group. No significant alterations in the expression of the antiapoptotic protein Bcl-2 were observed between NC, TP and silymarin treatment groups (Fig. 7 and Table II). By contrast, the expression of the proapoptotic protein Bax was significantly higher in the TP group compared with the NC group (P<0.01; Fig. 8 and Table II). However, silymarin significantly inhibited the expression of Bax and, therefore, the ratio of Bax/Bcl-2 was also lowered, compared with the TP group in a dose-dependent manner (P<0.01; Fig. 8 and Table II). As described above for western blot results, the changes in the levels of c-caspase-3 exhibited a similar pattern to Bax expression (Fig. 5). Furthermore, Cyt C levels in supernatants were measured by ELISA, and levels were significantly increased in the TP group compared with the NC group (P<0.01; Table II). However, silymarin pretreatment was observed to progressively reduce hepatic Cyt C levels in a dose-dependent manner compared with the TP group (P<0.01). These results are consistent with those for TUNEL staining and c-caspase-3 protein expression, indicating that an antiapoptotic effect may contribute to the protective action of silymarin in the liver of TP-treated rats.
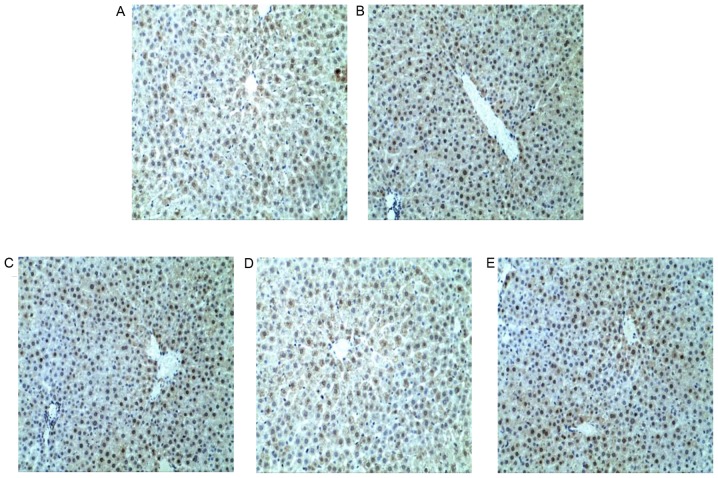
Figure 7.
Immunohistochemical staining of Bcl-2 in liver sections. Bcl-2 staining in (A) NC, (B) TP, (C) SLT, (D) SMT and (E) SHT groups. The positive area of Bcl-2 protein did not change significantly among all groups. Magnification, ×200. NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
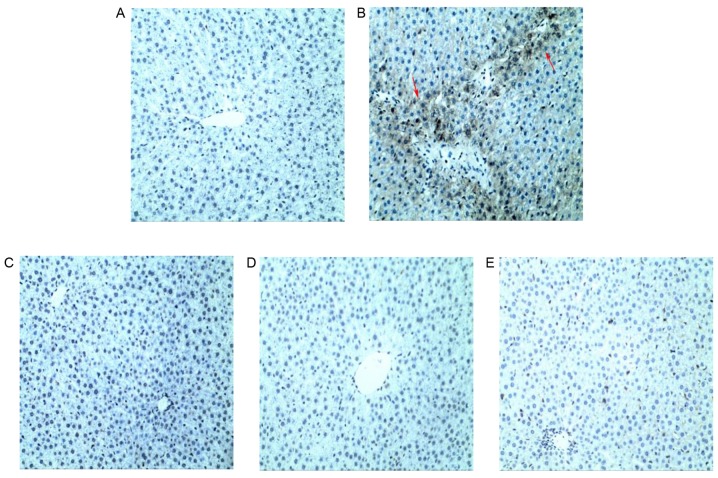
Figure 8.
Immunohistochemical staining of Bax in liver sections. (A) Bax staining in the NC group. (B) In the TP group, the Bax-positive area was markedly larger compared with the NC group. Bax staining in (C) SLT, (D) SMT and (E) SHT groups indicated that the expression of Bax was markedly reduced compared with the TP group in a dose-dependent manner. Magnification, ×200. Bax, Bcl-2-associated X; NC, normal control; TP, triptolide; SLT, low-dose silymarin treatment; SMT, middle-dose silymarin treatment; SHT, high-dose silymarin treatment.
