Abstract
Focal brain metabolic effects detected by proton magnetic resonance spectroscopy (MRS) in obsessive-compulsive disorder (OCD) represent prospective indices of clinical status and guides to treatment design. Sampling bilateral pregenual anterior cingulate cortex (pACC), anterior middle cingulate cortex (aMCC), and thalamus in 40 adult patients and 16 healthy controls, we examined relationships of the neurometabolites glutamate+glutamine (Glx), creatine+phosphocreatine (Cr), and choline-compounds (Cho) with OCD diagnosis and multiple symptom types. The latter included OC core symptoms (Yale-Brown Obsessive-Compulsive Scale--YBOCS), depressive symptoms (Montgomery-Åsberg Depression Rating Scale--MADRS), and general functioning (Global Assessment Scale--GAS). pACC Glx was 9.7% higher in patients than controls. Within patients, Cr and Cho correlated negatively with YBOCS and MADRS, while Cr correlated positively with the GAS. In aMCC, Cr and Cho correlated negatively with MADRS, while Cr in thalamus correlated positively with GAS. These findings present moderate support for glutamatergic and cingulocentric perspectives on OCD. Based on our prior metabolic model of OCD, we offer one possible interpretation of these group and correlational effects as consequences of a corticothalamic state of elevated glutamatergic receptor activity alongside below-normal glutamatergic transporter activity.
1. Introduction
Regional neurometabolic investigations of obsessive-compulsive disorder (OCD) using proton magnetic resonance spectroscopy (MRS) are clinically relevant to the development of objective diagnostic, symptomatic and prognostic indices, as well as to molecular and anatomic targeting in the design of behavioral, pharmacological, neurostimulatory, and neurosurgical interventions (Brennan et al., 2013).
Intriguing theories of OCD have emerged from such investigations. The best-known theory is the Glutamatergic Hypothesis of OCD (Rosenberg and Keshavan, 1998). It is supported by evidence from MRS, clinical trials, and genetics (reviewed by MacMaster et al., 2008; Pittenger et al. 2006, 2011), including some of our work (Feusner et al., 2009; O’Neill et al., 2013a). MRS evidence includes differences between OCD patients and healthy controls in levels of glutamate (Glu) or glutamate+glutamine (Glx), correlations of these levels with OCD symptoms, and changes in the levels after therapy. The Cingulocentric Theory of OCD (Middleton, 2009) is not as well known as the Glutamatergic Hypothesis. The Cingulocentric Theory updates classical cortico-striato-pallido-thalamo-cortical models of OCD. It is supported by OCD-related neuroimaging findings (reviewed in Saxena et al., 2009a), including our work (O’Neill et al., 2012, 2013a; Saxena et al., 2009b), in the cingulate gyrus. Such findings have been seen in the pregenual anterior cingulate cortex (pACC) and anterior middle cingulate cortex (aMCC) subregions of the cingulate gyrus. The thalamus forms neural circuits with pACC and aMCC and is also a frequent site of imaging findings in OCD (e.g., Mirza et al., 2006; Rosenberg et al., 2001; Saxena et al., 2009b; Smith et al., 2003). Finally, a third perspective on OCD is that it shares certain anatomic and physiological bases with attention deficit-hyperactivity disorder (ADHD), a condition that is, in a sense, its symptomatic opposite (Abramovitch et al., 2012; Carlsson, 2000). Pursuing this notion, we recently extended the Cingulocentric Theory to apply to ADHD (O’Neill et al., 2013b). The present investigation uses MRS to examine these glutamatergic, cingulocentric, and ADHD perspectives on OCD.
We measured neurometabolites in pACC, aMCC, and thalamus to determine whether they were affected by OCD diagnosis or OCD symptoms. Positive findings in these regions would represent possible consequences of dysfunction in cingulocentric brain circuits. Motivated by the Glutamatergic Hypothesis, Glu and Glx were our primary metabolite targets. Prior measurements of these in pACC or aMCC in OCD have yielded varying results, including below-normal (Rosenberg et al., 2004), normal (Simpson, et al. 2012), or above-normal (Zurowski et al., 2007) levels. To help resolve these disparities, our study methods took several steps to reduce untoward variation in metabolites. We measured absolute metabolite levels rather than ratios to Cr. We chose intermediate-sized acquisition voxels: not so large as to incur extensive partial-voluming and not so small as to induce unduly low signal-to-noise ratios. We acquired MRS bilaterally in the brain to detect any hemispheric asymmetries in metabolites. Finally, we restricted interpatient variability in medication, co-morbidities, and OCD subtype. (To restrict subtype, we recruited only patients with childhood-onset OCD and we excluded individuals with hoarding as their primary symptom.) Alongside Glu and Glx, MRS studies of ADHD have demonstrated notable effects involving Cr and Cho (O’Neill et al., 2013b; Perlov et al., 2009). To test for possible neurometabolic similarities between OCD and ADHD we therefore also measured Cr and Cho in pACC, aMCC and thalamus. Effects on Cr (Yücel et al., 2008; Starck et al., 2008) and Cho (Yücel et al., 2007; Starck et al., 2008; Kitamura et al., 2006; Mohammed et al., 2007; Yalçın et al., 2011; Lázaro et al., 2012; Whiteside et al., 2012; Weber et al., 2014) have been observed in OCD, including elevated Cho (Rosenberg et al., 2001; Smith et al., 2003) and Cr (Mirza et al., 2006) in the thalamus. But we were particularly interested in pACC Cr and Cho, since our recent pilot study (O’Neill and Feusner, 2015) showed that pretreatment levels of these metabolites predict long-term response of OCD to cognitive-behavioral therapy, a standard treatment. In summary, the present investigation compared Glu and Glx, Cr, and Cho in pACC, aMCC, and thalamus in OCD patients and healthy controls; within patients we also examined relationships of these metabolites with symptoms of four kinds: core obsessive-compulsive symptoms, depressive symptoms, anxious symptoms, and global functioning.
