Abstract
It has been reported that cornin may reduce neuronal death during cerebral ischemia; however, little is known about the molecular mechanism of the role of corninin autophagy in SH-SY5Y neuronal cells. In the present study, oxygen-glucose deprivation (OGD)-treated cells were used as a cerebral ischemia model in vitro. The results demonstrated that cornin was able to reduce neuronal cell loss, increase the apoptosis regulator Bcl-2/apoptosis regulator BAX ratio, and decrease the protein levels of caspase-3. In addition, cornin decreased the microtubule-associated proteins 1A/1B light chain 3B (LC3)-II/LC3-I ratio and beclin-1 protein expression, and resulted in an upregulation in phosphorylated (p)-RAC-α serine/threonine-protein kinase (Akt), p-protein kinase mTOR (mTOR) in OGD-treated SH-SY5Y cells. Additionally, it was observed that following inhibition of PI3K/Akt by LY294002, the levels of p-Akt and p-mTOR were markedly decreased, and the LC3-II/LC3-I ratio and beclin-1 were increased. Similarly, following inhibition of mTOR by rapamycin, LC3-II/LC3-I and Beclin-1 were significantly increased in SH-SY5Y cells. These results indicated that cornin protected SH-SY5Y cells against OGD-induced autophagy through the PI3K/Akt/mTOR pathway.
Keywords: cornin, oxygen-glucose deprivation, phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein kinase/protein kinase mTOR pathway, autophagy, SH-SY5Y cells
Introduction
Ischemic stroke has high morbidity and mortality rates and is a substantial burden for patients and society (1). A degree of progress has been made, including treatment with intravenous recombinant tissue plasminogen activator (rt-PA) (2,3) and recombinant T cell receptor ligand combined with rt-PA; however, the majority of clinical cases are treated with rt-PA thrombolytic therapy as the primary method. Due to the narrow therapeutic window of 4.5 h, therapeutic strategies for ischemic stroke remain unsatisfactory. A number of studies have reported that autophagy servesan important rolein cerebral ischemic injury in animal models and cellular models, by causing progressive degeneration of the brain (4,5).
Autophagy is a lysosomal degradation pathway which is essential for cell survival, proliferation, differentiation and homeostasis (6,7). Autophagy may be activated by potent extracellular stimuli, including starvation, viral infection, ischemia and hypoxia (8). However, excessive autophagy may induce cell death via direct autophagic cell death or indirect crosstalk with apoptosis (9,10). Protein kinase mTOR (mTOR) is an atypical serine/threonine protein kinase, and is important for growth regulation (11). mTOR is primarily regulated by the phosphatidylinositol 3-kinase (PI3K)/RAC-α serine/threonine-protein kinase (Akt)/mTOR signaling pathway, which serves important roles in the inhibition of cellular apoptosis, and the promotion of cell proliferation and cell survival (12,13). A number studies have demonstrated that the process of autophagy is negatively regulated by the activation of mTOR. It has been reported that autophagy may protect against toxicity and promote neuronal survival and plasticity, thereby leading to learning rescue and memory enhancement by activating mTOR protein biosynthesis (14,15). Therefore, retaining the PI3K/Akt/mTOR signaling pathway and inhibiting autophagy maybe a potential approach for the treatment ofischemic stroke.
Cornin is an iridoid glycoside isolated from the fruit of Verbena officinalis L. that has protective potential against cerebral ischemia injury and induces angiogenesis in vitro (16–18). However, the effect and molecular mechanism of cornin on autophagy in stroke remains unclear. The present study investigated whether cornin was able to inhibit autophagy through upregulation of the PI3K/Akt/mTOR signaling pathway in SH-SY5Y cells, and aimed to provide a novel theoretical basis for the treatment of ischemic stroke with cornin.