Discussion
The liver has a vital role in drug metabolism and is a target organ that is affected by toxicants (23). TWHF, TP and their preparations have been widely used for autoimmune and inflammatory diseases with pronounced efficacy. Unfortunately, their application has been increasingly restricted due to their potential adverse effects and acute toxicity, particularly hepatic toxicity. To date, various studies have been performed with the aim of identifying strategies to reduce the side effects of these agents (24–26). However, counteractive therapeutic approaches against TP-induced hepatotoxicity are yet to be established. Therefore, the development of effective strategies to counteract TP toxicity is urgently required. Certain herbal drugs, including Glycyrrhiza glabra L. (27), Curcuma longa L. (28) and Salvia miltiorrhiza Bunge (29), have been demonstrated to improve the regeneration of liver cells, which accelerates the healing process, and are therefore commonly employed in the management of various liver disorders. It is thought that Silybum marianum has been applied extensively for more than 2,000 years as a natural hepatoprotective agent for the treatment of hepatic diseases, including hepatitis and cirrhosis, and to protect the liver from toxic substances (10,30). At present, silymarin, the major active component of Silybum marianum, is a major ingredient in a variety of pharmaceutical preparations, Chinese and Western, that are used to treat liver dysfunction (12). The present study provides additional evidence to support our hypothesis that silymarin pretreatment may prevent TP-induced hepatotoxicity, which may occur by ameliorating oxidative stress, elevating antioxidant enzymes activities, and inhibiting lipid peroxidation, the inflammatory response and apoptosis.
Alterations in serum biochemical parameters and histological structure are directly indicative of pathological status in liver. Excessive levels of serum AST, ALT, ALP, TC and GGT, which are released from damaged hepatocytes into bloodstream, are associated with severe liver dysfunction in clinical practice (31). In the present study, a single administration of TP led to severe liver injury in rats in the TP group, as evidenced by significant increases in serum AST, ALT, ALP, TC and GGT compared with the NC group, which was consistent with previous studies (32,33). The results of the presents study also demonstrated that silymarin pretreatment significantly reduced TP-induced increases in the levels of these parameters, indicating improvements in hepatic function. Increases in these serum biomarkers were further validated by histopathological examination. TP caused obvious damage to the hepatic architecture and led to serious pathological abnormalities, including vacuole formation, inflammatory infiltration and focal necrosis. However, pretreatment with silymarin markedly reduced the presence of deteriorated hepatic cells. Hepatoprotective effects of silymarin were evidenced by the absence of cellular necrosis and inflammation in the liver section, particularly in rats treated with middle and high-dose silymarin.
Oxidative stress is a primary contributing factor in several pathological conditions, such as TP-induced liver injury, as it negatively affects cell membranes and function. Therefore, application of bioactive substances possessing antioxidative properties may be effective therapeutic agents for preventing and treating hepatotoxicity (34). It has been reported that silymarin functions as an antioxidant by scavenging oxygen free radicals and decreasing lipid peroxidation to accelerate hepatocyte regeneration with minimal adverse actions (35–38). The results of the present study were consistent with previous studies (39–41). The activities of antioxidant enzymes, including SOD, CAT, GST and GSH-Px, were decreased, while MDA production increased in the liver of rats in the TP group. When endogenous antioxidant systems were destroyed, an imbalance of oxidation and antioxidation occurred, which directly or indirectly damaged the intracellular proteins (42). Therefore, the mechanism of TP-induced liver injury may be associated with excessive levels of ROS and peroxides, which leads to induction of oxidative stress and lipid peroxidation (6). In the present study, pretreatment with silymarin, particularly high-dose silymarin, reversed the reduction in antioxidant enzymes activities to normal levels. These results indicate that silymarin may prevent TP from binding with hepatic lipids and proteins and thus inhibit lipid peroxidation and protect antioxidant enzymes. The capacity of silymarin for trapping free radicals has also been demonstrated in previous studies (37,43). Antioxidant action and anti-lipid peroxidation properties may be contributing factors in the protective effect of silymarin in TP-induced hepatocyte damage.