2. Methods
2.1. Subjects
Forty adults with DSM-IV OCD (Table 1) were recruited from UCLA and other clinics or by advertisement. All provided informed consent, and the UCLA Institutional Review Board approved the study. Diagnoses were established through interview by an author (JDF) experienced with OCD. Primary OCD and comorbid diagnoses were determined using the ADIS-IV Mini. Eligible participants scored ≥16 on the Yale-Brown Obsessive Compulsive Scale (YBOCS). All reported pediatric-onset (before age 18) OCD. Exclusions included psychotic disorder, bipolar disorder, lifetime substance dependence, or ADHD. We excluded individuals with hoarding as their primary symptom. Comorbid anxiety disorders were allowed if secondary to OCD. Comorbid major depressive disorder or dysthymic disorder was allowed, but individuals were excluded if the ADIS-IV clinical significance rating was ≥6 (severe). Altogether 27 patients had one or more such psychiatric comorbidity and 13 had none. Twelve participants were taking serotonin-reuptake inhibitors (6 fluoxetine, 1 escitalopram, 1 sertraline, 2 paroxetine, 2 fluvoxamine) and 28 were unmedicated; of medicated patients, none had any changes in dose or agent for 12 weeks before enrollment. Of the unmedicated OCD participants, 14 had taken psychiatric medications in the past and 14 were psychiatric medication-naïve. Other exclusions included IQ <80 on the Wechsler Abbreviated Scales of Intelligence (WASI) and medical conditions that affect cerebral metabolism including diabetes, thyroid disorders, a history of stroke, brain tumors, seizures, and multiple sclerosis.
Table 1.
Clinical characteristics of obsessive-compulsive disorder (OCD) and healthy groups.
| Participant characteristics | OCD (n = 40) | Control (n = 16) | P-value comparison |
|---|---|---|---|
| Mean age ± s.d. (in years) | 34.3 ± 11.8 | 32.1 ± 10.8 | 0.61a |
| Female sex (%) | 20 (50.0) | 5 (31.2) | 0.25b |
| Education ± s.d. (in years) | 15.6 ± 2.3 | 15.4 ± 2.5 | 0.55a |
| Handedness ± s.d. (Edinburgh Inventory) | 84.7 ± 20.9 | 82.6 ± 20.7 | 0.65a |
| Intelligence ± s.d. (WAIS) | 108.3 ± 10.4 | 110.2 ± 8.4 | 0.37a |
| General functioning ± s.d. (GAS) | 54.9† ± 7.5 | 85.2 ± 7.7 | <0.0005a |
| Anxious symptoms ± s.d. (HamA) | 12.6† ± 6.2 | 1.4 ± 1.1 | <0.0005a |
| Depressive symptoms ± s.d. (MADRS) | 15.7† ± 8.9 | 1.3 ± 1.2 | <0.0005a |
| Obsessive-compulsive symptoms ± s.d. (Y-BOCS) | 25.2 ± 4.3 | ||
| Obsessive-compulsive symptoms ± s.d.c (OCI-R) Total (distress) | 1.65 ± 0.64 | ||
| Washing ± s.d. | 1.82 ± 1.08 | ||
| Checking ± s.d. | 1.55 ± 0.89 | ||
| Doubting ± s.d. | 1.97 ± 1.11 | ||
| Ordering ± s.d. | 1.89 ± 1.10 | ||
| Obsessions ± s.d. | 1.64 ± 0.77 | ||
| Hoarding ± s.d. | 1.25 ± 1.06 | ||
| Neutralizing ± s.d. | 1.44 ± 0.95 | ||
| Current serotonin-reuptake inhibitor (%) | 12 (30.0) |
Abbreviations: GAS, Global Assessment Scale; HamA, Hamilton Anxiety Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; WAIS, Wechsler Abbreviated Scale of Intelligence; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; OCI-R, Obsessive-Compulsive Inventory Revised.
Mann-Whitney U-test.
χ2-test.
OCI-R data were missing for 1 participant
OCD participants were compared to 16 matched healthy controls. We recruited healthy controls via flyers and Internet ads. Healthy controls provided signed informed consent. Controls had no history of psychiatric disorder or substance abuse and no current major medical conditions or psychoactive medications.
The primary clinical measure was the YBOCS (patients only). Secondary measures included the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Anxiety Scale (HamA). General functionality and social and occupational performance were rated by the Global Assessment Scale (GAS; Endicott et al., 1976).
2.2. 1H MRS acquisition
Whole-brain MRI and localized MRS were acquired in 1-hr sessions at 3 T on a Siemens Trio with 12-channel phased-array head coil. Structural MRI was acquired with an axial-oblique (genu-splenium parallel) magnetization-prepared rapid gradient-echo (MPRAGE, 1×1×1 mm3) pulse-sequence. This MPRAGE and “coronal” and “sagittal” resliced copies were used to prescribe MRS. A neuroradiologist (NS) reviewed MRIs to exclude subjects with clinical abnormalities.
