Abstract
While aging is associated with clear declines in physical and cognitive processes, emotional functioning fares relatively well. Consistent with this behavioral profile, two core emotional brain regions, the amygdala and ventromedial prefrontal cortex, show little structural and functional decline in aging, compared with other regions. However, emotional processes depend on interacting systems of neurotransmitters and brain regions that go beyond these structures. This review examines how age-related brain changes influence processes such as attending to and remembering emotional stimuli, regulating emotion, recognizing emotional expressions, empathy, risk taking, impulsivity, behavior change and attentional focus.
INTRODUCTION
Aging is a multifaceted process that involves interacting brain regions and neurotransmitter systems that are not uniformly affected by aging. As should be expected given the variability in vulnerability to aging among brain regions and systems, some everyday abilities decline more in normal aging than others. Emotion is a fascinating domain within the study of aging, because emotional functions show less decline in normal aging than many other processes, and in some cases, are as or more effective in older adults than in younger adults. In this paper, I first review brain regions and neurotransmitter systems that play important roles in emotion and how these fare in aging. I then review age differences in various emotional tasks and processes and what is known about how they relate to age-related brain changes.
THE FATE OF EMOTION-RELATED BRAIN REGIONS AND MONOAMINERGIC NEUROTRANSMITTER SYSTEMS IN NORMAL AGING
Prefrontal Cortex
In the 1990’s, a frontal theory of aging emerged, accounting for older adults’ cognitive deficits by the greater decline in prefrontal than other brain regions in aging (West, 1996). But when it came to affect, the prefrontal theory of aging did not make sense. For instance, the famous lesion patient Phineas Gage had a tamping iron destroy part of the lower middle section of his prefrontal cortex (in the ventromedial prefrontal cortex or vmPFC; see Figure 1a) and lost the ability to control his emotions after his injury. Yet despite age-related prefrontal decline, emotional control impairments are not associated with normal aging.
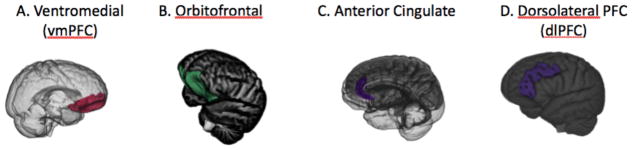
Figure 1.
Subdividing the prefrontal cortex is not simple and there are not always agreed-upon boundaries. However, in terms of understanding the aging and emotion literature, several subregions are important. First, the ventromedial prefrontal cortex (vmPFC; A) is the medial portion of the prefrontal cortex from the lower half of the prefrontal cortex, demarcated at the genu (or “knee” in Latin) of the corpus collosum. The orbitofrontal cortex (B) is the “floor” of the frontal cortex, which is found just above the eye orbits. The anterior cingulate cortex (C) is the medial portion of the prefrontal cortex that is adjacent to the corpus collosum. The vmPFC overlaps with the medial part of the orbitofrontal cortex and typically includes the ventral portion of the anterior cingulate (Clark et al., 2008). While the dorsolateral prefrontal region (D) is often defined as the middle frontal gyrus covering the lateral part of Brodmann’s areas 9 and 46 (e.g., Murray and Ranganath, 2007), some studies examining age differences in anatomical volume define it more broadly to include superior, middle and inferior frontal gyri spanning from the most dorsomedial point of the cortex down to the lateral orbital sulcus (e.g., Lövdén et al., 2013).
Indeed, the contrast between older adults and vmPFC lesion patients in their emotional behavior is striking. In many cases, vmPFC damage is associated with impulsive aggression, violence and anger (Grafman et al., 1996). Older adults, on the other hand, tend to be less prone to outbursts or feelings of anger than younger adults (Birditt and Fingerman, 2005, Charles and Carstensen, 2008, Phillips et al., 2006) and partner aggression is lower among older than younger adults (O’Leary and Woodin, 2005). Another characteristic of vmPFC lesions is the loss of ability to maintain secure attachments (Damasio et al., 1990). In contrast, deficits in attachment processes are not associated with aging—instead attachment anxiety is lower among older than younger adults (Chopik et al., 2013).
Phillips and Della Sala (1998) were the first to propose that the frontal theory of aging needed to be revised to accommodate such discrepancies. They proposed that dorsolateral prefrontal regions subserving fluid intelligence decline more in normal aging than orbitofrontal regions associated with emotional contributions to social behavior and decision making (see Figure 1B). They subsequently tested a modified version of their theory with vmPFC as the critical preserved region using a battery of tasks selected to tap either vmPFC or dorsolateral PFC (dlPFC) and found that age-related impairments were significantly more pronounced on the dlPFC tasks (MacPherson et al., 2002).
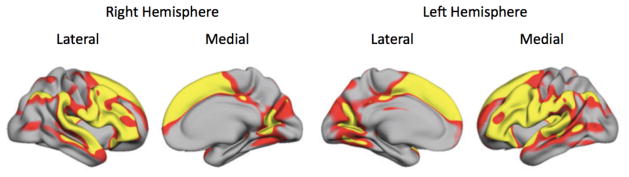
Subsequent findings that vmPFC declined in volume significantly less than dlPFC supported this hypothesis regarding differential rates of decline. For instance, an analysis aggregating across six different samples with a total of 883 participants found that there were highly significant negative correlations between age and cortical thickness in the superior, middle and inferior frontal gyri, but not in the anterior cingulate cortices or in vmPFC (Figure 2; Fjell and Walhovd, 2010).
Figure 2.
Regions shown in yellow were those that showed the largest decline in cortical thickness with age across a sample of 883 participants ranging in age from 18–94 (Fjell et al., 2009b).
Thus, the pattern of emotion-related findings and the structural data make a compelling case for relatively preserved vmPFC in aging. However, it should be noted that researchers using the Iowa Gambling Task, delay discounting, or facial emotion recognition as part of task batteries to assess ventromedial-dorsolateral distinctions in aging sometimes concluded that vmPFC functions are also significantly impaired in aging (Baena et al., 2010, Lamar and Resnick, 2004). As will be addressed in later sections, it is problematic to assume that these particular tasks depend on vmPFC.
Amygdala
The amygdala abuts the anterior end of the hippocampus (Figure 3A) and is a collection of nuclei that have somewhat distinct roles but together play a key role in emotion. In particular, the amygdala helps detect potentially emotionally relevant stimuli—relevant because they are novel, pose a threat, or are goal-relevant—and then modulates other brain systems to enhance attention and memory for those stimuli. It is clearly involved in anxiety and fear, but also plays a part in positive affect and motivation.
Figure 3.
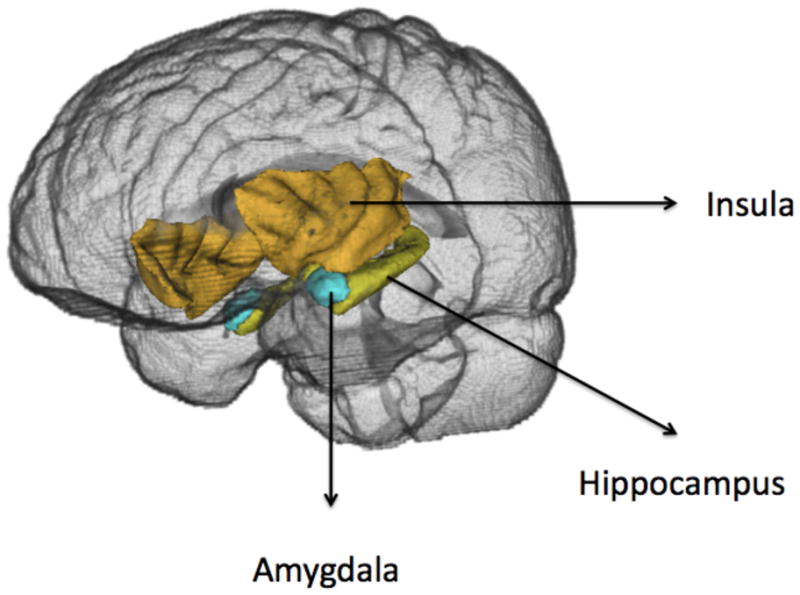
Locations of the left amygala, hippocampus and insula; the right amygdala, hippocampus and insula are shown in the background.
Findings regarding the structural integrity of the amygdala suggest that it is better maintained in aging than most other regions, although findings are not entirely consistent. In some structural MRI studies, the amygdala shows no significant differences in volume across the adult lifespan, in the context of decline in other structures (Jernigan et al., 2001, Jiang et al., 2014, Li et al., 2014, Shen et al., 2013). A post-mortem study also showed no significant decrease in volume with age (Brabec et al., 2010). Other studies reveal age-associated declines in amygdala volume that are less marked than in other brain regions (Good et al., 2001, Grieve et al., 2005, Kalpouzos et al., 2009, Long et al., 2012), while others show notable negative associations between amygdala volume and age (Allen et al., 2005b, Curiati et al., 2009, Fjell et al., 2013b, Mu et al., 1999, Walhovd et al., 2005).
Longitudinal studies examining amygdala volume are scarce, but nevertheless there already are conflicting findings. Amygdala and hippocampal volume showed similarly significant decline when assessed using an automated program (FreeSurfer; Fjell et al., 2009a) that held up when those who converted to Alzheimer’s disease 3–4 years later were excluded (Fjell et al., 2013a). In contrast, in two studies using hand tracing, there was no longitudinal amygdala decline detected (Cherbuin et al., 2011, Frodl et al., 2008).