Inflammation is another important pathological mechanism that may be involved in TP-induced hepatotoxicity. In a previous report, TP activated hepatic Kupffer cell external antibody CD68 to release substantial amounts of TNF-α (44), which causes normal cells to release IL-6, IL-10 and IL-1β into the bloodstream, and is involved in the pathogenesis of viral hepatitis, alcoholic or nonalcoholic fatty liver, and ischemia-reperfusion injury (22,45,46). TNF-α is one of the transcription factors of the mitogen-activated protein kinase (MAPK) signaling pathway, which has been reported to mediate inflammation and apoptosis (47). When triggered by inflammatory cytokines such as TNF-α, MAPK cascades are initiated via p38 and JNK pathways to regulate activator protein-1, which subsequently leads to inflammatory responses and cell apoptosis (48). Phosphorylation of p38 and JNK may also be induced by oxidative stress, which stimulates the production of proinflammatory mediators and establishes a vicious cycle that aggravates hepatic injuries (49). Previous reports have indicated that silymarin, by acting on a variety of molecular targets, including cytokines and enzymes (50), restricts excessive inflammation caused by restraint stress (22) and alcohol (51). The results of the present study are consistent with these previous studies. Silymarin effectively inhibited the protein expression levels of TNF-α, p-p38 and p-JNK, which was increased significantly by TP injection, and subsequently led to significant reductions in the production of inflammatory cytokines (IL-6, IL-10 and IL-1β) and reduced inflammatory infiltration in middle or high-dose silymarin treatment groups compared with the TP group. These results indicate that a potential anti-inflammatory effect of silymarin may also contribute to its ability to attenuate TP-induced hepatotoxicity.
Rapid secretion of TNF-α may also lead to the activation of mitochondria-controlled apoptosis, the cascade reaction that begins with the release of Cyt C due to reduced mitochondria membrane potential and destruction of the mitochondrial membrane (52), and results in the activation of caspase-3. ROS are thought to be responsible for damaging the mitochondrial membrane and releasing Cyt C (53). The release of Cyt C is also regulated by the Bcl-2-family of proteins that are situated on the mitochondrial membrane, of which Bcl-2 is the most typical antiapoptotic protein and Bax is the most typical proapoptotic protein (54). The ratio of Bcl-2/Bax determines whether a cell survives or undergoes cell death (54). When Bax expression is elevated by apoptosis-inducing factors, the reduced ratio leads to apoptosis. Additionally, JNK is also involved in mitochondrial intrinsic apoptotic pathways. JNK inhibits the expression of Bcl-2 and stimulates the expression of Bax. These key proteins cooperate to enhance the speed of apoptosis. Yang et al (55) previously demonstrated that TP-induced cell apoptosis is closely associated with the imbalance between Bcl-2 and Bax. Silymarin has been reported to maintain cell membrane fluidity and promote liver cell repair, which is thought to combat liver apoptosis (50). As expected, in the present study, TP-induced increases in the levels of Cyt C, Bax, JNK and c-caspase-3 were markedly prevented by silymarin treatment. Although the level of Bcl-2 did not change significantly between treatment groups, the alterations in the ratio of Bcl-2/Bax indicated the antiapoptotic effects of silymarin in a dose-dependent manner. These results were also verified by TUNEL staining observations. Qualitative and quantitative results of TUNEL staining demonstrated that the high-dose silymarin group had fewer TUNEL-positive cells and a lower apoptosis index compared with the TP group, which confirmed that inhibition of mitochondrial-controlled apoptosis may be the one of the protective mechanisms of silymarin against TP-induced hepatotoxicity.
Various studies have demonstrated that TP may be metabolized to three or four monohydroxylated metabolites, primarily by cytochrome P450 (CYP)3A4 and CYP3A2, respectively, in humans or rat liver microsomes during the phase I metabolic pathway (56–58). Therefore, hydroxylation and desaturation controlled by CYP3A were hypothesized to have important roles in TP detoxification (58). Further investigation of this potential mechanism is required in future studies.
In conclusion, the present study demonstrated that short-term oral administration of silymarin exerts pronounced effects in TP-induced liver injury. It was demonstrated to reduce lipid peroxidation and elevate antioxidant enzyme activity to enhance hepatic antioxidative defense systems, reduce excessive expression of proinflammatory cytokines, inhibit inflammatory signaling and ameliorate cell apoptosis to strengthen liver vitality. Silymarin exhibited satisfactory dose-dependent effects in preventing TP-induced hepatotoxicity and improving hepatic function. These results indicate that silymarin may be a potential candidate for treating TP-induced hepatotoxicity.
