Abstract
Background
Despite success of immune checkpoint and targeted therapy, many melanoma patients ultimately require further treatment. The combination of carboplatin, paclitaxel, and bevacizumab showed promising activity in a single-arm study. We performed a randomized phase II study to confirm efficacy and determine whether adding everolimus would increase its activity.
Methods
Through the North Central Cancer Treatment Group, 149 patients with unresectable stage IV melanoma were randomized from May 2010 to May 2014 to either carboplatin, paclitaxel, and bevacizumab (CPB) or the combination with everolimus (CPBE). The primary endpoint was progression-free survival (PFS), with secondary endpoints of overall survival (OS), response rate, and tolerability.
Results
The CPB and CPBE arms were balanced in age (median 59, 58 years), high LDH (48%, 51%), but unbalanced by sex (72%, 55% male, p= 0.03). Overall, there was no difference in PFS for CPB v CPBE, HR 1.14, (95% CI 0.81–1.62), p=0.44, median PFS 5.6 vs 5.1 months or median OS HR 1.16 (0.84–1.84), 14.5 vs 10.8m. Confirmed response rate was 13% for CPB, and 23% for CPBE (p=0.13). Toxicity was higher for CPBE vs CPB (83% Gr 3+, 14% 4+ vs 63% and 11%, respectively). Common grade 3+ toxicities were neutropenia, leukopenia, and fatigue in both arms with comparable frequency.
Conclusions
Both experimental arms demonstrated activity, with progression-free survival exceeding 5 months. However, the addition of everolimus to CPB failed to improve outcomes with increased toxicity. These findings replicate moderate antitumor activity of CPB, with future development possibly in combination with targeted or immunotherapy.
Keywords: Melanoma, chemotherapy, everolimus, uveal
Introduction
Treatment of metastatic melanoma has substantially transformed in the last decade, with improvement in outcomes with multiple approved immunotherapies targeting PD-1, CTLA4, and viral-based immunotherapy.1, 2,3, 4 These immunotherapies have produced durable responses in a substantial proportion of patients, with combinations showing even higher activity. Similarly, therapy targeted at the common activating V600 mutations in BRAF have proven effective, with high response rates, although responses are generally not as durable as with immunotherapy.5, 6 Still, not all patients respond to these therapies, and particularly BRAF wildtype patients who progress through initial immunotherapy are often left without a standard alternative second line treatment. In those instances, in the absence of available clinical trials, providers still use chemotherapy, with its modest activity, given the absence of other, perhaps more preferable, alternatives.
Background
Response rates to single-agent chemotherapy in melanoma are low (15–20%) and short-lived with no impact on overall survival7. Hodi et al, studied carboplatin and paclitaxel at (AUC 7.5, 175 mg/m2 respectively) on a 21 day cycle, in 15 chemo naïve patients.8 A 20% RR was seen, with 47% having stable disease. Toxicities were mostly hematologic. Zimpfer-Rechner et al, randomized patients to paclitaxel with or without carboplatin. Median PFS was reported as 8 weeks in both arms.9 Finally, a retrospective series by Rao et al, reported a 26% response rate with a median time to progression of 3 months.10 A phase III study confirmed 4- month PFS for carboplatin/paclitaxel in metastatic melanoma11, which is superior to historic numbers of 1.7 months in a large meta-analysis.12 The combination of carboplatin and paclitaxel is listed in National Comprehensive Cancer Network guidelines for treatment in second or later lines of therapy.13
Perez, et al14 examined the combination of carboplatin, paclitaxel, and bevacizumab for the treatment of stage IV unresectable melanoma, reporting a median PFS of approximately 6 months, with an overall survival of 12 months in the pre-immunotherapy and targeted therapy era. The most common severe (grade 3+) toxicities reported were neutropenia (49%), leukopenia (34%), thrombocytopenia (8%), anemia (8%), hypertension (6%), fatigue (6%), and nausea (6%).