Differences across studies may have to do with methods used to assess amygdala volume (e.g., FreeSurfer may not be so accurate for the amygdala; Morey et al., 2009) or differences in the people assessed. On balance across the cross-sectional and longitudinal studies, the evidence suggests there is some decline in the amygdala but that it is often less pronounced than that found in other regions such as the hippocampus, a region involved in memory processes adjacent to the amygdala.
One issue to keep in mind is that for the amygdala, bigger is not necessarily better. Greater amygdala volume can be associated with having had stressful experiences (Tottenham et al., 2010) and stressed people show reduced amygdala grey matter density after a stress reduction program (Hölzel et al., 2009). On the other hand, among both younger and older adults, greater amygdala volume is associated with the size and complexity of one’s social network (Bickart et al., 2011).
Furthermore, the intrinsic integrity of a brain region will have little impact without connections to other brain regions. Initial findings regarding the effects of aging on amygdala connectivity are intriguing. Resting state functional MRI reveals that the functional connectivity density of an amygdala-based network increases with age in contrast with age-related functional connectivity density decreases in nodes of other networks (such as the default mode network and dorsal attention network; Tomasi and Volkow, 2012).
Prefrontal-amygdala functional connectivity may reflect PFC regulation of anxiety and other emotional responses. Among older adults, amygdala connectivity with vmPFC (see Fig. 1) during processing emotion stimuli is associated with more positive memories (Sakaki et al., 2013), less negative evaluation of pictures (St. Jacques et al., 2010) and a more normative decline in cortisol over the course of a day (Urry et al., 2006). Thus, for older adults, medial PFC-amygdala functional connectivity during rest seems to be associated with more positive emotions or stress profiles, and thus far, there is no evidence for declines in functional connectivity for the amygdala (instead, the reverse is seen; Tomasi and Volkow, 2012).
The role of structural connectivity in older adults’ emotional well-being is less clear. Among younger adults, trait anxiety scores have been linked with the structural integrity of the uncinate fasciculus, a white matter tract that connects amygdala to ventrolateral PFC. Some studies find that better structural integrity predicts lower anxiety, whereas others report the opposite relationship (for review see Clewett et al., 2014). The uncinate fasciculus shows decreased white matter intensity in normal aging (Burzynska et al., 2010, Davis et al., 2009). If this plays a causal role in anxiety, one should see increased prevalence of anxiety with age that is not accounted for by other risk factors. This is not the case, as in population-based studies, there is no positive association with age and anxiety (Beekman et al., 1998, Schaub and Linden, 2000). Consistent with this, in a study that examined the relationship of anxiety to amygdala-ventral PFC in those aged 19–85 (with connectivity assessed for all of ventral PFC rather than just the uncinate fasciculus), better white matter structural integrity of the amygdala-ventral PFC pathway was not associated with lower anxiety either across the whole group or when age was accounted for (Clewett et al., 2014). One interesting possibility is that amygdala-vmPFC structural connectivity may be used to promote negative emotional processing among younger adults but positive emotional processing among older adults (Ford and Kensinger, 2014).
Functional neuroimaging indicates that, in older adults, the amygdala still activates robustly to novel salient stimuli, such as novel faces (Wright et al., 2007, Wright et al., 2008). As I will review in the later section on the age-related positivity effects, other studies have found age-related shifts in which type of emotional stimuli the amygdala activates most strongly to. In the meantime, the evidence reviewed in this section indicate that the amygdala shows relatively little decline in structure or function in normal aging.
Insula
The insula is a cortical area found within the sulcus separating the temporal lobe from the parietal and frontal lobes that receives interoceptive sensory information from the internal milieu of the body and helps regulate autonomic nervous system activity. It is involved in many aspects of emotion, including pain sensation, self-related and empathic feelings and risk and uncertainty processing.
Findings regarding adult age differences in insula volume vary. Cross-sectional studies using manual tracing find a moderate negative association with age that is not always significant (Allen et al., 2005a, Raz et al., 2010). Allen et al. argued that findings of more marked insula decline from voxel based morphometry studies (Good et al., 2001, Tisserand et al., 2004) may result from data smoothing artifacts at the border of the insula.
There have not been many longitudinal studies of insular volume, but two studies using similar tracing methods found somewhat different findings: in one, there was significant decline between the first and second assessment but not between the second and third assessment (Raz et al., 2010). In the other one, the insula showed substantially more shrinkage longitudinally but was also identified as the brain region with the most individual differences in longitudinal change across those studied (Persson et al., 2014). This high degree of individual difference in change may also help explain differences in findings across studies.
Consistent with the structural declines seen in the insula, older adults are less aware of visceral sensations than younger adults (for review see Mather, 2012). Older adults are also less vulnerable to pain associated with visceral pathology even though they are more vulnerable to neuropathic pain (Gagliese, 2009). Research is needed to test whether there are direct relationships between age-related decline in structure and sensation.
The insula also serves as the hub in a resting state network known as the salience network. The salience network responds to behaviorally relevant stimuli and facilitates dynamic interactions among other large-scale networks (Menon and Uddin, 2010). Resting-state fMRI studies reveal age-related declines in functional connectivity within the salience network (He et al., 2014, Meier et al., 2012, Onoda et al., 2012) and between the frontoinsular cortex and other resting state networks (He et al., 2014).
Thus, in summary, current evidence indicates the insula is a region that shows moderate age-related decline with notable individual differences.
Dopaminergic Influences
To understand emotional processing, it is important to also consider the role of neurotransmitter systems. Two that have critical roles in emotional processing and that have been identified as showing significant age-related changes are dopamine and norepinephrine. These two neurotransmitters have a similar structure and interacting effects on cognition. However, they also have distinct roles.
Dopamine has received much attention for its effects on reward processing, and is also important for learning, attention and movement control. Midbrain dopamine neurons typically activate briefly following unpredicted rewards. This response codes a prediction error, or the difference between obtained and predicted reward value (Schultz, 2013). These signals provide powerful tools to support learning about various states, rules and sequences in the world.
Aging clearly affects the dopaminergic system (for review see Li and Rieckmann, 2014). Dopaminergic neurons and dopamine transporters decrease in density. PET markers of dopamine system function show associations between these declines and executive function among older adults (Bäckman et al., 2010). Genes associated with dopaminergic system function are more strongly associated with individual differences in working memory and executive functions in late than in early life, suggesting that age-related decline in dopamine function makes genetic variation more influential (Colzato et al., 2013, Li et al., 2013, Nagel et al., 2008, Störmer et al., 2012).
Noradrenergic Influences
The locus coeruleus (LC) is a small nucleus in the brainstem that is the primary source of the brain’s noradrenaline. Its neurons fire at different rates depending on whether one is alert or drowsy and respond rapidly to arousing stimuli, such as loud noises and threatening stimuli. Although it is small, its neurons have particularly long axons and so can infuse large regions of the brain with noradrenaline whenever arousal increases.
The literature on age-related structural changes in the LC is mixed. For instance, in one study, postmortem counts of LC neurons decreased 40% across a 60 year period (Vijayashankar and Brody, 1979), whereas in another using different methods, younger and older nondemented adults showed no difference in LC neuron count or size (Mouton et al., 1994). A key factor is that both Alzheimer’s and Parkinson’s diseases target the LC, and in these disorders, LC damage occurs long before symptoms are marked enough to lead to a diagnosis (e.g., Braak et al., 2011).
Individual differences in both LC structure and noradrenaline levels are associated with cognitive function. In a postmortem study, neuronal density in the LC was more strongly correlated with cognitive decline in the few years right before death than neuronal integrity in the ventral segmental area, substantial nigra, or dorsal raphe nucleus (Wilson et al., 2013). In vivo, LC integrity (as assessed by structural imaging optimized to show the LC neuromelanin deposits) is correlated with verbal IQ scores (Clewett et al., in preparation). In addition, higher levels of cerebrospinal fluid noradrenaline are associated with poorer cognitive performance (Wang et al., 2013).
RELATIONS BETWEEN EMOTIONAL PROCESSING IN AGING AND BRAIN FUNCTION
In the previous sections, I outlined how key brain regions and neurotransmitter systems contributing to emotional functioning fare in normal aging. In the next sections, I turn to the question of how specific aspects of emotional behavior might relate to these age-related changes in the brain.
Positivity Effect
In 2003, we reported an age by valence interaction in attention (Mather and Carstensen, 2003) and memory (Charles et al., 2003). Relative to younger adults, older adults focused more on positive and less on negative stimuli, a pattern that became known as the age-related positivity effect (Mather and Carstensen, 2005). Many subsequent (but not all) studies found similar effects and a recent meta-analysis of 100 studies indicated that the age by valence interaction is a reliable effect in memory and in attention (Reed et al., 2014).
Older adults’ positivity effect could help maintain better moods (e.g. Isaacowitz et al., 2009b), and thereby help explain how longitudinal studies show that levels of negative affect tend to decrease whereas positive affect either tends to increase slightly or shows less decrease than negative affect with increasing age, at least until the 70’s (Carstensen et al., 2011, Charles et al., 2001, Gruenewald et al., 2008). But does the positivity effect actually result from emotion regulation processes? Or is it just a serendipitous side effect of age-related decline in brain mechanisms (in particular the amygdala) that support noticing and remembering negative, potentially threatening information (Cacioppo et al., 2011)?
As reviewed earlier, the amygdala does not show marked decline among healthy older adults, either in structure or function. Furthermore, it is relatively more responsive to positive than negative stimuli in older adults compared with younger adults (Erk et al., 2008, Ge et al., 2014, Kehoe et al., 2013, Leclerc and Kensinger, 2011, Mather et al., 2004, Waldinger et al., 2011, but see Moriguchi et al., 2011 for no age-by-valence effects in amygdala). Thus, the amygdala does not stop responding to emotional stimuli in later life, but instead, shifts which valence it is most responsive to.