Acknowledgements
The present study was supported by grants from the Hong Kong, Macao and Taiwan Science and Technology Cooperation Program of China (grant no. 2014DFH30010), the Science and Technology Planning Project of Guangdong Province (grant nos. 2013B090800052, 2013B090600007 and 2013B090600026), Guangdong International Cooperation Project (grant no. 2013508102016) and the Science and Technology Major Project of Guangdong Province (grant no. 2012A080205001).
References
- 1.Jia L, Feihai S, Cuiwen G, Wenwen W, Xiaozhe S, Xinlu F, Min H, Jing J, Zhiying H. Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS One. 2014;9:10. doi: 10.1371/journal.pone.0100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 3.Shu B, Duan W, Yao J, Huang J, Jiang Z, Zhang L. Caspase 3 is involved in the apoptosis induced by triptolide in HK-2 cells. Toxicol In Vitro. 2009;23:598–602. doi: 10.1016/j.tiv.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 5.Mei ZN, Lia XK, Wu QR, Yang XL. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol Res. 2005;51:345–351. doi: 10.1016/j.phrs.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Fu Q, Huang X, Shu B, Xue M, Zhang P, Wang T, Liu L, Jiang Z, Zhang L. Inhibition of mitochondrial respiratory chain is involved in triptolide-induced liver injury. Fitoterapia. 2011;82:1241–1248. doi: 10.1016/j.fitote.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: Release of Cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 8.Newmeyer DD. Hot papers-apoptosis-the release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis by R.M. Kluck, E. Bossy-Wetzel, D.R. Green, D.D. Newmeyer-Comments. Scientist. 1999;13:16–16. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 9.Shao LW, Huang LH, Yan S, Jin JD, Ren SY. Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol Lett. 2016;12:995–1000. doi: 10.3892/ol.2016.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: Past, present, future. Phytother Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 11.Pascual C, Gonz R, Armesto J, Muriel P. Effect of silymarin and silybinin on oxygen radicals. Drug Devel Res. 1993;29:73–77. doi: 10.1002/ddr.430290109. [DOI] [Google Scholar]
- 12.Crocenzi FA, Roma MG. Silymarin as a new hepatoprotective agent in experimental cholestasis: New possibilities for an ancient medication. Curr Med Chem. 2006;13:1055–1074. doi: 10.2174/092986706776360950. [DOI] [PubMed] [Google Scholar]
- 13.Arafa HMM. Uroprotective effects of curcumin in cyclophosphamide-induced haemorrhagic cystitis paradigm. Basic Clin Pharmacol Toxicol. 2009;104:393–399. doi: 10.1111/j.1742-7843.2009.00379.x. [DOI] [PubMed] [Google Scholar]
- 14.Avci H, Tunca R, Epikmen E. T, Birincioğlu SS, Sekkin SB Murat, Akşit H. Protective and antigenotoxic effects of Silymarin and Curcumin in experimental cyclophosphamide intoxication in rats. Kafkas Univ Vet Fak Derg. 2016;22:693–701. [Google Scholar]
- 15.Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology. 1999;30:1099–1104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 16.Hahn G, Lehmann HD, Kurten M, Uebel H, Vogel G. On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;18:698–704. [PubMed] [Google Scholar]
- 17.Rajasekaran A, Periyasamy M. Synthesis and evaluation of hepatoprotective activity of some new mannich bases bearing benztriazole moiety. J Chil Chem Soc. 2010;55:366–370. doi: 10.4067/S0717-97072010000300021. [DOI] [Google Scholar]
- 18.Jia R, Cao LP, Du JL, Xu P, Jeney G, Yin GJ. The protective effect of silymarin on the carbon tetrachloride (CCl4)-induced liver injury in common carp (Cyprinus carpio) In Vitro Cell Dev Biol-Anim. 2013;49:155–161. doi: 10.1007/s11626-013-9587-3. [DOI] [PubMed] [Google Scholar]
- 19.Pappachan JM, Antonio FA, Edavalath M, Mukherjee A. Non-alcoholic fatty liver disease: A diabetologist's perspective. Endocrine. 2014;45:344–353. doi: 10.1007/s12020-013-0087-8. [DOI] [PubMed] [Google Scholar]