Materials and methods
Drugs and reagents
Cornin (purity >99.0%; CAS no. 548-37-8; molecular formula, C17H24O10; molecular weight, 388.37) was provided by Shandong Engineering Research Center for Nature Drug (Shangdong, China) was dissolved in sterile physiological (0.9%) saline to make a stock solution. Dilutions were prepared according to the different administration doses.
The Akt (cat. no. 8805; 1:1,000 dilution), phosphorylated (p)-Akt (cat. no. 38449; 1:500 dilution), mTOR (cat. no. 2732; 1:2,000 dilution), p-mTOR (cat. no. 109268; 1:1,000 dilution), apoptosis regulator Bcl-2 (cat. no. 32124; 1:1,000 dilution), apoptosis regulator BAX (Bax) (cat. no. 32503; 1:1,000 dilution), β-actin (cat. no. 8226; 1:500 dilution), microtubule-associated proteins 1A/1B light chain 3B (LC3) (cat. no. 128025; 1:1,000 dilution) and Beclin-1 (cat. no. 62557; 1:2,000 dilution) antibodies were purchased from Abcam (Cambridge, UK). The following pharmacological agents were used: The PI3K/Akt inhibitor LY294002 and the mTOR inhibitor rapamycin (both Merck KGaA, Darmstadt, Germany).
Cell culture
The human neuroblastoma SH-SY5Y cell line was obtained from the Institute of Basic Medical Sciences of Chinese Academy of Medical Sciences (Beijing, China). Short-tandem repeat analysis was performed by Shanghai Saily Biotechnologies Co., Ltd. (Shanghai, China) to ascertain that the cell line used in the present study was of human origin (sample no. 20160921-01). SH-SY5Y cells were maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences) and antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) in a humidified atmosphere of 5% CO2 at 37°C. The control group was treated with dimethyl sulfoxide (DMSO). In order to study the mechanism of the effect of cornin on autophagy, SH-SY5Y cells were incubated with cornin (9 µM) for 24 h prior to OGD, followed by 10 µM LY294002 or 10 µM rapamycin for 6 h.
In vitro OGD model
In order to simulate OGD in vitro, SH-SY5Y cells were incubated in a hypoxia solution for 6 h. The hypoxia solution contained 0.9 mM NaH2PO4, 6.0 mM NaHCO3, 1.0 mM CaCl2, 1.2 mM MgSO4, 40 mM sodium lactate, 20 mM HEPES, 98.5 mM NaCl and 10.0 mM KCl (pH adjusted to 6.8), and was bubbled with N2 for 30 min prior to application. The O2 pressure of the hypoxia solution was adjusted to 64.0 kPa. Hypoxic conditions were produced by placing the plates of cultured SH-SY5Y cells in a hypoxic incubator (Kendro; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with the oxygen adjusted to 1.0% and the CO2 to 5.0%. Prior to hypoxia, SH-SY5Y cells were pretreated with various concentrations (3, 9 and 27 µM) of cornin for 24 h. Normal culturing (DMEM containing 2% FBS under 20% oxygen and 5% CO2) served as the negative control, and the hypoxia solution culture served as the control.
Determination of cell viability and lactate dehydrogenase (LDH) leakage
SH-SY5Y cells were incubated with or without cornin in the hypoxia solution for 6 h and cell viability was assessed using an MTT assay. MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into the cells and the cells were incubated for 4 h in 37°C. Following the removal of the medium, DMSO was added to dissolve the blue-colored formazan product. The absorbance was measured at a wavelength of 490 nm to determine the optical density value of each well. LDH, an indicator of cellular injury, was detected according to manufacturer's protocol of the LDH assay kit (Beijing Zhongsheng Bioreagent, Beijing, China). The formula LDH leakage rate (%)=Ae/At ×100 was used, in which Ae indicated extracellular LDH (cell culture fluid) and At indicated intracellular and extracellular LDH (cell lysate).