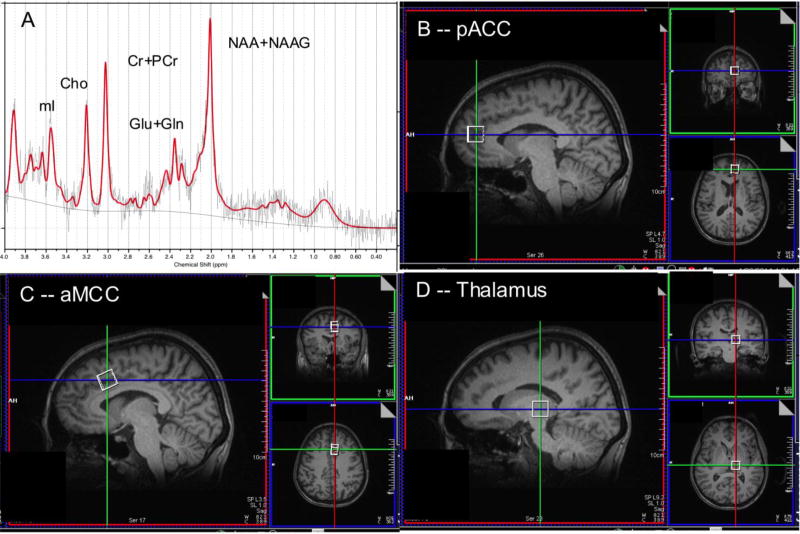
Single-voxel water-suppressed point-resolved spectroscopy (PRESS) MRS (TR/TE=2000/30 ms, 96 NEX) was acquired bilaterally from three sites. Each PRESS excitation volume (“voxel”) measured 15×15×15 mm3, adjusted to maximize gray matter. Each scan was followed by an identical non-water-suppressed acquisition (8 NEX). We placed pairs of voxels in pACC, aMCC, and thalamus, as in Fig. 1 (details in Appendix). In addition to its target volume-of-interest (VOI), each voxel contained modica of gray matter from adjoining regions, white matter, and CSF. Each MRS voxel was shimmed locally using the Trio automatic shimming routine.
Fig. 1.
[A] sample MR spectrum from left pregenual anterior cingulate cortex (pACC) with principal resonances for N-acetyl-aspartate+N-acetyl-aspartylyglutamate (NAA+NAAG = tNAA), glutamate+glutamine (Glu+Gln = Glx), creatine+phosphocreatine (Cr), choline-compounds (Cho), and myo-inositol (mI). [B] parasagittal, coronal and axial-oblique T1w MRI sections showing position of MRS acquisition volume (“voxel”) for left pACC. [C] the same for left anterior middle cingulate cortex (aMCC) voxel. [D] The same for left thalamus voxel. Voxels were acquired at homologous sites in the right cerebral hemisphere.
2.3. MR post-processing
Each MPRAGE was segmented into gray matter, white matter, and CSF using FSL FAST (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST). The volume percent of each tissue in each MRS voxel was calculated and metabolite levels were corrected for CSF-content. CSF-correction was performed by dividing the uncorrected metabolite level by a factor of one minus the volume-fraction CSF present inside the MRS voxel. For the thalamic VOIs, tissue-content values are reported for CSF only due to poor gray-matter/white-matter segmentation.
MR spectra were fit with LCModel yielding levels of N-acetyl-aspartate+N-acetyl-aspartyl-glutamate (tNAA), Glu, Glx, Cr, Cho, and myo-inositol (mI) referenced to unsuppressed water. The intensity of the water signal used for quantitation was obtained from the non-water-suppressed PRESS acquisition. Values for each of the individual neurometabolite signals were rejected if the LCModel %SD (Cramer-Rao Lower Bound) exceeded 20%. Spectra with obvious artifact or poor quality (linewidth >0.1 ppm, signal-to-noise <3) were rejected in toto.
2.4. Statistical analysis
Non-parametric statistics were adopted for a conservative approach. This was achieved with non-parametric tests (Mann-Whitney U, Spearman) or by rank-transforming data before parametric tests (e.g., repeated-measures analysis-of-variance—R-ANOVA). MR endpoints did not differ significantly between left and right hemispheres for the three VOI pairs. Therefore, left+right (“midline”) averages were used for each pair. Groups were compared for sex, age, education, handedness, IQ, MADRS, HamA, and GAS and for differences in MRS voxel tissue-content at each site. Within OCD patients: metabolites were compared between male and female subgroups to determine effects of sex; correlations were run to determine effects of age and tissue-content on metabolites; and medicated and unmedicated subgroups were compared to determine effects of medication on metabolites.
An omnibus approach was taken to multiple comparisons by conducting R-ANOVA for each metabolite with the three midline VOIs as within-subjects factor and diagnosis (OCD, control) as between-subjects factor. Similarly, within the OCD sample, omnibus testing was applied with each of Y-BOCS, MADRS, HamA, and GAS as between-subjects variable. Where there was a significant main effect, it was followed-up by post-hoc protected tests at each site. The post-hoc test was univariate ANOVA for between-group comparisons of metabolite levels and Spearman correlation for within-group relationships between metabolite levels and clinical scales within the OCD sample. Covariates were added to ANOVA (rendering it ANCOVA) when significant between-group differences in sex, age, or MRS voxel tissue-content were demonstrated; sex, age, MRS voxel tissue-content, or use of psychiatric medication were partialled-out of Spearman correlations when these variables significantly affected metabolites. Criterion for significance was p<0.05.
3. Results
3.1. Demographic and clinical variables, MRS voxel tissue content
The groups were well matched for age, handedness, IQ, and education (Table 1) and did not differ significantly in sex. As expected, MADRS (Z=5.7, N=55, p<0.0005) and HamA (Z=5.6, N=55, p<0.0005) were higher for OCD than controls, while GAS (Z=−5.7, N=54, p<0.0005) was lower (Table 1). MRS voxel gray-matter, white-matter, or CSF-content did not differ significantly between groups at any site (Table 2).
Table 2.