Other findings regarding the positivity effect also do not fit an amygdala decline account. The amygdala draws attention to emotionally salient stimuli and helps create more long-lasting memory traces of those stimuli. Like younger adults, older adults show an advantage in noticing and detecting potentially threatening or arousing stimuli (Hahn et al., 2006, Leclerc and Kensinger, 2008b, Knight et al., 2007, LaBar et al., 2000, Mather and Knight, 2006), suggesting intact amygdala detection functionality. When stimuli are presented in pairs and looking patterns are assessed, the positivity effect in visual attention does not emerge until after the first look or detection of the stimuli, at a point when controlled processes can exert an influence (Isaacowitz et al., 2009a, Knight et al., 2007), suggesting that a key component of the age-related positivity effect is that older adults are more likely to disengage from negative stimuli. Consistent with this, whereas younger adults are significantly slower to disengage from negative than positive distractors, older adults are slightly better at disengaging from negative than positive distractors (Hahn et al., 2006) or no-longer-relevant stimuli (Ashley and Swick, 2009).
Also arguing against the amygdala-decline account are findings that that the age-by-valence positivity effect interactions in memory and pleasantness ratings are stronger for low than high arousal emotional words (Kensinger, 2008, Streubel and Kunzmann, 2011). Likewise, younger and older adults showed similar amygdala activation in response to high arousal negative stimuli but for low arousal negative stimuli older adults showed decreased amygdala activity compared with younger adults, along with increased activity in the anterior cingulate cortex (Dolcos et al., 2014). Greater anterior cingulate cortex activity was associated with less negative ratings of low arousal negative pictures for older but not for younger adults (see also Ge et al., 2014 for similar links between vmPFC/anterior cingulate activity and rating intensity). Thus, across studies, older adults’ memory, ratings, and amygdala activity resembles that of younger adults more when stimuli arousal is high than low.
The positivity effect modulation by arousal fits with a regulation account of the age difference, as once someone has reacted to a stimulus, emotion regulation tends to be more difficult and less successful for higher arousal stimuli (Sheppes, 2014). Thus, if older adults generally attempt to regulate their responses to emotional stimuli after processing them, the biggest age differences should be seen for those stimuli that offer the highest opportunity for modulation by regulatory strategies: negative or positive stimuli that are not so arousing.
Consistent with a regulatory account, compared with younger adults, older adults tend to show a greater increase in prefrontal activation for emotional than neutral stimuli (for reviews see Mather, 2012, Nashiro et al., 2012a, St. Jacques et al., 2009). In particular, ventromedial PFC and adjacent anterior cingulate cortex seem to play an especially important role in maintaining older adults’ well-being. Greater activity in these regions (in tandem with less activity in the amygdala) during processing negative emotional stimuli has been linked with greater positivity, better emotional stability, and a better diurnal stress profile among older adults (Dolcos et al., 2014, Sakaki et al., 2013, Urry et al., 2006, Williams et al., 2006). Likewise, there are links between well-being and responses in these regions during processing positive stimuli. When doing a cueing task, older adults were more distracted than younger adults by task-irrelevant positive faces (but not by sad or fearful faces) and showed greater activity in anterior cingulate during the distracting positive faces than did younger adults (Brassen et al., 2011). This anterior cingulate activity was associated with greater emotional stability.
Across studies, it seems clear that activity in these medial frontal cortex regions is associated with age differences in positivity, but it is not as clear when older adults will show greater or less activity than younger adults. While studies using the International Affective Pictures System images or emotional faces have found greater medial PFC activation to negative than positive stimuli among older compared with younger adults (Leclerc and Kensinger, 2011, Williams et al., 2006), studies using words or object images have found age-related reversals in vmPFC regions in the opposite direction: older adults show more medial PFC activity during processing positive than negative stimuli and vice versa for older adults (Leclerc and Kensinger, 2008a, Leclerc and Kensinger, 2010, Leclerc and Kensinger, 2011). Medial PFC could be employed both to engage more deeply with positive stimuli (for instance, by relating it to oneself) and to reinterpret or to distract oneself from negative stimuli. Thus, it may vary across stimuli sets (and also depend on orienting instructions) whether the desire to disengage from negative stimuli or to engage with positive stimuli is the stronger driving motivation during the session.
Behavioral studies provide further support for the notion that older adults engage prefrontal resources in order to help increase positivity and/or diminish negativity of attention and memory by showing that higher executive function is associated with higher positivity among older adults but not younger adults (Isaacowitz et al., 2009b, Knight et al., 2007, Mather and Knight, 2005, Petrican et al., 2008, Sasse et al., 2014, Simón et al., 2013, but see Foster et al., 2013).
Why might older adults be more likely than younger adults to deploy cognitive resources to regulate emotions? Socioemotional selectivity theory posits that everyone has some sense of time left in life and that as time is perceived as more limited, people prioritize emotional goals more (Carstensen et al., 2006), which in turn should lead to more focus on regulating emotion when confronted with emotional stimuli (Kryla-Lighthall and Mather, 2009). One prediction from this perspective that has not received support is that one’s perceived time left in life should predict the positivity effect (Demeyer and De Raedt, 2013, Foster et al., 2013). However, individual differences in depression and optimism likely also influence perceived time left in life and should be associated with a lower positive-to-negative ratio in attention and memory. This opposing correlation could obscure the effects of any concurrent lifespan changes. Thus, manipulations of time perspective provide a cleaner way to examine if there is a relationship between time perspective and the positivity effect. Indeed, studies manipulating time perspective have shown that shifting to a more limited time perspective increases positivity in emotion perception (Kellough and Knight, 2012) and memory (Barber et al., 2015).
In summary, the age-related positivity effect cannot be accounted for by age-related amygdala decline. The effect is often associated with age-related differences in how medial PFC responds to positive versus negative stimuli, which is consistent with a regulatory account.
Emotion Regulation
The aging and emotion regulation literature can be confusing, as researchers who focus on everyday outcomes and behaviors portray older adults as being better at regulating emotions, yet in the laboratory when given a structured emotion regulation task, there is no clear evidence of greater emotion regulation skill among older adults (for reviews see Mather, 2012, Mather and Ponzio, in press, Silvers et al., 2013). How can we reconcile these different perspectives? First, insofar as older adults focus more on emotional goals, they should allocate more resources to regulate emotions throughout their everyday lives, which should improve emotional outcomes. In other words, older adults may be chronic emotion regulators whereas younger adults are more sporadic (e.g., Mather and Johnson, 2000). Second, different emotion regulation strategies rely on different brain regions, and older adults may compensate for lateral prefrontal declines by shifts in their “go-to” or preferred emotion regulation strategy. Thus, laboratory-based experiments may fail to capture the regulatory strategies that older adults use in their everyday lives. Indeed, there is an age difference in preferences such that with greater age, people tend to prefer distraction more and reappraisal less (Scheibe et al., in press).
Reappraisal (involving changing one’s interpretation of an emotional stimulus) is a cognitively demanding strategy that, in younger adults, tends to recruit dorsal and lateral PFC regions as well as the posterior parietal lobe but does not typically recruit the vmPFC (Buhle et al., 2013). In a couple of neuroimaging studies, older adults activated left ventrolateral PFC less than younger adults when reappraising to diminish the impact of negative emotional pictures (Opitz et al., 2012, Winecoff et al., 2011). Across two regulation strategies (reappraisal and distraction), older adults showed less dlPFC activity for regulation trials than younger adults (Allard and Kensinger, 2014). Thus, one pattern now seen across a few studies is less lateral PFC activity during instructed emotion regulation for older than younger adults. Also, older adults are less effective than younger adults at disengaging brain regions associated with the default mode network during reappraisal (Martins et al., 2014).
On the one hand, older adults’ preference for using distraction rather than reappraisal is surprising given that selective attention shares some dlPFC mechanisms with working memory and declines in working memory are one hallmark of aging. Also, older adults are less effective at avoiding being distracted by external salient stimuli (Madden et al., 2014). Yet voluntary attention shifting, or top-down attention, shows little influence of age (Greenwood et al., 1993, Madden, 2007). Parietal cortex plays a key role in top-down attention, and fewer age differences tend to be seen in functional activity in parietal cortex than in other regions during cognitive tasks (Spreng et al., 2011). Well-maintained voluntary control over attention selection may make distraction an attractive emotion regulation option for older adults. In particular, older adults do well at using expectations or cues to guide subsequent attention (Madden, 2007), which suggests that preparing to implement distraction when given a cue that something emotional is about to occur would be a particularly effective strategy for older adults. In contrast, without an external cue, proactive control is likely a computationally costly strategy for lateral PFC (Braver et al., 2014) and so may not be effective for older adults.
Recognizing Others’ Emotions
Emotions are conveyed in many ways, but faces are often the most specific and clear signal of emotions. Both face identity (Germine et al., 2011) and emotion recognition abilities decline with age (Ruffman et al., 2008). One question is whether the age-related declines in recognizing emotions are just a function of some of the general declines in face processing. For instance, brain imaging studies reveal that older adults show less neural specialization for faces in the ventral visual cortex (Goh et al., 2010) and declines in the contributions of fusiform face area to identifying and remembering faces (Dennis et al., 2008, Grady et al., 2000). In addition, the white matter tracts passing through the right fusiform gyrus are more reduced in their structural integrity among older adults than white matter more generally, and the integrity of these tracts is associated with the ability to discriminate two similar faces (Thomas et al., 2008).