Western blot analysis
SH-SY5Y cells were cultured for 24 h, washed twice with ice-cold PBS and lysed in NP40 lysis buffer (BioSource International, Inc., Camarillo, CA, USA) (50 mM Tris, pH 7.4; 250 mM NaCl; 5 mM EDTA; 50 mM NaF; 1 mM Na3VO4; 1% NP-40; and 0.02% NaN3) supplemented with 1 mM phenylmethylsulfonyl fluoride and 1X protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Equal amounts of cellular protein (40 µg) were separated by 12 or 10% SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% (w/v) skimmed milk for 2 h at room temperature. The membranes were then incubated with the following specific antibodies: Anti-Akt (cat. no. 8805; 1:1,000 dilution), anti-p-Akt (cat. no. 38449; 1:500 dilution), anti-mTOR (cat. no. 2732; 1:2,000 dilution), anti-p-mTOR (cat. no. 109268; 1:1,000 dilution), anti-LC3 (cat. no. 128025; 1:1,000 dilution), anti-beclin-1 (cat. no. 62557; 1:2000 dilution), anti-Bcl-2 (cat. no. 32124; 1:1,000 dilution), anti-Bax (cat. no. 32503; 1:1,000 dilution), anti-caspase-3 (cat. no. 13847; 1:1,000 dilution) and anti-β-actin (cat. no. 8226; 1:500 dilution) as a loading control at 4°C overnight. Subsequently, they were incubated for 2 h at room temperature with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (cat no. A0208; 1:5,000 dilution; Beyotime Institute of Biotechnology). Protein bands were visualized using enhanced chemiluminescence regent (EMD Millipore, Billerica, MA, USA). The optical densities of the bands were scanned and quantified using a Gel Doc2000 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data were normalized against those of the corresponding β-actin bands. Results are expressed as a fold increase compared with the control.
Statistical analysis
All of the experiments were performed in triplicate. Quantitative data from experiments are expressed as the mean ± standard deviation. Significance was determined by one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of cornin on cultured SH-SY5Y cells against OGD-induced cell death
The cell viability of OGD-treated SH-SY5Y cells was markedly decreased compared with the normal cultured cells. However, the cell viability of OGD-treated cells was markedly increased following treatment with cornin (3–27 µM), as presented in Table I. In order to further investigate the protective effect of cornin, the LDH leakage rate was estimated. A significant increase in the LDH leakage rate in SH-SY5Y cells was observed following OGD. Incubation with various concentrations of cornin significantly inhibited the OGD-induced LDH release in a concentration-dependent manner.
Table I.
Effects of cornin on viability and LDH leakage in SH-SY5Y cells exposed to oxygen-glucose deprivation.
| Groups | OGD | Content, µM | Cell viability, % | LDH leakage, % |
|---|---|---|---|---|
| Normal | − | − | 91.1±2.9 | 3.0±0.9 |
| Control | + | _ | 51.1±3.5a | 22.9±3.2a |
| + | 3 | 63.7±4.0b | 18.6±1.6b | |
| Cornin | + | 9 | 72.2±2.7b | 16.7±1.7b |
| + | 27 | 62.2±3.2b | 19.2±2.2b |
Following 6 h OGD, followed by 24 h incubation with cornin, cell viability and LDH leakage were assessed. Values are presented as the mean ± standard deviation (n=6). Significance was determined by one-way analysis of variance followed by Dunnett's test.
P<0.01 vs. normal group
P<0.01 vs. control group. OGD, oxygen-glucose deprivation; LDH, lactate dehydrogenase.