MRS voxel tissue composition and neurometabolite levels (IU).
| Endpoint | OCD n=40 | Healthy control n=16 |
|---|---|---|
| pregenual anterior cingulate cortex | ||
| volume% gray matter | 60.9 (6.8) | 59.5 (5.5) |
| volume% white matter | 29.7 (8.6) | 30.3 (5.9) |
| volume% CSF | 9.3 (4.6) | 10.2 (5.5) |
| tNAA | 6.7 (0.6) | 6.8 (1.1) |
| Glu | 9.1 (1.0) | 8.8 (1.4) |
| Glx | 12.9 (1.7)a | 11.8 (1.5) |
| Cr | 6.1 (0.5) | 6.2 (0.7) |
| Cho | 1.7 (0.2) | 1.7 (0.3) |
| mI | 5.4 (0.9) | 5.8 (1.1) |
| anterior middle cingulate cortex | ||
| volume% gray matter | 56.3 (8.2) | 52.8 (6.7) |
| volume% white matter | 34.7 (10.7) | 38.9 (8.5) |
| volume% CSF | 9.1 (5.6) | 8.3 (6.1) |
| tNAA | 7.4 (0.6) | 7.3 (0.4) |
| Glu | 9.4 (1.3) | 8.8 (1.1) |
| Glx | 12.1 (1.5) | 11.6 (2.0) |
| Cr | 5.6 (0.6) | 5.5 (0.4) |
| Cho | 1.6 (0.2) | 1.6 (0.3) |
| mI | 5.4 (0.6) | 5.5 (0.7) |
| thalamus | ||
| volume% CSF | 0.0 (0.0) | 0.0 (0.0) |
| tNAA | 7.9 (0.7) | 8.1 (0.8) |
| Glu | 7.6 (1.1) | 7.5 (0.8) |
| Glx | 10.8 (1.7) | 10.3 (1.5) |
| Cr | 5.8 (0.5) | 5.7 (0.6) |
| Cho | 1.6 (0.2) | 1.6 (0.2) |
| mI | 4.4 (0.8) | 4.5 (0.9) |
p<0.05 Mann-Whitney U-test for between-group difference.
Within OCD patients, no metabolite levels differed significantly between male and female subgroups or varied significantly with age (all p>0.05, n.s.) In aMCC, tNAA (r=−0.40, p=0.013), Glu (r=−0.65, p<0.0005), Glx (r=−0.46, p=0.003), and Cr (r=−0.45, p=0.004) varied with white-matter content. In pACC, only Glu (r=−0.40, p=0.012) and Glx (r=−0.31, p=0.05) varied with white matter. In aMCC, mean Glu (MWU=78.0, N=39, p=0.05), Glx (MWU=52.0, N=39, p=0.006), Cr (MWU=38.0, N=39, p=0.001), Cho (MWU=60.0, N=39, p=0.012), and mI (MWU=56.0, N=39, p=0.008) were lower in medicated than non-medicated patients. Use of medication and white-matter content were, accordingly, applied as covariates or partials in the relevant tests. In pACC and thalamus, there were no significant differences between medicated and non-medicated patients in any metabolite (all p>0.05).
3.2. MRS neurometabolite levels
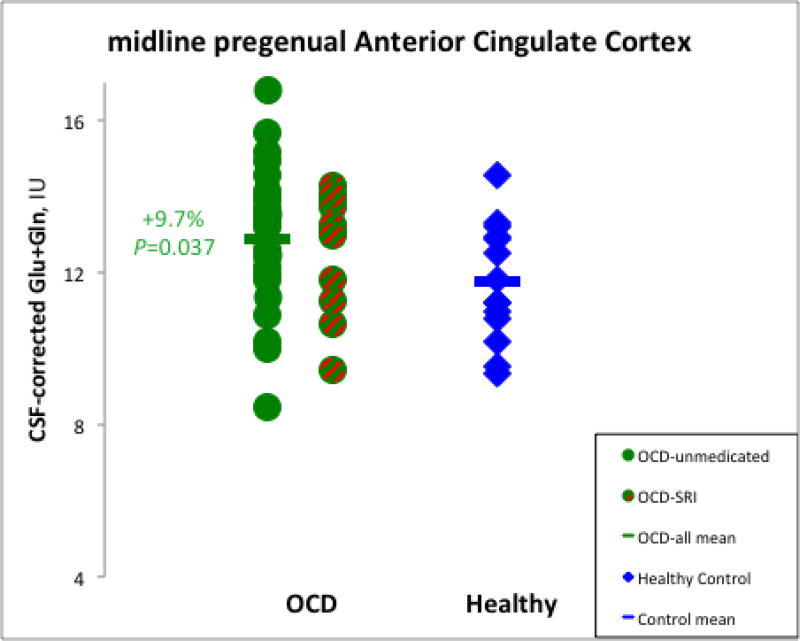
Metabolite levels for OCD patients and controls are in Table 2. R-ANOVA for Glx across VOIs showed a significant main effect of diagnosis (F=4.5, d.f.=1,53, p=0.038). Post-hoc protected ANCOVA covarying white matter confirmed that Glx in pACC was higher for OCD patients than controls (9.7%; F=4.6, d.f.=1,54, p=0.037; Fig. 2). This difference remained significant when age and education were added as covariates and the comparison was restricted to unmedicated patients (10.8%; F=4.8, d.f.=1,38, p=0.036). There were no other significant between-group metabolite differences. Also, there were no significant metabolite differences between the patient subgroups with and without psychiatric comorbidities (all p>0.05).
Fig. 2.