- 20.Voroneanu L, Nistor I, Dumea R, Apetrii M, Covic A. Silymarin in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. 2016;2016:5147468. doi: 10.1155/2016/5147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcaraz-Contreras Y, Mendoza-Lozano RP, Martínez-Alcaraz ER, Martínez-Alfaro M, Gallegos-Corona MA, Ramírez-Morales MA, Vázquez-Guevara MA. Silymarin and dimercaptosuccinic acid ameliorate lead-induced nephrotoxicity and genotoxicity in rats. Hum Exp Toxicol. 2016;35:398–403. doi: 10.1177/0960327115591373. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Oh DS, Oh JY, Son TG, Yuk DY, Jung YS. Silymarin prevents restraint stress-induced acute liver injury by ameliorating oxidative stress and reducing inflammatory response. Molecules. 2016;21:443. doi: 10.3390/molecules21040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrell MD, Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev. 2011;43:317–334. doi: 10.3109/03602532.2011.577781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol. 2015;162:238–243. doi: 10.1016/j.jep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Li XJY, Jiang ZZ, Zhang LY. Triptolide: Progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155:67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Yang GH, Wang L, Yu XT, Chen H. Protective effect of 18 beta-glycyrrhetinic acid against triptolide-induced hepatotoxicity in rats. Evid Based Complement Alternat Med. 2017;2017:3470320. doi: 10.1155/2017/3470320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dastagir G, Rizvi MA. Glycyrrhiza glabra L. (Liquorice) Pak J Pharm Sci. 2016;29:1727–1733. [PubMed] [Google Scholar]
- 28.Adaramoye OA, Odunewu AO, Farombi EO. Hepatoprotective effect of Curcuma longa L. in D-galactosamine induced liver injury in mice: Evidence of antioxidant activity. Afr J Med Med Sci. 2010;(Suppl 39):S27–S34. [PubMed] [Google Scholar]
- 29.Wan JM, Sit WH, Lee CL, Fu KH, Chan DK. Protection of lethal toxicity of endotoxin by Salvia miltiorrhiza BUNGE is via reduction in tumor necrosis factor alpha release and liver injury. Int Immunopharmacol. 2006;6:750–758. doi: 10.1016/j.intimp.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Wellington K, Jarvis B. Silymarin: A review of its clinical properties in the management of hepatic disorders. Biodrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Sidhu S, Chopra K, Khan MU. Hepatoprotective effect of trans-Chalcone on experimentally induced hepatic injury in rats: Inhibition of hepatic inflammation and fibrosis. Can J Physiol Pharmacol. 2016;94:879–887. doi: 10.1139/cjpp-2016-0071. [DOI] [PubMed] [Google Scholar]
- 32.Ali MHH, Messiha BAS, Abdel-Latif HAT. Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats. Pharm Biol. 2016;54:1198–1208. doi: 10.3109/13880209.2015.1060247. [DOI] [PubMed] [Google Scholar]
- 33.Xing H, Jia K, He J, Shi C, Fang M, Song L, Zhang P, Zhao Y, Fu J, Li S. Establishment of the Tree Shrew as an alcohol-induced fatty liver model for the study of alcoholic liver diseases. PLoS One. 2015;10:12. doi: 10.1371/journal.pone.0128253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao LJ, Li HD, Yan M, Li ZH, Gong H, Jiang P, Deng Y, Fang PF, Zhang BK. The protective effects of isoliquiritigenin and glycyrrhetinic acid against triptolide-induced oxidative stress in HepG2 cells involve Nrf2 activation. Evid Based Complement Alternat Med. 2016;2016:8912184. doi: 10.1155/2016/8912184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 36.Surai PF. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants (Basel) 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng B, Meng R, Huang B, Shen SM, Bi Y, Zhu DL. Silymarin alleviates hepatic oxidative stress and protects against metabolic disorders in high-fat diet-fed mice. Free Radic Res. 2016;50:314–327. doi: 10.3109/10715762.2015.1116689. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Hong RT, Tian TL. Silymarin's protective effects and possible mechanisms on alcoholic fatty liver for rats. Biomol Ther. 2013;21:264–269. doi: 10.4062/biomolther.2013.