Effect of cornin on the expression of cellular apoptosis-associated proteins in SH-SY5Y cells
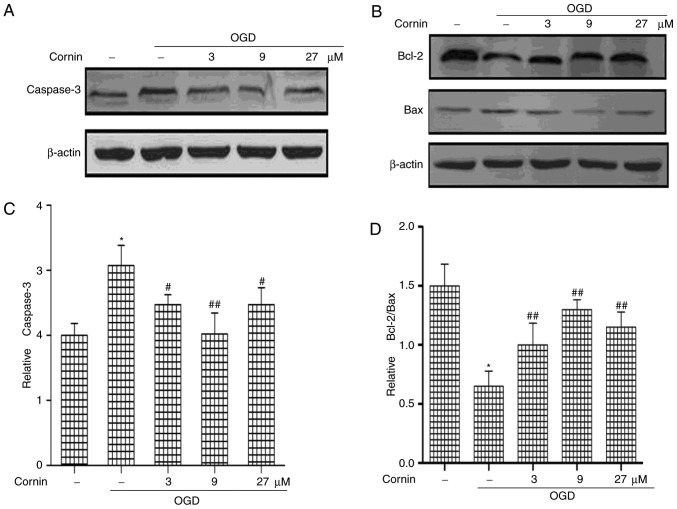
The Bcl-2 and caspase families are the important mediators of apoptosis. Bcl-2, caspase-3, and Bax protein levels were determined to elucidate whether cornin was able to protect SH-SY5Y cells from OGD-induced apoptosis. The protein levels of Caspase-3 and Bax were significantly increased, and Bcl-2 was decreased, by treatment with OGD, compared with normal cells. However, cornin increased Bcl-2, and decreased Bax and caspase-3 levels significantly in OGD-treated cells (Fig. 1).
Figure 1.
Effect of cornin on the apoptosis of SH-SY5Y cells. Cells were treated with cornin (3, 9 and 27 µM) for 24 h prior to exposure to OGD for 6 h. (A) Caspase-3, (B) Bcl-2 and Bax protein expression was obtained by western blot analysis. The results for (C) caspase-3, (D) Bcl-2 and Bax were quantified, and are expressed as the mean ± standard deviation. n=3. *P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. OGD group. OGD, oxygen-glucose deprivation; Bcl-2, apoptosis regulator Bcl-2; Bax, apoptosis regulator BAX.
Protective effect of cornin is associated with inhibited autophagy in OGD-treated SH-SY5Y cells
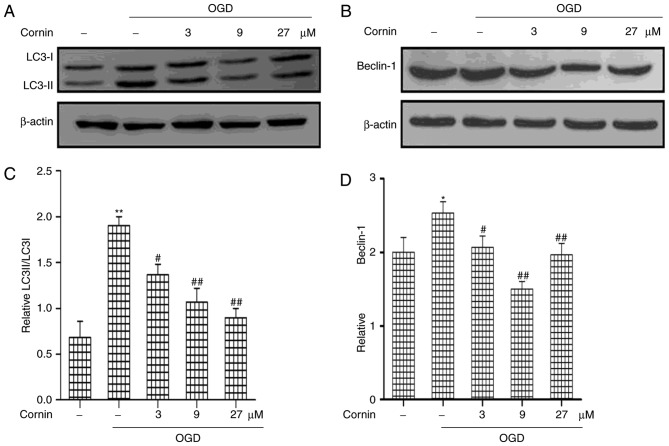
In order to determine the protective mechanisms of cornin in OGD-treated cells, the present study analyzed the effects of cornin on autophagy in the protection of SH-SY5Y cells. Previous studies have demonstrated that autophagy activation is involved in ischemic stroke (19,20); however, the role of cornin in regulating autophagy in ischemic stroke has not been clearly defined. In the model used in the present study, the regulatory effect of cornin on LC3 and beclin-1, which are frequently used as indicators of autophagy, was investigated. LC3 and beclin-1 expression in the OGD model were detected by western blot analysis. As presented in (Fig. 2), beclin-1 and the ratio of LC3-II/LC3-I increased in the OGD model compared with the control group. This effect was significantly inhibited by treatment with cornin for 24 h prior to OGD, in a concentration-dependent manner.
Figure 2.