However, the emotion deficits cannot be explained simply by declines in general face processing abilities, as aging has a different impact on recognizing different emotions (Ruffman et al., 2008). Older adults tend to be worse than younger adults at identifying fear and sadness and sometimes also anger. In contrast, they show little impairment at recognizing happiness, surprise and disgust. Indeed, they sometimes are better than younger adults at recognizing disgust.
What might explain the finding that older adults show no impairment and instead even better disgust recognition than younger adults? The recognition and experience of disgust is more closely linked with the insula than any other brain region. Thus, older adults’ ability to recognize others’ disgust expressions suggests age-related maintenance or even increased insular involvement in face processing. Consistent with this, older adults showed more insular cortex activity than younger adults during successful encoding of fearful faces (Fischer et al., 2010) and during rating emotions (Keightley et al., 2007). However, it is hard to reconcile better insula function in late life with the age-related declines seen in the insula (as described in insula section above).
A more plausible possibility is that age-related shifts in general face processing strategies cause the selective enhancement in disgust recognition. Compared with younger adults, older adults are less likely to look at someone’s eyes and more likely to look at their mouth or nose (Circelli et al., 2013, Firestone et al., 2007, Heisz and Ryan, 2011, Murphy and Isaacowitz, 2010, Sullivan et al., 2007, Wong et al., 2005) and are worse than younger adults at detecting configural changes in the eye region than in the mouth/nose region of the face (Chaby et al., 2011, Slessor et al., 2013). The age difference in top-bottom bias is seen for both neutral and emotional faces but not for scenes (Circelli et al., 2013).
Research with younger adults indicates that fear, sadness and anger are more recognizable from the top half of the face whereas happiness and (especially) disgust are more recognizable from the bottom half of the face (Calder et al., 2000). Surprise showed no strong top-bottom bias (Calder et al., 2000). These differences correspond with the pattern of impairment/lack thereof for older adults’ emotion recognition. Indeed, among older adults, whereas looking more at the top half of a face predicted better recognition of anger, fear and sadness, looking more at the bottom half of a face predicted better recognition of disgust, and happiness and surprise showed no significant relationship (Wong et al., 2005).
If intact perception of facial disgust expressions is due to a general shift in face processing mechanisms and not to stronger influence of brain regions specialized for perceiving disgust, then perception of disgust should not be selectively maintained in aging in other sensory modalities. This appears to be the case, as aging is associated with impairments in perceiving which emotion others express verbally, and disgust is as impaired as the other emotions (e.g., Lambrecht et al., 2012).
These age-related shifts in face processing may stem from changes in the brain networks involved in face processing. A network of brain regions contributes to perceiving and interpreting eye gaze, including the superior temporal sulci, the medial prefrontal cortex and the amygdala. Existing research does not provide sufficient information to indicate whether a particular component of this network involved in processing eye gaze is particularly affected in aging. The one fMRI study to compare younger and older adults while categorizing emotion from pictures of eyes had a small sample and did not see the typical behavioral impairments among the older adults (Castelli et al., 2010).
In summary, aging is associated with declines in recognizing facial expressions of some emotions more than others and a slight improvement in recognizing disgust. These shifts do not appear to be due to selective declines in brain regions more involved in perceiving one emotion than another (if such segregation exists; for arguments against see Lindquist et al., 2012), but instead due to changes in how older adults process faces. Such face processing changes may be due to age-related changes in the structure and function of face processing regions, but clear brain linkages have yet to be established.
Empathy
To successfully navigate the social world, it is important not just to recognize what emotions others are experiencing, but also to be able to predict how the emotion will influence their actions. To make such predictions, we rely on both the capacity to personally share (and thereby simulate) the emotions of others and the ability to understand how the perspective of others may differ from our own. These affective and cognitive aspects of empathy rely on dissociable neural circuits and so may be affected differently by aging.
Current models suggest that the insula is involved in simulating the feelings of others via embodied representations of their emotional states (e.g., Bernhardt and Singer, 2012). On the other hand, the cognitive aspects of empathy (requiring “theory-of-mind”) rely on a neural network including the medial prefrontal cortex, posterior superior temporal sulcus, temporoparietal junction, and temporal pole (Bzdok et al., 2012).
Self-report assessments of empathy show either no age-related differences in empathy (Diehl et al., 1996, Eysenck et al., 1985) or declines (Chen et al., 2014, Phillips et al., 2002, Schieman and Van Gundy, 2000). When emotional and cognitive empathy are distinguished using separate sub scales, cognitive but not affective empathy is lower among older than younger adults (Bailey et al., 2008, Beadle et al., 2012, but see Khanjani et al., 2015). A limitation of these studies is their cross-sectional design. A study that examined both cross-sectional and longitudinal age effects found that older cohorts tended to report less empathy, but that there was no decline in self-reported empathy in individuals over a 12-year period, suggesting that cross-sectional findings of age differences in empathy may be due to cohort rather than age effects (Grühn et al., 2008). Considered together, these self-report findings provide little clear evidence of age-related decline in empathy.
Going beyond self-report measures by using measures of emotion perception, emotion congruency with the speaker and sympathetic body and facial responses, one study found that older adults tended to be more perceptive and empathic for a more self-relevant social loss theme than for a life transition theme, and performed as well as or better than younger adults for this theme (Richter and Kunzmann, 2011). Thus, as in other domains, older adults perform quite well when given self relevant contexts (Hess, 2005).
Theory-of-mind tests tap some of the processes needed for cognitive empathy. As outlined in the previous section, older adults are less likely to attend to eyes than younger adults. They also tend to be impaired at a common theory-of-mind test using pictures of eyes (Bailey et al., 2008, Khanjani et al., 2015, Phillips et al., 2002, Slessor et al., 2007), although their impairment also extends to inferring age and gender based on eyes (Slessor et al., 2007) so does not seem to be emotion specific. Likewise, when given stories testing the ability to make theory-of-mind inferences about the beliefs of others, older adults are impaired, but they tend to be similarly impaired at making inferences about physical or mechanical causation (German and Hehman, 2006, Happé et al., 1998, Slessor et al., 2007, but see Maylor et al., 2002). These studies suggest that age-related impairments in cognitive empathy likely stem from broader impairments in cognitive function (Moran, 2013). Consistent with this, older adults showed less activation in the dorsomedial PFC than younger adults while performing three different mentalizing tasks (Moran et al., 2012), whereas there were no consistent age differences in activity within the regions typically associated with cognitive empathy.
Another fMRI study compared younger, middle-aged and older adults’ responses to brief animations of hands or feet in painful or non painful situations, and in dyads or alone (Chen et al., 2014). Across age groups, there was a significant effect of social context, with greater activity in the medial prefrontal cortex, posterior STS, posterior cingulate cortex and fusiform gyrus in the dyad trials. In contrast, in the right anterior insula, the response to seeing others in pain (compared with no pain) decreased across age groups, with older adults showing no significant effect. However, such changes were not significantly associated with insular gray matter changes (Chen et al., 2014), suggesting that structural volume in itself is not a strong predictor. Older adults also showed less insula activation than younger adults when offered unfair divisions of money in an Ultimatum Game study (Harlé and Sanfey, 2012). These findings suggest that insula is less involved in interpreting social situations among older than younger adults.
In summary, initial evidence suggests that older adults show less involvement of the insula in situations in which one might simulate the feelings of others, and also some evidence that although they show similar cognitive empathy brain networks, decreased involvement of brain regions such as dlPFC that support executive function more generally may contribute to declines in making inferences about the mental states of others. Self-reported empathy scales do not necessarily reflect these changes.
Iowa Gambling Task
As mentioned earlier, research using behavioral tasks to discriminate dlPFC from either ventromedial or orbital PFC in contributing to age differences have yielded mixed results (Baena et al., 2010, Lamar and Resnick, 2004, MacPherson et al., 2002). A key issue that has not been fully appreciated is that the tasks used to tap vmPFC function also rely on other regions in the PFC and elsewhere. In this and the next section, I focus on the two decision tasks that have received the most attention in the aging literature and discuss what can and what cannot be gleaned from the current findings about the neural underpinnings.
The Iowa Gambling Task demonstrated that patients with vmPFC lesions have deficits in decision making that relate to impaired integration of emotional signals (Bechara et al., 1996). When given this gambling task, patients with vmPFC lesions are less likely to learn to avoid decks of cards that typically yield positive outcomes but occasionally lead to a large loss. Yet the vmPFC’s role in making decisions and the underlying mechanisms of the Iowa Gambling Task have been the subject of much debate (e.g., Buelow and Suhr, 2009, Maia and McClelland, 2004).
Despite uncertainty about what processes it taps, the Iowa Gambling Task has been the most highly utilized task to probe for adult age differences in decision making, and across studies, older adults are more impaired at avoiding the risky decks in this task (Mata et al., 2011). But does this impairment reflect vmPFC impairment? Not necessarily. DlPFC lesion patients also show deficits on the Iowa Gambling Task (Fellows and Farah, 2005a). This suggests that impairments in working memory that allow on-going information to be updated and integrated may contribute to declines in performance on the task among some older adults.
Indeed, there are indications that older adults’ impairments on the task relate to impairments in learning from the decks over time. First of all, older adults’ deficits tend to be stronger in later blocks than in initial ones (Denburg et al., 2005, Zamarian et al., 2008), with impairments sometimes seen in the later blocks even when there is no overall main effect of age (Baena et al., 2010, Isella et al., 2008). This pattern suggests a learning deficit. Also, older adults show more recency effects and more rapid forgetting in the task (Wood et al., 2005). Thus, impairments in memory processes may account for the age impairments in this task seen in some studies.