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu HP, Gu XZ, Xu QT, Kang WY. Antioxidant activity in vitro and hepatoprotective effect of Phlomis maximowiczii in vivo. Afr J Tradit Complement Altern Med. 2014;11:46–52. doi: 10.4314/ajtcam.v11i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simeonova R, Vitcheva V, Kondeva-Burdina M, Krasteva I, Manov V, Mitcheva M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. Biomed Res Int. 2013;2013:757126. doi: 10.1155/2013/757126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan L, Gu XZ, Yin ZH, Kang WY. Antioxidant activities in vitro and hepatoprotective effects of Nelumbo nucifera leaves in vivo. Afr J Tradit Complement Altern Med. 2014;11:85–91. doi: 10.4314/ajtcam.v11i3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185–194. doi: 10.1055/s-2002-30106. [DOI] [PubMed] [Google Scholar]
- 43.Desplaces A, Choppin J, Vogel G, Trost W. The effects of silymarin on experimental phalloidine poisoning. Arzneimittel-Forschung. 1975;25:89–96. [PubMed] [Google Scholar]
- 44.Woo K, Stewart SG, Kong GS, Finch-Edmondson ML, Dwyer BJ, Yeung SY, Abraham LJ, Kampmann SS, Diepeveen LA, Passman AM, et al. Identification of a thalidomide derivative that selectively targets tumorigenic liver progenitor cells and comparing its effects with lenalidomide and sorafenib. Eur J Med Chem. 2016;120:275–283. doi: 10.1016/j.ejmech.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Imanishi H, Scales WE, Campbell DA. Tumor necrosis factor alpha alters the cytotoxic effect of hydrogen peroxide in cultured hepatocytes. Biochem Biophys Res Commun. 1997;230:120–124. doi: 10.1006/bbrc.1996.5901. [DOI] [PubMed] [Google Scholar]
- 46.Auguet T, Vidal F, López-Dupla M, Broch M, Gutiérrez C, Olona M, Oltra C, Aguilar C, González E, Quer JC, et al. A study on the TNF-alpha system in Caucasian Spanish patients with alcoholic liver disease. Drug Alcohol Depend. 2008;92:91–99. doi: 10.1016/j.drugalcdep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5:439–447. doi: 10.1016/S1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 49.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26:173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 50.Jin YW, Zhao X, Zhang H, Li QS, Lu GD, Zhao XG. Modulatory effect of silymarin on pulmonary vascular dysfunction through HIF-1 alpha-iNOS following rat lung ischemia-reperfusion injury. Exp Ther Med. 2016;12:1135–1140. doi: 10.3892/etm.2016.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zholobenko A, Modriansky M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia. 2014;97:122–132. doi: 10.1016/j.fitote.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Volbracht C, Leist M, Kolb SA, Nicotera P. Apoptosis in caspase-inhibited neurons. Mol Med. 2001;7:36–48. [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 54.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Yang F, Zhuo L, Ananda S, Sun TY, Li SX, Liu L. Role of reactive oxygen species in triptolide-induced apoptosis of renal tubular cells and renal injury in rats. J Huazhong Univ Sci Technolog Med Sci. 2011;31:335–341. doi: 10.1007/s11596-011-0377-4. [DOI] [PubMed] [Google Scholar]
- 56.Tai T, Huang X, Su YW, Ji J, Su Y, Jiang Z, Zhang L. Glycyrrhizin accelerates the metabolism of triptolide through induction of CYP3A in rats. J Ethnopharmacol. 2014;152:358–363. doi: 10.1016/j.jep.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 57.Han FM, Peng ZH, Wang JJ, Chen Y. In vivo effect of triptolide combined with glycyrrhetinic acid on rat cytochrome P450 enzymes. Yao Xue Xue Bao. 2013;48:1136–1141. [PubMed] [Google Scholar]
- 58.Ye X, Li W, Yan Y, Mao C, Cai R, Xu H, Yang X. Effects of cytochrome P4503A inducer dexamethasone on the metabolism and toxicity of triptolide in rat. Toxicol Lett. 2010;192:212–220. doi: 10.1016/j.toxlet.2009.10.028. [DOI] [PubMed] [Google Scholar]