Effects of cornin on the protein expression of LC3 and beclin-1. SH-SY5Y cells were treated with cornin (3, 9 and 27 µM) for 24 h and cell lysates were subjected to immunoblot analysis for detecting the levels of (A) LC3II, LC3I and (B) beclin-1. β-actin was used as cell lysates loading controls. The results for (C) LC3 II/LC 3 I and (D) beclin-1 are expressed as the mean ± standard deviation. n=3. *P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. OGD group. OGD, oxygen-glucose deprivation; LC3, microtubule-associated proteins 1A/1B light chain 3B.
Cornin affects OGD-induced autophagy, involving the activation of PI3K/Akt/mTOR pathway
Previous studies demonstrated that cornin inhibited OGD-induced cell damages by activating PI3K/Akt signaling (16,18). Therefore, the present study aimed to further examine whether cornin may affect OGD-induced autophagy through the Akt/mTOR pathway. SH-SY5Y cells were treated with 3, 9, or 27 µM cornin, and control groups were treated with DMSO. A total of 24 h subsequently, the phosphorylation levels of Akt and mTOR were examined using western blotting. The results demonstrated that cornin was able to upregulate the protein levels of p-Akt, and p-mTOR under OGD, as presented in Fig. 3.
Figure 3.
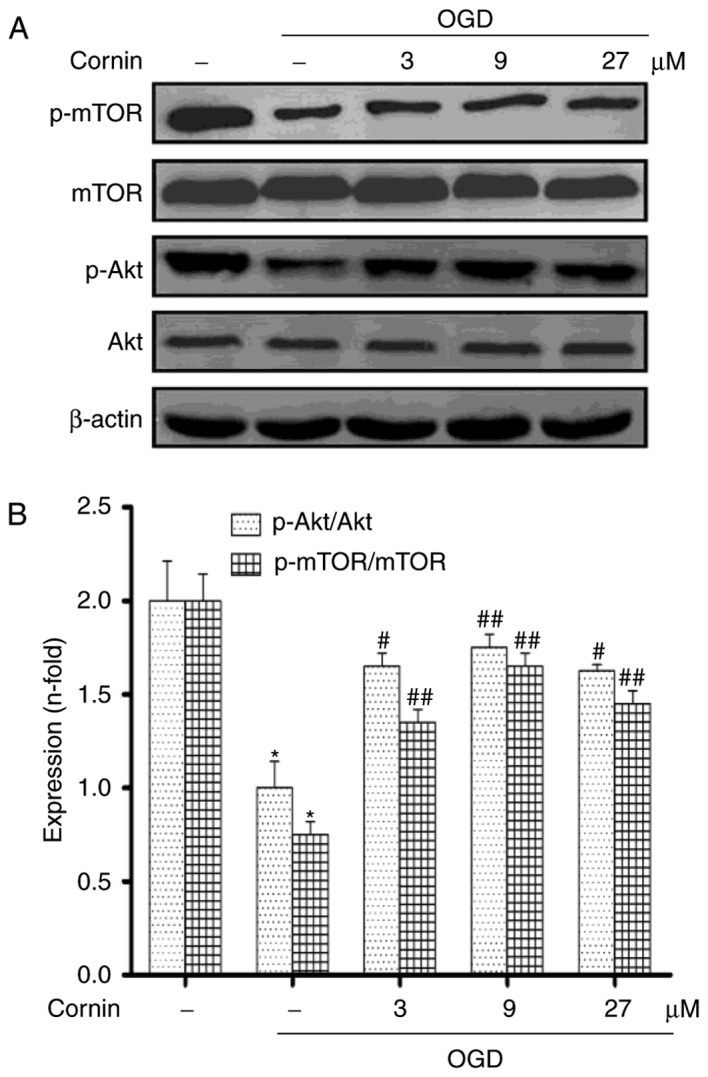
Effect of cornin on the protein expression of Akt, p-Akt, mTOR and p-mTOR. SH-SY5Y cells were treated with cornin (3, 9 and 27 µM) for 24 h and cell lysates were subjected to immunoblot analysis for detecting the levels of (A) AKT, p-Akt, mTOR, p-mTOR. β-actin was used as the cell lysate loading control. (B) Densitometric analysis was performed. The results are expressed as the mean ± standard deviation. n=3. *P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. OGD group. OGD, oxygen-glucose deprivation; Akt, RAC-α serine/threonine-protein kinase; mTOR, protein kinase mTOR; p, phosphorylated.