The Iowa Gambling Task has also revealed an emotional difference across age groups even when performance is equivalent. Whereas younger adults weighted previous losses more heavily than previous wins in their card deck choices, older adults weighted wins and losses evenly (Wood et al., 2005). In addition, healthy younger adults typically produce larger anticipatory skin conductance responses before selecting from a “bad” than a “good” deck in the task (Bechara et al., 1996). In contrast, older adults who completed the task successfully showed the opposite pattern, with larger anticipatory skin conductance responses before selecting from a “good” than a “bad” deck (Denburg et al., 2006). These findings indicate that the emotional nature of decision strategies in this task shift with age to focus more on the positive than on the negative outcomes, a shift seen in other contexts as well, such as during decision search (Löckenhoff and Carstensen, 2007, Mather et al., 2005), deciding among risky gambles (Mather et al., 2012), and anticipating wins or losses (Nielsen et al., 2008).
Delay Discounting
Would you prefer $10 now or $15 in a month? Given evidence from vmPFC patients that vmPFC lesions lead to impulsive behavior (Berlin et al., 2004), one might think that vmPFC should help people be more patient and wait for delayed rewards instead of taking less valuable immediate rewards. But although vmPFC patients are less likely than controls to think about far future events, they do not show differences in how much they value present versus future monetary rewards on a delay discounting task (Fellows and Farah, 2005b). Thus vmPFC seems to influence how likely one is to think about one’s own distant future rather than how much one values rewards in the future.
Older adults typically show less delay discounting than younger adults (Green et al., 1994, Eppinger et al., 2012, Reimers et al., 2009), although such differences can be reduced by controlling for income levels (Green et al., 1996). In addition, the animal literature shows consistent decreases in delay discounting with age (e.g., Roesch et al., 2012, Simon et al., 2010), although a critical difference is that delay discounting tasks for animals require the animal to learn about the delays and rewards via repeated experience. With humans, most delay discounting tasks simply describe the amount of money and delay in text format on each trial and do not present outcomes until the end of the session. One interesting finding to note is that, when two options differ only in delay (not in reward amount) older rats are impaired at learning to avoid the longer delay option, but that when the two options differ in reward amount (and not in delay), they are not impaired (Roesch et al., 2012). Thus, learning about differences in time duration may be more impaired in aging than learning about differences in amount (c.f., Zanto et al., 2011).
Among healthy participants between the ages of 63 and 93, those with greater structural thickness in the vmPFC exhibited less delay of gratification (Drobetz et al., 2014). Likewise, in rats trained in the delay discounting task before lesioning, orbitofrontal lesions resulted in less “impulsive” choices of a smaller sooner reward over a larger later reward than did sham lesions (Winstanley et al., 2004). Thus, the orbitofrontal cortex may help integrate information about the delay into the representation of value for an option. Failure to integrate that information makes the delayed option seem more attractive and older adults may be impaired at this integration of time delay information into value estimations.
However, in initial functional neuroimaging studies with humans, there is no indication that vmPFC changes are associated with delay discounting changes in aging. For instance, in two fMRI studies, there were no significant age differences in medial PFC regions associated with temporal discounting (Eppinger et al., 2012, Samanez-Larkin et al., 2011). Furthermore, in a study of 123 older adults, functional connectivity between fronto-insular seed regions and PFC did not differ for those older adults with high versus low delay discounting (Han et al., 2013). Thus, further work is needed to evaluate the role of vmPFC in age differences in delay discounting.
Studies also suggest a role for other neural systems. For instance, the same study with the negative structural correlation with vmPFC found that greater dlPFC cortical surface area predicted greater delay of gratification (Drobetz et al., 2014). Also, younger adults showed significantly greater striatal activation when choosing immediate over delayed choice options, whereas older adults showed no significant difference (Eppinger et al., 2012, Samanez-Larkin et al., 2011), suggesting that age-related reductions in dopaminergic reward sensitivity may be involved. In the next section, we review age-related changes in dopaminergic influences over reward processing.
Reward Processing
Dopaminergic coding of reward prediction error seems to be disrupted in older adults. Older adults show reduced prediction-error-related activity in the vmPFC and ventral striatum (a region encompassing the nucleus accumbens that is involved in reward processing) in tasks requiring learning about which response to make to specific stimuli (Eppinger et al., 2013, Samanez-Larkin et al., 2014). Older adults also show impairments on behavioral reward learning tasks (e.g., Mell et al., 2005), especially those involving more computationally demanding model-based strategies (Worthy et al., 2014). Both the prediction error signal in the ventral striatum and probabilistic reward learning performance are enhanced when older adults receive the dopamine precursor L-DOPA (Chowdhury et al., 2013).
Age-related impairments may be specific to situations in which reward prediction errors support learning, as older adults show as much influence of reward as younger adults in other contexts. Striatal regions still respond robustly to rewarding outcomes (e.g., Samanez-Larkin et al., 2014, Schott et al., 2007, Vink et al., 2015). Recognition and source memory are enhanced by positive compared with negative feedback (Eppinger et al., 2010, Mather and Schoeke, 2011) and by reward anticipation (Spaniol et al., 2014) in older adults as much as in younger adults. Task reaction times are also speeded up by potential rewards as much for older as younger adults (Vink et al., 2015).
Emotion and Behavior Change
Why do we have emotions, in particular negative emotions? One overarching potential function of emotions is to trigger behavior change (Frijda and Parrott, 2011, Oatley and Johnson-Laird, 1987). Yet emotions often enhance learning associations (Mather, 2007). Thus, strong emotion during learning a contingency (e.g., whenever I select this option, I get a reward) could impair later behavior change that requires suppressing the learned association.
Reversal learning research indicates that orbitofrontal brain regions are critical for updating knowledge about choice outcomes and so should support flexible behavior. Yet while orbitofrontal lesions alone impair performance, when there is a concurrent amygdala lesion, orbitofrontal lesions to no longer impede learning contingency reversals (Stalnaker et al., 2007). This suggests that orbitofrontal cortex facilitates flexible updating by opposing amygdala stabilization of the prior contingency. Consistent with the notion that orbitofrontal cortex works against amygdala strengthening emotional associations, orbitofrontal activity is greater during reversal of emotional than neutral outcomes, with negative functional connectivity seen between orbitofrontal cortex and amygdala (Nashiro et al., 2012b). Older adults show similar effects of emotion as younger adults both in an fMRI reversal task (Nashiro et al., 2013b) and when updating simple associations between stimuli (Nashiro et al., 2013a), suggesting that prefrontal-amygdala interactions mediating flexible updating remain intact in later life.
Being able to flexibly change behavior may also depend on the locus coeruleus and the noradrenaline it releases (Aston-Jones and Cohen, 2005). Not much is known yet about how age-related changes in the LC-noradrenaline system might relate to the likelihood of exploring new options versus remaining fixated on current choices, but it is an interesting avenue for future research, especially given findings of less exploratory behavior (Mata et al., 2013) and less information seeking during decision making (Mather, 2006) among older than younger adults.
Arousal and Cognitive Selectivity
As outlined in our recent GANE model (Mather, under review), the LC increases the selectivity of processing through a variety of mechanisms, including shunting blood flow and metabolic resources to highly active regions and away from other regions. At local synapses, norepinephrine interacts with the brain’s primary excitatory neurotransmitter, glutamate, to amplify activity at the most highly active neurons while inhibiting activation elsewhere. These modulatory effects of the LC are especially potent in moments of high emotional arousal, when people tend to focus on whatever is most salient and ignore the rest. Thus, noradrenaline prepares the brain for targeted action by supporting processing in brain regions that are most highly active at that moment. This model accounts for findings that arousal increases the selectivity of attention and memory, favoring salient and high priority items (e.g., Lee et al., 2014, Sakaki et al., 2014). Initial evidence indicates that older adults show similar enhancement of bottom-up salience under arousal (Sutherland and Mather, in press, Lee et al., in preparation), but that arousal does not increase selectivity for older adults, but instead broadly enhances processing of both low and high priority items (Lee et al., in preparation).
CONCLUSIONS
Emotion processes depend on interacting systems of neurotransmitters and specific brain regions. Changes with age in these systems vary in nature and in degree.
The amygdala and the vmPFC, two brain regions that play key roles in emotion processing, show less structural and functional decline than many other brain regions. In addition, they show shifts in processing that support favoring positive over negative stimuli in attention and memory. Although older adults do show different performance than younger adults on tests that have previously been identified with the vmPFC, such as facial emotion expression recognition and Iowa Gambling Task performance, the differences in performance are better explained by other factors than age-related vmPFC decline, such as reduced focus on eyes during face processing and declines in dlPFC working memory and learning processes.
The insula shows both structural and functional decline in aging that initial evidence suggests may be linked to age-related changes in empathic processes. Yet despite the role of the insula in feeling disgust, older adults tend to recognize disgusted facial expressions as well or better than younger adults, potentially due to their greater focus on noses and mouths rather than eyes when looking at faces.
Age-related decline in the dopaminergic system is associated with impaired reward prediction error computation, but when new learning is not involved, reward anticipation and response are well maintained in aging.
The noradrenergic system is vulnerable to pathology that slowly progresses throughout the life span and may trigger compensatory release of noradrenaline. Older adults often show as much of an arousal response to emotional stimuli as younger adults, but this arousal seems to have less of a targeted effect on cognitive processing.
In general, shifts in the relative efficacy of different brain systems may contribute to changes in the strategies older adults use to cope with difficult emotional situations and maintain effective emotional processing. The relative lack of decline in core emotion-related brain regions likely plays a key role in explaining older adults’ well-maintained emotional functioning, in particular the ability to maintain positive affect and minimize negative affect in everyday life.
SUMMARY POINTS.
Amygdala and vmPFC structure and function are relatively well-maintained in healthy aging.