We hypothesized that the addition of everolimus would improve clinical outcomes of CPB regimen as outlined below. First, given the tremendous complexity and redundancy in metabolic pathways in melanoma, aberrant pathways must likely be targeted in multiple ways in order to provide optimal inhibition. Multiple studies have demonstrated the adverse effects of vascular endothelial growth factor (VEGF) expression in melanoma including its association with worsened prognosis15, chemotherapy resistance16, and immunosuppression.17 Bevacizumab is a monoclonal antibody against VEGF-A, and as such effective at reducing VEGF-A levels, but not VEGF-C which is important in lymphangiogenesis and possibly VEGF-induced immune suppression18. mTOR, the target of everolimus, can induce expression of VEGF-C, and inhibition of mTOR with rapamycin has been shown to potently reduce VEGF-C expression in a murine skin flap model19 and murine tumor xenografts.20 A phase II study combining everolimus and bevacizumab in metastatic melanoma patients showed a PFS of 4 months and good tolerability.21
We constructed a randomized, phase II study to explore the combinations of carboplatin, paclitaxel, bevacizumab, with and without everolimus to explore the activity of the combination. Unlimited targeted therapy and immunotherapy were allowed prior to enrollment.
Methods
Study Design
This North Central Cancer Treatment group (NCCTG, now part of the Alliance) clinical trial N0879 was a randomized phase II trial of 149 patients with unresectable stage IV melanoma to assess addition of everolimus to carboplatin, paclitaxel, and bevacizumab l This study was conducted in accordance with Mayo Clinic Cancer Center Data and Safety Monitoring Board (MCCC DSMB), CTCAE version 4.0, and the Mayo Clinic IRB. All patients provided written informed consent before any study procedure.
Study Population
Patients were enrolled in this study from May 2010 to May 2014. This study was available to all eligible patients, regardless of race, gender, or ethnic origin. Inclusion criteria required histologic proof of stage IV malignant melanoma not amenable to surgery,, measurable disease, life expectancy of ≥ 4 months, age ≥18 years, adequate blood counts and organ function, and ECOG performance score 0–1, and no more than one prior chemotherapy based regimen for metastatic melanoma (no prior taxane-based regimens). Prior adjuvant non-taxane based chemotherapy and/or adjuvant immunotherapy were allowed and there was no limit on the number of prior biologic, immunologic or targeted therapies. Exclusion criteria were prior mTOR or VEGF directed therapy, brain metastases, major comorbidities, or contraindications to bevacizumab.
Random Assignment
A 12 patient “run-in” (six eligible patients were randomized to each to the regimens) phase was conducted to assess for any unexpected toxicities from the addition of everolimus to the CPB regimen. Full study enrollment was suspended until each of these 12 patients was followed for at least 2 cycles of treatment to monitor for development of dose limiting toxicities. Once the study was open to enrollment, a dynamic allocation procedure allocated an equal number of patients to one of the two treatment regimens. The stratification factors that were balanced between the two treatment arms were M-stage (M1a, M1b, M1c), LDH elevation (Y/N), and prior cytotoxic chemotherapy for metastatic disease.
Treatment Protocol
Patients were randomized to Treatment Arm A (CPB) with carboplatin AUC 5 IV day 1 with repeat every 28 days, paclitaxel 80 mg/m2 IV days 1,8, and 15, and bevacizumab 10mg/kg IV days 1 and 15 (Figure 1). Treatment Arm B (CPBE) with the above regimen and everolimus 5mg days 1–5, 8–12, 15–19, and 22–26 with repeat every 28 days. Due to neutropenia and other related toxicities, the everolimus dose was decreased to 5 mg three times weekly (Addendum 5, October 1, 2010). With the initial dose of everolimus, four patients had grade 3 neutropenia, four patients had grade 3 leukopenia, and 1 patient had grade 2 myalgia (1 pt). To maintain the starting doses of all therapies on CPBE, the starting dose of everolimus was reduced to an initial dose for 5 mg 3 times weekly. This was chosen due to the half-life of everolimus (25–30 hours), and in addition, there is demonstrated evidence of continued successful target inhibition in a patient whose everolimus dose was 5mg three times weekly when used in a 3-drug combination that was well tolerated.22 In addition, the maximum carboplatin dose for newly enrolled patients was capped at 750 mg.