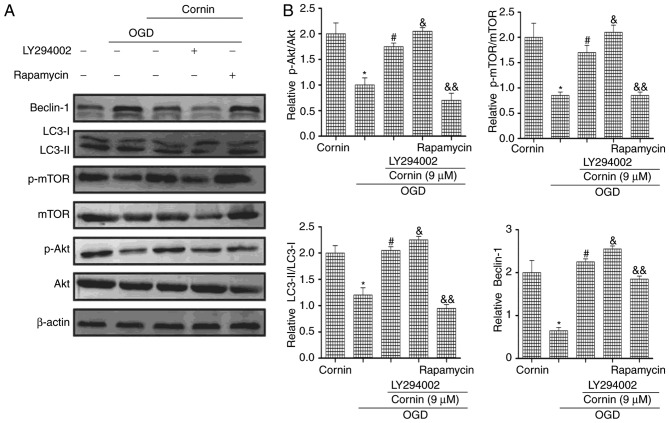
To investigate whether cornin affected OGD-induced autophagy via the PI3K/Akt/mTOR signaling pathway, the PI3K/Akt inhibitor LY294002 and the mTOR inhibitor rapamycin were used in western blotting. The protein expression levels of p-Akt, p-mTOR, LC3-II/LC3-I and beclin-1 were decreased by treatment with LY294002. Rapamycin increased mTOR phosphorylation, and increased LC3-II/LC3-I and beclin-1 protein expression, as presented in (Fig. 4). These results of the present study indicated that cornin reduced OGD-induced autophagy by affecting Akt and mTOR phosphorylation levels.
Figure 4.
Cornin decreased OGD-induced autophagy via activation of the PI3K/Akt/mTOR signaling pathway in vitro. SH-SY5Y cells were pretreated with a selective PI3K/Akt inhibitor (LY294002; 10 µM) and a mTOR inhibitor (rapamycin; 10 µM) for 6 h following incubation with cornin (9 µM) for 24 h. (A) Western blot analysis was performed. (B) Densitometric analysis was subsequently performed and the results are expressed as the mean ± standard deviation. n=3. *P<0.05 vs. control group; #P<0.05 vs. OGD group; &P<0.05, &&P<0.01 vs. cornin 9 µM group. OGD, oxygen-glucose deprivation; PI3K, phosphatidylinositol 3-kinase; Akt, RAC-α serine/threonine-protein kinase; mTOR, protein kinase mTOR; LC3, microtubule-associated proteins 1A/1B light chain 3B; p, phosphorylated.
Discussion
The principal findings of the present study indicated that cornin exerted protective effects against OGD-induced autophagy in SH-SY5Y cells. Additionally, treatment with cornin markedly activated the expression of p-Akt and p-mTOR. The increased levels of LC3II/LC3I and beclin-1 were decreased following treatment with cornin. Following inhibition of PI3K/Akt with LY294002, p-Akt, p-mTOR, LC3II/LC3I and beclin-1 were significantly decreased in SH-SY5Y cells. Similarly, following inhibition of mTOR with rapamycin, LC3II/LC3I and beclin-1 were significantly increased in SH-SY5Y cells. The results of the present study suggested that cornin was involved in the modulation of OGD-induced autophagy on PI3K/Akt/mTOR signaling.
It is known that autophagy is a highly conserved pathway for degradation and servesan important role in cerebral ischemic injury. However, excessive autophagy may exacerbate cellular damage and result in autophagic cell death or apoptosis (21,22). The present study demonstrated reduced cell viability, increased LDH leakage, and upregulation of caspase-3 protein in the OGD model, which indicated that cornin may exert protective effects on OGD-induced apoptosis.