Increased vmPFC (along with decreased amygdala) activity when confronted with negative stimuli is associated with better everyday emotional outcomes among older adults.
Age-related declines in lateral PFC may be related to shifts in preferred emotion regulation strategies.
Selective sparing of recognition of facial disgust along with declines in recognition of fear, sadness and anger is better accounted for by changes in general face processing strategies than by aging effects on specific brain regions.
Although reward learning processes are impaired among older adults, responses to rewarding outcomes are maintained.
Potential age-related decreases in insular contributions to interoception and simulating the emotions of others deserve further examination.
Emotion interferes with updating associations in older adults as well as in younger adults, with similar amygdala-PFC involvement.
Acronyms and Definitions List
- vmPFC
ventromedial prefrontal cortex
- dlPFC
dorsolateral prefrontal cortex
- LC
locus coeruleus
References
- Allard ES, Kensinger EA. Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Frontiers in psychology. 2014:5. doi: 10.3389/fpsyg.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Methods for studying the aging brain: volumetric analyses versus VBM. Neurobiology of Aging. 2005a;26:1275–1278. [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005b;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ashley V, Swick D. Consequences of emotional stimuli: age differences on pure and mixed blocks of the emotional Stroop. Behavioral and Brain Functions. 2009:5. doi: 10.1186/1744-9081-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Baena E, Allen PA, Kaut KP, Hall RJ. On age differences in prefrontal function: the importance of emotional/cognitive integration. Neuropsychologia. 2010;48:319–333. doi: 10.1016/j.neuropsychologia.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Bailey PE, Henry JD, Von Hippel W. Empathy and social functioning in late adulthood. Aging and Mental Health. 2008;12:499–503. doi: 10.1080/13607860802224243. [DOI] [PubMed] [Google Scholar]
- Barber SJ, Opitz PC, Martins B, Sakaki M, Mather M. Thinking about a limited future reduces the negativity of younger and older adults’ recall. Support for Socioemotional Selectivity Theory. 2015 doi: 10.3758/s13421-016-0612-0. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle JN, Paradiso S, Kovach C, Polgreen L, Denburg NL, Tranel D. Effects of age-related differences in empathy on social economic decision-making. International Psychogeriatrics. 2012;24:822–833. doi: 10.1017/S1041610211002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Bremmer MA, Deeg DJ, Van Balkom AJ, Smit JH, De Beurs E, Van Dyck R, Van Tilburg W. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. International journal of geriatric psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Berlin H, Rolls E, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual review of neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature neuroscience. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL. Do we get better at picking our battles? Age group differences in descriptions of behavioral reactions to interpersonal tensions. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2005;60B:P121–P128. doi: 10.1093/geronb/60.3.p121. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Brabec J, Rulseh A, Hoyt B, Vizek M, Horinek D, Hort J, Petrovicky P. Volumetry of the human amygdala - An anatomical study. Psychiatry Research-Neuroimaging. 2010;182:67–72. doi: 10.1016/j.pscychresns.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Brassen S, Gamer M, Buchel C. Anterior cingulate activation is related to a positivity bias and emotional stability in successful aging. Biological Psychiatry. 2011;70:131–137. doi: 10.1016/j.biopsych.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, Adcock RA, Barch DM, Botvinick MM, Carver CS. Mechanisms of motivation–cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA. Construct validity of the Iowa gambling task. Neuropsychology review. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013:bht154. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson CG, Bechara A, Tranel D, Hawkley LC. Could an aging brain contribute to subjective well-being?: The value added by a social neuroscience perspective. In: TODOROV A, FISKE ST, PRENTICE D, editors. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind. New York: Oxford University Press; 2011. [Google Scholar]
- Calder AJ, Young AW, Keane J, Dean M. Configural information in facial expression perception. Journal of Experimental Psychology: Human perception and performance. 2000;26:527. doi: 10.1037//0096-1523.26.2.527. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation and emotion. In: BIRREN JE, SCHAIE KW, editors. Handbook of the Psychology of Aging. 6. San Diego: Academic Press; 2006. [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Brooks KP, Nesselroade JR. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychology and aging. 2011;26:21. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli I, Baglio F, Blasi V, Alberoni M, Falini A, Liverta-Sempio O, Nemni R, Marchetti A. Effects of aging on mindreading ability through the eyes: An fMRI study. Neuropsychologia. 2010;48:2586–2594. doi: 10.1016/j.neuropsychologia.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Chaby L, Narme P, George N. Older adults’ configural processing of faces: role of second-order information. Psychology and aging. 2011;26:71. doi: 10.1037/a0020873. [DOI] [PubMed] [Google Scholar]
- Charles ST, Carstensen LL. Unpleasant situations elicit different emotional responses in younger and older adults. Psychology and Aging. 2008;23:495–504. doi: 10.1037/a0013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. [PubMed] [Google Scholar]
- Chen Y-C, Chen C-C, Decety J, Cheng Y. Aging is associated with changes in the neural circuits underlying empathy. Neurobiology of aging. 2014;35:827–836. doi: 10.1016/j.neurobiolaging.2013.10.080. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Sachdev PS, Anstey KJ. Mixed handedness is associated with greater age-related decline in volumes of the hippocampus and amygdala: the PATH through life study. Brain and Behavior. 2011;1:125–134. doi: 10.1002/brb3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopik WJ, Edelstein RS, Fraley RC. From the cradle to the grave: Age differences in attachment from early adulthood to old age. Journal of personality. 2013;81:171–183. doi: 10.1111/j.1467-6494.2012.00793.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Düzel E, Dolan RJ. Dopamine restores reward prediction errors in old age. Nature neuroscience. 2013;16:648–653. doi: 10.1038/nn.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circelli KS, Clark US, Cronin-Golomb A. Visual scanning patterns and executive function in relation to facial emotion recognition in aging. Aging, Neuropsychology, and Cognition. 2013;20:148–173. doi: 10.1080/13825585.2012.675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Bachman S, Mather M. Age-related reduced prefrontal-amygdala structural connectivity is associated with lower trait anxiety. Neuropsychology. 2014;28:631. doi: 10.1037/neu0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Lee TH, Greening SG, Ponzio A, Margalit E, Mather M. Neuromelanin Marks the Spot: Identifying a Locus Coeruleus Biomarker of Cognitive Reserve in Healthy Aging. doi: 10.1016/j.neurobiolaging.2015.09.019. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Van Den Wildenberg WP, Hommel B. The genetic impact (C957T-DRD2) on inhibitory control is magnified by aging. Neuropsychologia. 2013;51:1377–1381. doi: 10.1016/j.neuropsychologia.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Curiati P, Tamashiro J, Squarzoni P, Duran F, Santos L, Wajngarten M, Leite C, Vallada H, Menezes P, Scazufca M. Brain structural variability due to aging and gender in cognitively healthy Elders: results from the Sao Paulo Ageing and Health study. American journal of neuroradiology. 2009;30:1850–1856. doi: 10.3174/ajnr.A1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer I, De Raedt R. Attentional bias for emotional information in older adults: The role of emotion and future time perspective. PloS one. 2013;8:e65429. doi: 10.1371/journal.pone.0065429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61:19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology-Learning Memory and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Coyle N, Labouvie-Vief G. Age and sex differences in strategies of coping and defense across the life span. Psychology and Aging. 1996;11:127–139. doi: 10.1037//0882-7974.11.1.127. [DOI] [PubMed] [Google Scholar]
- Dolcos S, Katsumi Y, Dixon RA. The role of arousal in the spontaneous regulation of emotions in healthy aging: a fMRI investigation. Frontiers in psychology. 2014:5. doi: 10.3389/fpsyg.2014.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetz R, Hänggi J, Maercker A, Kaufmann K, Jäncke L, Forstmeier S. Structural brain correlates of delay of gratification in the elderly. Behavioral neuroscience. 2014;128:134. doi: 10.1037/a0036208. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Herbert M, Kray J. We remember the good things: Age differences in learning and memory. Neurobiology of Learning and Memory. 2010;93:515–521. doi: 10.1016/j.nlm.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PloS one. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, Cohen JD. Reduced striatal responses to reward prediction errors in older compared with younger adults. The Journal of Neuroscience. 2013;33:9905–9912. doi: 10.1523/JNEUROSCI.2942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Walter H, Abler B. Age-related physiological responses to emotion anticipation and exposure. NeuroReport. 2008;19:447–452. doi: 10.1097/WNR.0b013e3282f5d92f. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and individual differences. 1985;6:613–619. [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005a;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005b;43:1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Firestone A, Turk-Browne NB, Ryan JD. Age-related deficits in face recognition are related to underlying changes in scanning behavior. Aging Neuropsychology and Cognition. 2007;14:594–607. doi: 10.1080/13825580600899717. [DOI] [PubMed] [Google Scholar]
- Fischer H, Nyberg L, Backman L. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex. 2010;46:490–497. doi: 10.1016/j.cortex.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Mcevoy L, Holland D, Dale AM, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer’s disease. The Journal of Neuroscience. 2013a;33:8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, Mcevoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. The Journal of Neuroscience. 2009a;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009b;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiology of aging. 2013b;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Kensinger EA. The relation between structural and functional connectivity depends on age and on task goals. Frontiers in human neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SM, Davis HP, Kisley MA. Brain responses to emotional images related to cognitive ability in older adults. Psychology and aging. 2013;28:179. doi: 10.1037/a0030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH, Parrott WG. Basic emotions or ur-emotions? Emotion Review. 2011;3:406–415. [Google Scholar]
- Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Möller H-J, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. Journal of psychiatry & neuroscience: JPN. 2008;33:423. [PMC free article] [PubMed] [Google Scholar]