Elevated levels of glutamate+glutamine measured by magnetic resonance spectroscopy (MRS) in pregenual anterior cingulate cortex (pACC) of OCD patients relative to healthy controls. Levels did not differ significantly for patients treated and not-treated with serotonin reuptake inhibitors. (SRIs). IU=Institutional Units
3.3. Associations between metabolite levels and clinical scales
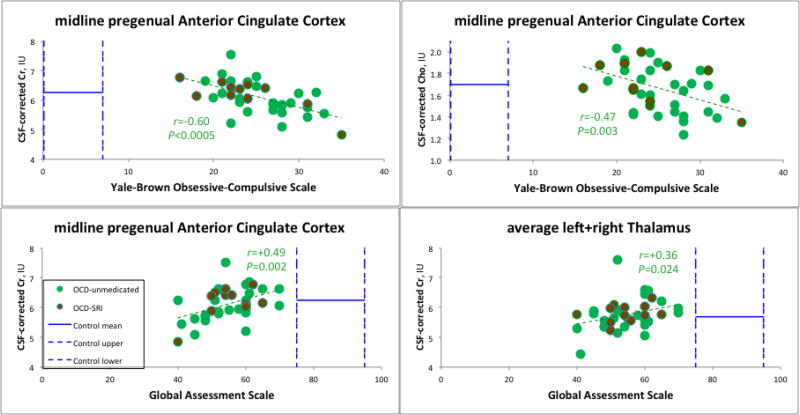
Within the OCD sample, R-ANOVA for Cr across sites yielded a significant effect of YBOCS (F=13.2, d.f.=1,37, p=0.001). Post-hoc, YBOCS had a strong negative correlation with Cr in pACC (r=−0.60, p<0.0005), i.e., patients with more severe OC symptoms had lower Cr (Fig. 3). This correlation remained significant when age and education were partialled-out and only unmedicated patients were examined (r=−0.45, p=0.021). R-ANOVA for Cho similarly yielded a significant effect of YBOCS (F=6.4, d.f.=1,37, p=0.016). Post-hoc, pACC Cho also correlated negatively with YBOCS (r=−0.47, p=0.003; Fig. 3). This correlation remained significant when age and education were partialled-out and only unmedicated patients were examined (r=−0.40, p=0.037).
Fig. 3.
MRS Cr and Cho drop with increasing OCD symptoms; Cr rises with increasing functioning. pACC Cr and Cho levels in OCD are close to normal means (horizontal solid blue bars), yet vary significantly with psychiatric symptoms. Symptoms themselves are outside normal ranges, indicated by vertical dashed blue bars marking measured control (GAS) or standard subclinical (YBOCS) ranges. [A] Cr and [B] Cho in pACC drop with increasing obsessive-compulsive symptoms. Cr in pACC [C] and thalamus [D] rises with increasing global functioning. (Not shown) Cr and Cho in pregenual cingulate and also anterior middle cingulate similarly drop with increasing depressive symptoms. For abbreviations, see Figs. 1–2.
R-ANOVA further yielded significant effects of MADRS on Cr (F=8.7, d.f.=1,36, p=0.006) and Cho (F=6.3, d.f.=1,36, p=0.017). In post-hoc tests, MADRS correlated negatively with Cr in pACC (r=−0.36, p=0.025). But this correlation was no longer significant when age and education were partialled-out and only unmedicated patients were examined (r=−0.19, p=0.361 n.s.) MADRS correlated negatively with aMCC Cr (r=−0.44, p=0.008) partialling white matter and medication. This correlation remained significant when age and education were additionally partialled-out and only unmedicated patients were examined (r=−0.52, p=0.009). MADRS also correlated negatively with Cho in pACC (r=−0.34, p=0.038) and in aMCC (r=−0.41, p=0.012) partialling medication. These correlations dropped to trends when age and education were additionally partialled-out and only unmedicated patients were examined (pACC: r=−0.35, p=0.09 trend; aMCC: r=−0.39, p=0.062 trend). All results were in the direction of patients with more severe depressive symptoms having lower Cr and Cho. Cr and Cho also decreased with increasing anxiety symptoms (HamA), but none of these correlations were significant.
R-ANOVA for Cr revealed a significant effect of GAS (F=12.3, d.f.=1,36, p=0.001). Post-hoc, GAS correlated significantly with Cr in pACC (r=+0.49, p=0.002; Fig. 3) and thalamus (r=+0.36, p=0.024; Fig. 3), i.e., patients with lower global functioning had lower Cr. The pACC correlation remained significant when age and education were additionally partialled-out and only unmedicated patients were examined (r=+0.41, p=0.043), while the thalamic correlation dropped to a trend (r=+0.38, p=0.084 trend).
Within the healthy control sample, there were no significant correlations between regional metabolite levels and scores on the MADRS, HamA, or GAS (all p>0.05). (Y-BOCS was not assessed in controls.)
4. Discussion
This MRS investigation of OCD had four major findings: 1) elevated Glx in patients compared with healthy controls in pACC; 2) Cr and Cho in pACC inversely associated with OC symptom severity; 3) Cr and/or Cho in pACC and aMCC inversely associated with depressive symptoms; and 4) Cr positively associated with global functioning in pACC and thalamus. These findings offer moderate support for glutamatergic, cingulocentric, and ADHD-affine notions of OCD. Our recent model (Fig. S1; O’Neill et al., 2013b) of OCD as a disease with high Glu receptor activity and low Glu transporter activity in cingulocentric circuits seeks to explain the co-occurrence of the above Glx, Cr, and Cho findings.