LC3 is a an important indicator of the occurrence of autophagy. During autophagy, the cytosolic form LC3-I is converted to the phosphatidylethanolamine-conjugated form LC3-II to promote autophagosome formation (23). Therefore, an increase in the expression of LC3-II has been used to indicate the activation of autophagy (24). Beclin-1, an autophagy-associated protein, may mediate other autophagicproteins attached to autophagosome membranes and decrease LC3-II accumulation. Beclin-1 is an additional import indicator of the degree of autophagy (25,26). In the present study, LC3-II/ LC3-I and Beclin-1 were increased in SH-SY5Y cells subjected to 6 h of OGD, whereas cornin reduced them in a concentration-dependent manner. These results indicated that the anti-apoptotic protective effect of cornin may be associated with a decreased in the autophagy induced by OGD.
In order to elucidate the mechanisms underlying the regulatory effect of cornin on OGD-induced autophagy in SH-SY5Y cells, the present study investigated the effects of cornin on the activation of the PI3K/Akt/mTOR pathway. mTOR, one of the downstream targets of the PI3K/Akt signaling pathway, is a key regulator of cell growth, proliferation, autophagy and survival (27,28). Previous studies have suggested that PI3K/Akt/mTOR signaling is important for nerve vascular unit survival during ischemia (29,30). The present study demonstrated that treatment with cornin markedly activated the phosphorylationof Akt and mTOR. The PI3K/Akt inhibitor LY294002 significantly abrogated the increased phosphorylation of Akt and mTOR, and reduced the increased levels of LC3-II/LC3-I and beclin-1 following treatment with cornin. Additionally, the inhibition of mTOR by rapamycin strengthened the occurrence of autophagy.
In conclusion, the results of the present study suggested that cornin may be a novelway to regulate cerebral ischemia-induced autophagy in neurons. Although the data from the present study provided cellular and pharmacological proof of principle for the use of cornin in vitro, in vivo validation of these mechanisms remains to be obtained. The present findings provided novel evidence for the regulatory mechanism of autophagy and may provide a theoretical basis for the development of cornin as a treatment for cerebral ischemia.
Acknowledgements
The present study was supported by Binzhou Medical University Science and Technology Program (grant no. BY2016KJ11), and financially supported in part by the National Natural Science Foundation of China (grant no. 31570352).
References
- 1.Endres M, Dirnagl U. Ischemia and stroke. Adv Exp Med Biol. 2002;513:455–473. doi: 10.1007/978-1-4615-0123-7_17. [DOI] [PubMed] [Google Scholar]
- 2.Bas DF, Ozdemir AO, Colak E, Kebapci N. Higher insulin resistance level is associated with worse clinical response in acute ischemic stroke patients treated with intravenous thrombolysis. Transl Stroke Res. 2016;7:167–171. doi: 10.1007/s12975-016-0453-y. [DOI] [PubMed] [Google Scholar]
- 3.Mandava P, Shah SD, Sarma AK, Kent TA. An outcome model for intravenous rt-pa in acute ischemic stroke. Transl Stroke Res. 2015;6:451–457. doi: 10.1007/s12975-015-0427-5. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q, Harris VA, Kumar S, Mansour HM, Black SM. Autophagy in neonatal hypoxia ischemic brain is associated with oxidative stress. Redox Biol. 2015;6:516–523. doi: 10.1016/j.redox.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Levine B. Autophagic cell death: The story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puyal J, Clarke PG. Targeting autophagy to prevent neonatal stroke damage. Autophagy. 2009;5:1060–1061. doi: 10.4161/auto.5.7.9728. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZQ, Yang Y, Lu T, Luo P, Li J, Wu JP, Tang ZZ, Lu QP, Duan QH. Protective effect of autophagy inhibition on ischemia-reperfusion-induced injury of N2a cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33:810–816. doi: 10.1007/s11596-013-1203-y. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchiyama Y, Koike M, Shibata M. Autophagic neuron death in neonatal brain ischemia/hypoxia. Autophagy. 2008;4:404–408. doi: 10.4161/auto.5598. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, Su L, Zhang Y. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One. 2012;7:e46092. doi: 10.1371/journal.pone.0046092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82:1245–1266. doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei H, Li Y, Han S, Liu S, Zhang N, Zhao L, Li S, Li J. cPKCgamma-modulated autophagy in neurons alleviates ischemic injury in brain of mice with ischemic stroke through Akt-mTOR pathway. Transl Stroke Res. 2016;7:497–511. doi: 10.1007/s12975-016-0484-4. [DOI] [PubMed] [Google Scholar]
- 13.Mao XY, Zhou HH, Li X, Liu ZQ. Huperzine A alleviates oxidative glutamate toxicity in hippocampal ht22 cells via activating bdnf/trkb-dependent PI3K/akt/mtor signaling pathway. Cell Mol Neurobiol. 2016;36:915–925. doi: 10.1007/s10571-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao C, Cai Y, Zhang X, Huang H, Wang J, Wang Y, Tong X, Wang J, Wu J. Ischemic preconditioning mediates neuroprotection against ischemia in mouse hippocampal CA1 neurons by inducing autophagy. PLoS One. 2015;10:e0137146. doi: 10.1371/journal.pone.0137146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L, Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al. bFGF regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the PI3K/Akt/mTOR pathway. Sci Rep. 2015;5:9287. doi: 10.1038/srep09287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang Z, Jiang W, Luan H, Zhao F, Zhang S. Cornin induces angiogenesis through PI3K-Akt-eNOS-VEGF signaling pathway. Food Chem Toxicol. 2013;58:340–346. doi: 10.1016/j.fct.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Zhang G, Kang Z, Xu Y, Jiang W, Zhang S. Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin pathway. Arch Pharm Res. 2016;39:133–142. doi: 10.1007/s12272-015-0652-1. [DOI] [PubMed] [Google Scholar]
- 18.Jiang WL, Zhang SP, Zhu HB, Jian Hou, Tian JW. Cornin ameliorates cerebral infarction in rats by antioxidant action and stabilization of mitochondrial function. Phytother Res. 2010;24:547–552. doi: 10.1002/ptr.2978. [DOI] [PubMed] [Google Scholar]
- 19.Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, Fu W, Zhang J, Wu W, Zhang X, Chen YG. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12:781–790. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 20.Xingyong C, Xicui S, Huanxing S, Jingsong O, Yi H, Xu Z, Ruxun H, Zhong P. Upregulation of myeloid cell leukemia-1 potentially modulates beclin-1-dependent autophagy in ischemic stroke in rats. BMC Neurosci. 2013;14:56. doi: 10.1186/1471-2202-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18:250–260. doi: 10.1111/j.1755-5949.2012.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 23.Larsen KE, Sulzer D. Autophagy in neurons: A review. Histol Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Singh R, Aschner M. Methods for the detection of autophagy in Mammalian cells. Curr Protoc Toxicol. 2016;69(20):12.1–20.12.26. doi: 10.1002/cptx.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 26.Mei Y, Glover K, Su M, Sinha SC. Conformational flexibility of BECN1: Essential to its key role in autophagy and beyond. Protein Sci. 2016;25:1767–1785. doi: 10.1002/pro.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widlund AL, Baur JA, Vang O. mTOR: More targets of resveratrol? Expert Rev Mol Med. 2013;15:e10. doi: 10.1017/erm.2013.11. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–114. doi: 10.1016/j.biopha.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi: 10.1016/j.ejphar.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Yang Y, Hu Z, Ling S, Fang M. Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front Neuroanat. 2015;9:48. doi: 10.3389/fnana.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]