- Gagliese L. Pain and aging: the emergence of a new subfield of pain research. The Journal of Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Ge R, Fu Y, Wang D, Yao L, Long Z. Age-related alterations of brain network underlying the retrieval of emotional autobiographical memories: an fMRI study using independent component analysis. Frontiers in human neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TP, Hehman JA. Representational and executive selection resources in ‘theory of mind’: Evidence from compromised belief-desire reasoning in old age. Cognition. 2006;101:129–152. doi: 10.1016/j.cognition.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition. 2011;118:201–210. doi: 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Mcintosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and nondegraded face processing. Cognitive Neuropsychology. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown H, Salazar A. Frontal lobe injuries, violence, and aggression a report of the vietnam head injury study. Neurology. 1996;46:1231–1231. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: The role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Haxby JV. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31:471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Mroczek DK, Ryff CD, Singer BH. Diverse pathways to positive and negative affect in adulthood and later life: an integrative approach using recursive partitioning. Developmental psychology. 2008;44:330. doi: 10.1037/0012-1649.44.2.330. [DOI] [PubMed] [Google Scholar]
- Grühn D, Rebucal K, Diehl M, Lumley M, Labouvie-Vief G. Empathy across the adult lifespan: Longitudinal and experience-sampling findings. Emotion. 2008;8:753. doi: 10.1037/a0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologica. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Han SD, Boyle PA, Yu L, Fleischman DA, Arfanakis K, Bennett DA. Ventromedial PFC, parahippocampal, and cerebellar connectivity are associated with temporal discounting in old age. Experimental gerontology. 2013;48:1489–1498. doi: 10.1016/j.exger.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG, Winner E, Brownell H. The getting of wisdom: theory of mind in old age. Developmental psychology. 1998;34:358. doi: 10.1037//0012-1649.34.2.358. [DOI] [PubMed] [Google Scholar]
- Harlé KM, Sanfey AG. Social economic decision-making across the lifespan: an fMRI investigation. Neuropsychologia. 2012;50:1416–1424. doi: 10.1016/j.neuropsychologia.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Human brain mapping. 2014;35:3446–3464. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisz JJ, Ryan JD. The effects of prior exposure on face processing in younger and older adults. Frontiers in Aging Neuroscience. 2011:3. doi: 10.3389/fnagi.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM. Memory and aging in context. Psychological bulletin. 2005;131:383. doi: 10.1037/0033-2909.131.3.383. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Social cognitive and affective neuroscience. 2009:nsp034. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Allard ES, Murphy NA, Schlangel M. The time course of age-related preferences toward positive and negative stimuli. Journals of Gerontology Series B- Psychological Sciences and Social Sciences. 2009a;64B:188–192. doi: 10.1093/geronb/gbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging. 2009b;24:989–994. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Mapelli C, Morielli N, Pelati O, Franceschi M, Appollonio IM. Age-related quantitative and qualitative changes in decision making ability. Behavioural Neurology. 2008;19:59–63. doi: 10.1155/2008/893727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sachdev P, Lipnicki DM, Zhang H, Liu T, Zhu W, Suo C, Zhuang L, Crawford J, Reppermund S. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. NeuroImage. 2014;86:203–211. doi: 10.1016/j.neuroimage.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C, Barré L, Constans J-M, Viader F, Eustache F. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kehoe EG, Toomey JM, Balsters JH, Bokde AL. Healthy aging is associated with increased neural processing of positive valence but attenuated processing of emotional arousal: an fMRI study. Neurobiology of aging. 2013;34:809–821. doi: 10.1016/j.neurobiolaging.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL. Age-related differences in brain activity underlying identification of emotional expressions in faces. Social Cognitive and Affective Neuroscience. 2007;2:292–302. doi: 10.1093/scan/nsm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellough JL, Knight BG. Positivity effects in older adults’ perception of facial emotion: The role of future time perspective. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012;67B:150–158. doi: 10.1093/geronb/gbr079. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Age differences in memory for arousing and nonarousing emotional words. Journal of Gerontology: Psychological Sciences. 2008;63:P13–P18. doi: 10.1093/geronb/63.1.p13. [DOI] [PubMed] [Google Scholar]
- Khanjani Z, Mosanezhad Jeddi E, Hekmati I, Khalilzade S, Etemadi Nia M, Andalib M, Ashrafian P. Comparison of Cognitive Empathy, Emotional Empathy, and Social Functioning in Different Age Groups. Australian Psychologist. 2015;50:80–85. [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kryla-Lighthall N, Mather M. The role of cognitive control in older adults’ emotional well-being. In: BERNGTSON V, GANS D, PUTNEY N, SILVERSTEIN M, editors. Handbook of Theories of Aging. 2. New York: Springer Publishing; 2009. [Google Scholar]
- Labar KS, Mesulam MM, Gitelman DR, Weintraub S. Emotional curiousity: Modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2000;38:1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiology of Aging. 2004;25:553–558. doi: 10.1016/j.neurobiolaging.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Lambrecht L, Kreifelts B, Wildgruber D. Age-related decrease in recognition of emotional facial and prosodic expressions. Emotion. 2012;12:529–539. doi: 10.1037/a0026827. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive Affective & Behavioral Neuroscience. 2008a;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008b;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience. 2010;5:560–576. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural processing of emotional pictures and words: A comparison of young and older adults. Developmental Neuropsychology. 2011;36:519–538. doi: 10.1080/87565641.2010.549864. [DOI] [PubMed] [Google Scholar]
- Lee TH, Greening SG, Ponzio A, Clewett D, Mather M. Age-related changes on the effect of emotional arousal on biased competition of visual processing in preparation.
- Lee TH, Sakaki M, Cheng R, Velasco R, Mather M. Emotional arousal amplifies the effects of biased competition in the brain. Social cognitive and affective neuroscience. 2014:nsu015. doi: 10.1093/scan/nsu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Papenberg G, Nagel IE, Preuschhof C, Schröder J, Nietfeld W, Bertram L, Heekeren HR, Lindenberger U, Bäckman L. Aging magnifies the effects of dopamine transporter and D2 receptor genes on backward serial memory. Neurobiology of aging. 2013;34:358.e1–358.e10. doi: 10.1016/j.neurobiolaging.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Li S-C, Rieckmann A. Neuromodulation and aging: implications of aging neuronal gain control on cognition. Current opinion in neurobiology. 2014;29:148–158. doi: 10.1016/j.conb.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Li W, Tol MJ, Li M, Miao W, Jiao Y, Heinze HJ, Bogerts B, He H, Walter M. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Human brain mapping. 2014;35:238–247. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: Motivational manipulations can reduce age differences. Psychology and Aging. 2007;22:134–146. doi: 10.1037/0882-7974.22.1.134. [DOI] [PubMed] [Google Scholar]
- Long X, Liao W, Jiang C, Liang D, Qiu B, Zhang L. Healthy aging: an automatic analysis of global and regional morphological alterations of human brain. Academic radiology. 2012;19:785–793. doi: 10.1016/j.acra.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Schmiedek F, Kennedy KM, Rodrigue KM, Lindenberger U, Raz N. Does variability in cognitive performance correlate with frontal brain volume? NeuroImage. 2013;64:209–215. doi: 10.1016/j.neuroimage.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Madden DJ. Aging and visual attention. Current Directions in Psychological Science. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Parks EL, Davis SW, Diaz MT, Potter GG, Chou Y-H, Chen N-K, Cabeza R. Age mediation of frontoparietal activation during visual feature search. NeuroImage. 2014;102:262–274. doi: 10.1016/j.neuroimage.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Mcclelland JL. A reexamination of the evidence for the somatic marker hypothesis: What participants really know in the Iowa gambling task. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins B, Ponzio A, Velasco R, Kaplan J, Mather M. Dedifferentiation of emotion regulation strategies in the aging brain. Social cognitive and affective neuroscience. 2014:nsu129. doi: 10.1093/scan/nsu129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R, Josef AK, Samanez-Larkin GR, Hertwig R. Age differences in risky choice: a meta-analysis. Annals of the New York Academy of Sciences. 2011;1235:18–29. doi: 10.1111/j.1749-6632.2011.06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R, Wilke A, Czienskowski U. Foraging across the life span: is there a reduction in exploration with aging? Frontiers in neuroscience. 2013:7. doi: 10.3389/fnins.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. A review of decision-making processes: Weighing the risks and benefits of aging. In: CARSTENSEN LL, HARTEL CR, editors. When I’m 64. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield SL, Wais P, Ochsner KN, Gabrieli JDE, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Quieting most activity while igniting local hot spots: How norepinephrine amplifies the effects of priority in perception and memory under review. [Google Scholar]
- Mather M, Johnson MK. Choice-supportive source monitoring: Do our decisions seem better to us as we age? Psychology and Aging. 2000;15:596–606. doi: 10.1037//0882-7974.15.4.596. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M, Mccaffrey M. The allure of the alignable: Younger and older adults’ false memories of choice features. Journal of Experimental Psychology: General. 2005;134:38–51. doi: 10.1037/0096-3445.134.1.38. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61:P54–P57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Mather M, Mazar N, Gorlick M, Lighthall NR, Ariely D. Risk preferences and aging: The “Certainty Effect” in older adults’ decision making. Psychology and Aging. 2012;27:801–816. doi: 10.1037/a0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Ponzio A. Emotion and aging. In: BARRETT LF, LEWIS M, HAVILAND-JONES J, editors. Handbook of Emotions. in press. [Google Scholar]
- Mather M, Schoeke A. Positive outcomes enhance incidental learning for both younger and older adults. Frontiers in Neuroscience. 2011;5:129. doi: 10.3389/fnins.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylor EA, Moulson JM, Muncer AM, Taylor LA. Does performance on theory of mind tasks decline in old age? British Journal of Psychology. 2002;93:465–485. doi: 10.1348/000712602761381358. [DOI] [PubMed] [Google Scholar]