Support for the Glutamatergic Hypothesis was restricted. A small (~10%) Glx excess was observed in one VOI (pACC) only and no significant correlations with symptoms. Effects of OCD on Glu or Glx were noted in prior studies in caudate, white matter, or posterior middle cingulate (Yücel et al., 2008; Whiteside et al., 2006, 2012; Rosenberg et al., 2000, 2004). Of studies of pACC or aMCC, some (Kitamura et al., 2006; Ebert et al., 1997; Sumitani et al., 2007) did not quantify Glu or Glx; others (Yalçın et al., 2011; Bédard et al., 2011; Besiroglu et al., 2011; Fan et al., 2010) expressed results as ratios (e.g., Glx/Cr). Two studies of pACC using absolute metabolites (Lázaro et al., 2012; Simpson et al., 2012) found no effects of OCD on Glx. MRS voxels in these studies, however, were larger (12–18.8 cc) than ours (3.4 cc), implying possible obscurant partial-voluming effects. Using a 3-cc voxel, Rosenberg et al. (2004) observed below-normal pACC Glx in pediatric OCD. Two more recent pediatric studies, one using a 3-cc voxel straddling pACC/aMCC (Ortiz et al., 2015), the other using 4-cc bilateral voxels in pACC (Starck et al., 2008), found no difference in Glx between patients and controls. These results disagree variously with our observation of elevated Glx. Our patients, though having pediatric-onset OCD, were scanned as adults; so perhaps Glx is higher in adult patients. Zurowski et al. (2007) measured above-normal pACC Glu in adult OCD. Thus, our Glx finding supports glutamatergic theories of OCD (Carlsson, 2000; Rosenberg et al., 2000; Pittenger et al., 2006), but not definitively. An elevation of the Glu subcomponent of Glx in pACC in OCD patients would have made a stronger case for the Glutamatergic Hypothesis, but such was not observed in our study. The absence of significant Glu elevation in the presence of significantly elevated Glx may result from the relative ease of fitting the latter compared to the well-known difficulty of extracting the former during spectral fitting. Thus, notable elevation of Glu may have been present in pACC in patients but variability in fitting may have rendered it difficult to prove statistically. An alternative mentioned by Brennan et al. (2015) is that the Gln subcomponent of Glx may be above-normal in OCD. Their 2D-MRS measurements of Gln argue against this possibility, although even with 2D techniques Gln is challenging to assay in vivo.
The present results reinforce the Cingulocentric Theory of OCD, particularly the link between pACC and mood disturbance. Most results were in pACC, a region associated with anxiety and other negative emotions (Middleton 2009) and the site of prior OCD neuroimaging findings (Saxena et al., 2009b). The aMCC is also the site of OCD findings (Saxena et al., 2009b) and is active in fear and volition (Middleton 2009), key mental functions in OCD. Only two significant relationships were demonstrated between aMCC metabolites and symptoms (MADRS), although metabolite relationships with the MADRS fell in significance when age and education were added as covariates and only unmedicated patients were considered. Future studies of aMCC may wish to use purely unmedicated subjects and smaller MRS voxels containing less white matter. The latter is challenging given the narrow geometry of aMCC, relative to pACC. The thalamus is a key node in the Cingulocentric Theory. Its various subnuclei exert arousal, motor, sensory, affective, and cognitive functions (Mitchell et al., 2014), all impacting everyday activities. Since our MRS voxels sampled all subnuclei together, it is perhaps not surprising that the only significant metabolite correlation in the thalamus was with the GAS, a metric of global functioning.
Similar to ADHD, prominent effects involving Cr and Cho were observed in this OCD study. Most previously reported effects of OCD on Cr (Rosenberg et al., 2001; Mirza et al., 2006; Yücel et al., 2008; Starck et al., 2008; Whiteside et al., 2012) and Cho (Rosenberg et al., 2001; Yücel et al., 2008; Starck et al., 2008; Smith et al., 2003; Kitamura et al. 2006; Mohammed et al., 2007; Zurowski et al., 2007; Yalçın et al., 2011; Lázaro et al., 2012; Whiteside et al., 2012; Weber et al., 2014) were in white matter or striatum, regions not scanned here. In bilateral pACC, one investigation (Yalçın et al., 2011) found lower Cho/Cr in pediatric OCD than controls, a result difficult to interpret due to use of ratios. Elevated Cr (Mirza et al., 2006) and Cho (Rosenberg et al., 2001; Smith et al., 2003) were also measured in thalamus in pediatric OCD. These effects, however, were restricted to mesial thalamus and may be difficult to detect in our MRS voxels, which spanned lateral and mesial thalamus. Our present findings importantly link Cr and Cho to dominant and disabling affective symptoms of OCD, resonating with our previous pilot finding of the ability of pACC Cr and Cho to predict long-term post-treatment symptoms (O’Neill and Feusner, 2015).
Our model (Appendix Fig. S1) may unite the above findings. It proposes that MRS Glu or Glx is elevated in OCD due to longer lifetime of Glu molecules. Glu molecules are detained longer extracellularly by overly profuse N-methyl-D-aspartate (NMDA) and other glutamatergic receptors and enter the neuron for catalysis more slowly due to scarce transporters. The stronger extracellular Glu presence and more abundant NMDA receptors underlie clinical symptoms. Briefly, more NMDA-activation implies more long-term potentiation (LTP). More LTP means more memory associations, many involving anxiety and other negative affect and the behavioral actions that (temporarily) relieve these feelings. This manifests as stronger obsessive-compulsive symptoms (higher YBOCS). LTP likewise strengthens the repetitive negative thoughts and “learned helplessness” of depression, manifesting as higher MADRS. Obsessive-compulsive and depressive symptoms in turn drive down global functioning. Lower Cr with increasing OC and depressive symptoms is also accounted for by our model. Glu receptor stimulation associated with more severe symptoms accelerates neuronal energy production via glycolysis and oxidative metabolism (Fig. S1). This drives the creatine-phosphocreatine equilibrium to the right, promoting extracellular export of phosphocreatine. This lowers creatine and phosphocreatine, and thence the MRS Cr signal (which represents the sum of both compounds). Our model also accounts for lower MRS Cho with greater OC and depressive symptoms. The more severe the symptoms, the greater the neuronal energy needs. To meet these needs, less glucose is diverted from glycolysis to synthesis of choline-containing molecules (Fig. S1), resulting in lower MRS Cho. In addition, overproduction of Glu receptors requires greater phospholipid membrane synthesis, draining the pool of free lipid monomers and reducing MRS Cho.