- Meier TB, Desphande AS, Vergun S, Nair VA, Song J, Biswal BB, Meyerand ME, Birn RM, Prabhakaran V. Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage. 2012;60:601–613. doi: 10.1016/j.neuroimage.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM. Effect of aging on stimulus-reward association learning. Neuropsychologia. 2005;43:554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM. Lifespan development: The effects of typical aging on theory of mind. Behavioural brain research. 2013;237:32–40. doi: 10.1016/j.bbr.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. The Journal of Neuroscience. 2012;32:5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, Labar KS, Styner M, Mccarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, Barrett LF. Differential Hemodynamic Response in Affective Circuitry with Aging: An fMRI Study of Novelty, Valence, and Arousal. Journal of Cognitive Neuroscience. 2011;23:1027–1041. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Pakkenberg B, Gundersen HJG, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. Journal of chemical neuroanatomy. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR American Journal of Neuroradiology. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Age effects and gaze patterns in recognising emotional expressions: An in-depth look at gaze measures and covariates. Cognition & Emotion. 2010;24:436–452. [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. The Journal of neuroscience. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S-C, Von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in human neuroscience. 2008:2. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Huffman D, Mather M. Both younger and older adults have difficulty updating emotional memories. Journal of Gerontology: Psychological Sciences. 2013a;68:224–227. doi: 10.1093/geronb/gbs039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? Gerontology. 2012a:156–163. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Nga L, Mather M. Differential brain activity during emotional vs. non-emotional reversal learning. Journal of Cognitive Neuroscience. 2012b;24:1794–1805. doi: 10.1162/jocn_a_00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Nga L, Mather M. Age-related similarities and differences in brain activity underlying reversal learning. Frontiers in Integrative Neuroscience. 2013b doi: 10.3389/fnint.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, Knutson B, Carstensen LL. Affect dynamics, affective forecasting, and aging. Emotion. 2008;8:318–330. doi: 10.1037/1528-3542.8.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’leary KD, Woodin EM. Partner aggression and problem drinking across the lifespan: How much do they decline? Clinical Psychology Review. 2005;25:877–894. doi: 10.1016/j.cpr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Oatley K, Johnson-Laird PN. Towards a cognitive theory of emotions. Cognition and Emotion. 1987;1:29–50. [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. Journal of cognitive neuroscience. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging. 2012;33:645–655. doi: 10.1016/j.neurobiolaging.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N. Regional brain shrinkage over two years: Individual differences and effects of pro-inflammatory genetic polymorphisms. NeuroImage. 2014;103:334–348. doi: 10.1016/j.neuroimage.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychology and Aging. 2008;23:585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Phillips L, Henry J, Hosie J, Milne A. Age, anger regulation and well-being. Aging and Mental Health. 2006;10:250–256. doi: 10.1080/13607860500310385. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Della Sala S. Aging, intelligence, and anatomical segregation in the frontal lobes. Learning and Individual Differences. 1998;10:217–243. [Google Scholar]
- Phillips LH, Maclean RDJ, Allen R. Age and the understanding of emotions: Neuropsychological and sociocognitive perspectives. Journals Of Gerontology Series B-Psychological Sciences And Social Sciences. 2002;57:P526–P530. doi: 10.1093/geronb/57.6.p526. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikels JA. Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychology and Aging. 2014;29:1. doi: 10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personality and Individual Differences. 2009;47:973–978. [Google Scholar]
- Richter D, Kunzmann U. Age differences in three facets of empathy: performance-based evidence. Psychology and aging. 2011;26:60. doi: 10.1037/a0021138. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G. Willingness to wait and altered encoding of time-discounted reward in the orbitofrontal cortex with normal aging. The Journal of Neuroscience. 2012;32:5525–5533. doi: 10.1523/JNEUROSCI.0586-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, Mather M. Emotion strengthens high priority memory traces but weakens low priority memory traces. Psychological Science. 2014:25. doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. Journal of cognitive neuroscience. 2013;25:1206–1224. doi: 10.1162/jocn_a_00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, Mcclure SM. Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in neuroscience. 2011:5. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Worthy DA, Mata R, Mcclure SM, Knutson B. Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:672–682. doi: 10.3758/s13415-014-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse LK, Gamer M, Büchel C, Brassen S. Selective Control of Attention Supports the Positivity Effect in Aging. PloS one. 2014;9:e104180. doi: 10.1371/journal.pone.0104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub R, Linden M. Anxiety and anxiety disorders in the old and very old—results from the Berlin Aging Study (BASE) Comprehensive psychiatry. 2000;41:48–54. doi: 10.1016/s0010-440x(00)80008-5. [DOI] [PubMed] [Google Scholar]
- Scheibe S, Sheppes G, Staudinger UM. Distract or reappraise? Age-related differences in emotion-regulation choice. Emotion. doi: 10.1037/a0039246. in press. [DOI] [PubMed] [Google Scholar]
- Schieman S, Van Gundy K. The personal and social links between age and self-reported empathy. Social Psychology Quarterly. 2000:152–174. [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duezel E. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Current opinion in neurobiology. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kassir MA, Wu J, Zhang Q, Zhou S, Xuan SY, Li Q, Ye Y, Hu J. MR volumetric study of piriform-cortical amygdala and orbitofrontal cortices: the aging effect. PloS one. 2013;8:e74526. doi: 10.1371/journal.pone.0074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G. Emotion regulation choice: theory and findings. Handbook of Emotion Regulation 2014 [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN, Silvers J. The neuroscience of emotion regulation: basic mechanisms and their role in development, aging, and psychopathology. The Handbook of Cognitive Neuroscience. 2013;1:52–78. [Google Scholar]
- Simon NW, Lasarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiology of aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón T, Suengas AG, Ruiz-Gallego-Largo T, Bandrés J. Positive bias is a defining characteristic of aging to the same extent as declining performance. International Journal of Psychology. 2013;48:704–714. doi: 10.1080/00207594.2012.718781. [DOI] [PubMed] [Google Scholar]
- Slessor G, Phillips LH, Bull R. Exploring the specificity of age-related differences in theory of mind tasks. Psychology and Aging. 2007;22:639. doi: 10.1037/0882-7974.22.3.639. [DOI] [PubMed] [Google Scholar]
- Slessor G, Riby DM, Finnerty AN. Age-related differences in processing face configuration: The importance of the eye region. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68:228–231. doi: 10.1093/geronb/gbs059. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Schain C, Bowen HJ. Reward-enhanced memory in younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2014;69:730–740. doi: 10.1093/geronb/gbt044. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience & Biobehavioral Reviews. 2011;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: Fronto-amygdalar differences during emotional perception and episodic memory. Journal of the International Neuropsychological Society. 2009;15:819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Störmer VS, Passow S, Biesenack J, Li S-C. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: Insights from molecular genetic research and implications for adult cognitive development. Developmental psychology. 2012;48:875. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- Streubel B, Kunzmann U. Age differences in emotional reactions: Arousal and age-relevance count. Psychology and Aging. 2011;26:966–978. doi: 10.1037/a0023424. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Ruffman T, Hutton SB. Age differences in emotion recognition skills and the visual scanning of emotion faces. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2007;62:P53–P60. doi: 10.1093/geronb/62.1.p53. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, Mather M. Negative arousal increases stimulus priority in older adults. Experimental Aging Research. doi: 10.1080/0361073X.2015.1021644. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. Journal of Cognitive Neuroscience. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Functional connectivity density and the aging brain. Molecular psychiatry. 2012;17:471–471. [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, Mccarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. Quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. Journal of Neuropathology and Experimental Neurology. 1979;38:490–497. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Vink M, Kleerekooper I, Van Den Wildenberg WP, Kahn RS. Impact of aging on frontostriatal reward processing. Human brain mapping. 2015 doi: 10.1002/hbm.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldinger RJ, Kensinger EA, Schulz MS. Neural activity, neural connectivity, and the processing of emotionally valenced information in older adults: links with life satisfaction. Cognitive Affective & Behavioral Neuroscience. 2011;11:426–436. doi: 10.3758/s13415-011-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wang LY, Murphy RR, Hanscom B, Li G, Millard SP, Petrie EC, Galasko DR, Sikkema C, Raskind MA, Wilkinson CW. Cerebrospinal fluid norepinephrine and cognition in subjects across the adult age span. Neurobiology of aging. 2013 doi: 10.1016/j.neurobiolaging.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B, Cronin-Golomb A, Neargarder S. Patterns of visual scanning as predictors of emotion identification in normal aging. Neuropsychology. 2005;19:739–749. doi: 10.1037/0894-4105.19.6.739. [DOI] [PubMed] [Google Scholar]
- Wood S, Busemeyer J, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: Evidence from the Iowa gambling task. Psychology and Aging. 2005;20:220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]
- Worthy D, Cooper J, Byrne K, Gorlick M, Maddox WT. State-based versus reward-based motivation in younger and older adults. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:1208–1220. doi: 10.3758/s13415-014-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biological Psychiatry. 2007;62:1388–1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. NeuroImage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarian L, Sinz H, Bonatti E, Gamboz N, Delazer M. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology. 2008;22:645–657. doi: 10.1037/0894-4105.22.5.645. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Pan P, Liu H, Bollinger J, Nobre AC, Gazzaley A. Age-related changes in orienting attention in time. The Journal of Neuroscience. 2011;31:12461–12470. doi: 10.1523/JNEUROSCI.1149-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]