Our study had limitations. Some patients were taking psychiatric medication. In adult OCD, it is challenging to recruit drug-free samples. Within our medicated subsample, heterogeneity was mitigated in that all subjects were on serotonin-reuptake inhibitors only. These patients were symptomatic (YBOCS >16) despite ≥12 weeks at constant dose. Serotonin-reuptake inhibitor use did affect several metabolites. Interestingly, these effects were confined to aMCC, a region where earlier studies (Sumitani et al., 2007; Jang et al., 2006) noted effects of serotonin-reuptake inhibitors on metabolites. For major study findings, moreover, endpoints for medicated and unmedicated patients were well intermixed (Figs. 2–3). The aMCC was also the site of effects of white matter on metabolites. While our MRS voxels were small enough for lateralized acquisition, they were too large to sample pure pACC and aMCC gray matter and contained, on average, over one-third white matter. Smaller voxels are called for in future studies. As is common in OCD, depressive symptoms were present in the patient sample, and comorbid anxiety and depressive disorders. Cr and Cho correlated with depression. This is perhaps unsurprising in view of the heterogeneity of mood and anxiety disorders and the overlapping nature of symptoms across these disorders. Depression in all patients, however, was secondary to OCD and did not surpass moderate severity. To exclude such symptoms or comorbidities entirely would make the sample poorly representative of typical clinical populations. A better approach might be to compare OCD patients with non-OCD depressed patients, as in Rosenberg et al. (2004) and Mirza et al. (2006), who observed similar metabolite findings for pediatric patients with OCD or major depressive disorder. Finally, one MRS finding was expressed as Glx rather than Glu. This finding was consistent with Zurowski et al. (2007), who did measure Glu. Shorter echo-time (15–20 ms) pulse-sequences could enable superior segregation of Gln from Glu. Notwithstanding, this study had several strengths. It included all adult patients with pediatric-onset illness, and at most secondary depression or anxiety. A standard pulse-sequence (single-voxel PRESS) was used to acquire MRS in small (3.4-cc) voxels of known tissue content. MR spectra were fit with the widely accepted LCModel package and corrected for CSF. With these strengths and limitations in mind, our MRS study adds support to neurobiological models implicating pACC and aMCC in OCD (Saxena et al. 2009b). Findings are also interpretable in terms of our model (O’Neill et al., 2013b) of OCD as a disorder of Glu metabolism, possibly including receptor activation and/or transport.
Supplementary Material
Highlights.
MRS glutamate+glutamine in pregenual anterior cingulate is elevated in adult OCD
MRS choline- and creatine compounds in cingulate and thalamus vary with severity of symptoms
Results give some evidence for OCD as a glutamatergic, cingulocentric, and ADHD-like disorder
Our model of OCD explains the co-occurrence of glutamatergic, choline and creatine effects
Acknowledgments
This study was supported by NIMH grants R01 MH085900 (to Drs. O’Neill and Feusner) and R01 MH081864 (to Dr. O’Neill and Dr. J.C. Piacentini).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has financial disclosures to report.
References
- Abramovitch A, Dar R, Hermesh H, Schweiger A. Comparative neuropsychology of adult obsessive–compulsive disorder and attention deficit/hyperactivity disorder: Implications for a novel executive overload model of OCD. Journal of Neuropsychololgy. 2012;6(2):161–191. doi: 10.1111/j.1748-6653.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- Bédard M-J, Chantal S. Brain magnetic resonance spectroscopy in obsessive–compulsive disorder: The importance of considering subclinical symptoms of anxiety and depression. Psychiatry Research: Neuroimaging. 2011;192:45–54. doi: 10.1016/j.pscychresns.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Besiroglu L, Sozen M, Ozbebir Ö, Avcu S, Selvi Y, Bora A, Atli A, Unal O, Bulut MD. The involvement of distinct neural systems in patients with obsessive–compulsive disorder with autogenous and reactive obsessions. Acta Psychiatrica Scandinavica. 2011;124:141–151. doi: 10.1111/j.1600-0447.2011.01726.x. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Rauch SL, Jensen JE, Pope HG., Jr A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol Psychiatry. 2013;73(1):24–31. doi: 10.1016/j.biopsych.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ, Pope HG, Jr, Jenike MA, Baker JT, Killgore WDS, Hudson JI, Jensen JE, Rauch SL. An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology. 2015;40:1866–1876. doi: 10.1038/npp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ML. On the role of cortical glutamate in obsessive-compulsive disorder and attention-deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatrica Scandinavica. 2000;102:401–413. doi: 10.1034/j.1600-0447.2000.102006401.x. [DOI] [PubMed] [Google Scholar]
- Ebert D, Speck O, König A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Research: Neuroimaging. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Fan Q, Tan L, You C, Wang J, Ross CA, Wang X, Zhang T, Jianqi L, Chen K, Xiao Z. Increased N-Acetylaspartate/creatine ratio in the medial prefrontal cortex among unmedicated obsessive–compulsive disorder patients. Psychiatry and Clinical Neuroscience. 2010;64:483–490. doi: 10.1111/j.1440-1819.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Kerwin L, Saxena S, Bystritsky A. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacology Bulletin. 2009;42(1):81–93. [PubMed] [Google Scholar]
- Jang JH, Kwon JS, Jang DP, Moon W-J, Lee J-M, Ha TH, Chung EC, Kim IY, Kim SI. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naïve patients with obsessive compulsive disorder. American Journal of Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive–compulsive disorder: a magnetic resonance spectroscopy study at 3-Tesla. Acta Psychiatrica Scandinavica. 2006;114:101–108. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Lázaro L, Bargallo N, Andrés S, Falcón C, Morer A, Junque C, Castro-Fornieles J. Proton magnetic resonance spectroscopy in pediatric obsessive–compulsive disorder: Longitudinal study before and after treatment. Psychiatry Reserach: Neuroimaging. 2012;201:17–24. doi: 10.1016/j.pscychresns.2011.01.017. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, O’Neill J, Rosenberg DR. Brain imaging in pediatric obsessive compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1262–1272. doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA, Smith MA, Schlund MW, Nestadt G, Barker PB, Hoehn-Saric R. Proton magnetic resonance spectroscopy in obsessive-compulsive disorder: A pilot investigation comparing treatment responders and non-responders. Psychiatry Res: Neuroimaging. 2007;156:175–179. doi: 10.1016/j.pscychresns.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Middleton FA. The contribution of anterior cingulate-basal ganglia circuitry to complex behavior and psychiatric disorders. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; New York: 2009. pp. 619–632. [Google Scholar]
- Mirza Y, O’Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, Bhandari R, Boyd C, Rose M, Ivey J, Renshaw PF, Rosenberg DR. Increased medial thalamic creatine/phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. Journal of Child Neurolology. 2006;21(2):106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. Journal of Neuroscience. 2014;34(46):15340–15346. doi: 10.1523/JNEUROSCI.3289-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Feusner JD. Cognitive-behavioral therapy for OCD: Access to treatment, prediction of long-term outcome with neuroimaging. Psychology Research and Behavior Management. 2015;8:211–223. doi: 10.2147/PRBM.S75106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Gorbis E, Feusner JD, Yip JC, Chang S, Maidment KM, Levitt JG, Salamon N, Ringman JM, Saxena S. Effects of intensive cognitive-behavioral therapy on cingulate neurochemistry in obsessive-compulsive disorder. Journal of Psychiatry Research. 2013a;47(4):494–504. doi: 10.1016/j.jpsychires.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Levitt JG, Alger JR. Magnetic resonance spectroscopy studies of attention deficit hyperactivity disorder. In: Blüml S, Panigrahy A, editors. MR Spectroscopy of Pediatric Brain Disorders. Springer; New York: 2013b. pp. 229–276. [Google Scholar]
- O'Neill J, Piacentini JC, Chang S, Levitt JG, Rozenman M, Bergman L, Salamon N, Alger JR, McCracken JT. MRSI correlates of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36:161–168. doi: 10.1016/j.pnpbp.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz AE, Ortiz AG, Falcon C, Morer A, Plana MT, Bargalló N, Lázaro L. 1H-MRS of the anterior cingulate cortex in childhood and adolescent obsessive–compulsive disorder: A case-control study. European Neuropsychopharmacology. 2015;25:60–68. doi: 10.1016/j.euroneuro.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, Hesslinger B, Buechert M, Henning J, Ebert D, Tebartz van Elst L. Spectroscopic findings in attention-deficit/hyperactivity disorder: Review and meta-analysis. World Journal of Biological Psychiatry. 2009;10(4):355–365. doi: 10.1080/15622970802176032. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch M, Wegner R, Teitelbaum C, Krystal JH, Coric V. Glutamate dusfunction in obsessive-compulsive disorder and the potential clinical utility of glutamate-modulating agents. Primary Psychiatry. 2006;13(10):65–77. [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacology and Therapeutics. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Amponsah A, Sullivan A, Macmillan S, Moore GJ. Increased medial thalamic choline in pediatric obsessive-compulsive disorder as detected by quantitative in vivo spectroscopic imaging. Journal of Child Neurology. 2001;16:636–641. doi: 10.1177/088307380101600902. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive–compulsive disorder. Biological Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgeratd KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(9):1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee P, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Saxena S, O’Neill J, Rauch SL. The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; New York: 2009a. pp. 587–618. [Google Scholar]
- Saxena S, Gorbis E, O’Neill J, Baker SK, Mandelkern MA, Maidment KM, Chang S, Salamon N, Brody AL, Schwartz JM, London ED. Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Molecular Psychiatry. 2009b;14(2):197–205. doi: 10.1038/sj.mp.4002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, Kegeles LS. Investigation of cortical glutamate–glutamine and γ-aminobutyric acid in obsessive–compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2012;37(12):2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, Bhandari R, Moore GJ, Rosenberg DR. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: A magnetic resonance spectroscopy study. Biological Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jönsson L, Lundberg S, Ivarsson T, Ribbelin S, Ekholm S, Carlsson A, Forssell-Aronsson E, Carlsson M. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. Journal of Neural Transmission. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive–compulsive disorder patients. Psychiatry Research: Neuroimaging. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Weber AM, Soreni N, Stanley JA, Greco A, Mendlowitz S, Szatmari P, Schachar R, Mannasis K, Pires P, Swinson R, Noseworthy MD. Proton magnetic resonance spectroscopy of prefrontal white matter in psychotropic naïve children and adolescents with obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2014;222(1–2):67–74. doi: 10.1016/j.pscychresns.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Abramowitz JS, Port JD. The effect of behavior therapy on caudate N-acetyl-l-aspartic acid in adults with obsessive–compulsive disorder. Psychiatry Reserach: Neuroimaging. 2012;201:10–16. doi: 10.1016/j.pscychresns.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive–compulsive disorder and anxiety. Psychiatry Res: Neuroimaging. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yalçın Ö, Şener Ş, Konuş Boyunağa ÖL, Sarıpınar EG, Oğur T, Güney E, Akin Sari B, Işeri E. Comparing brain magnetic resonance spectroscopy findings of pediatric treatment-naive obsessive-compulsive disorder patients with healthy controls. Turkish Journal of Psychiatry. 2011;22(4):222–229. [PubMed] [Google Scholar]
- Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, Clarke K, Phillips ML, Kyrios M, Velakoulis D, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64(8):946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, Velakoulis D, Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Australia-New Zealand Journal of Psychiatry. 2008;42(6):467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Zurowski B, Weber-Fahr W, Freyer T, Wahl K, Büchert M, Kuel AK, Hohagen F, Braus DF, Voderholzer U, Kordon A. Neurochemical abnormalities in patients with obsessive-compulsive disorder diminish in the course of behavior therapy. Society of Neuroscience Abstracts. 2007 (abstract 450.10) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.