Figure 1.
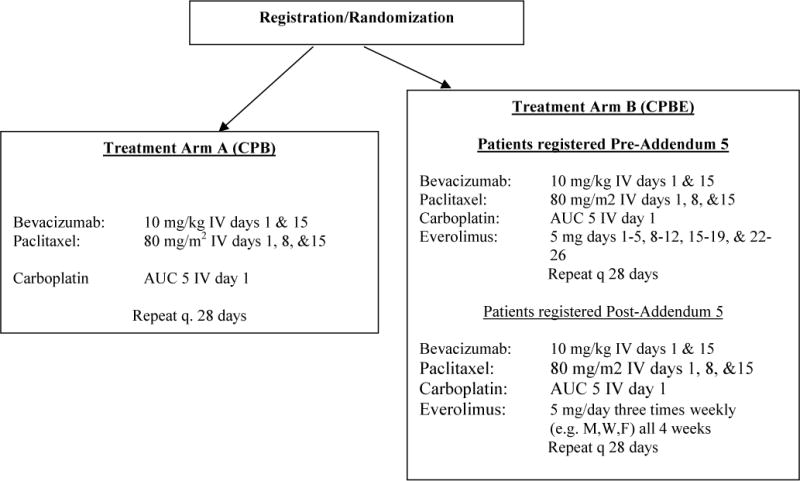
Patients were randomized into one of two arms, carboplatin, paclitaxel and bevacizumab with or without everolimus (CPB or CPBE). The CPBE arm was amended for excessive toxicity after the first 20 patients.
Outcome Measures
The primary end point was progression free survival with the addition of everolimus to the combination of carboplatin, paclitaxel, and bevacizumab. The secondary objectives were to estimate the confirmed tumor response rate, estimate the distribution of overall survival time, and assess the safety profile of each of the treatment regimens. We examined the impact of common genetic variants and site origin on outcomes from therapy.
Statistical Analysis
The design had a one-sided alpha=0.10 significance level with 83% power HF = 0.67. This was an intention to treat analysis with all eligible patients being analyzed to the treatment arm to which they were randomized for the primary outcome.
Out of 149 patients enrolled in the study, one patient was later determined to be ineligible. This left 148 patients in a modified ITT analysis set (75 in arm CPB and 73 in CPBE). Of these 148 patients, 6 patients cancelled participation prior to receiving any study treatment (71 were treated in each arm).
Overall survival time was defined as the time from registration to death due to any cause. The distribution of PFS and OS were estimated with the Kaplan-Meier method. A stratified logrank test and Cox partial likelihood score test assessed whether the distribution of PFS or OS times differ with respect to treatment regimen having adjusted for M stage (M1a, M1b, M1c) and prior chemotherapy in the metastatic setting. A confirmed tumor response was defined to be a CR or PR (by the RECIST v1.1 criteria), and the proportion of tumor responses was estimated by the number of confirmed tumor responses divided by the total number of evaluable patients with a 90% confidence interval. When measuring treatment response, a subsequent scan was obtained 8 weeks following initial documentation of an objective status of either complete response (CR) or partial response (PR). In the case of stable disease (SD), follow-up measurements must have met the SD criteria at least once after study entry at a minimum interval of 8 weeks.
The CTEP version 4.0 of the NCI Common Terminology Criteria for Adverse Events (CTCAE) was utilized for adverse event reporting. The study team reviewed the toxicities, which were submitted for consideration by the MCCC DSMB, CTEP, and the IRB. The information was submitted to the MCCC DSMB every 6 months until all patients were off study treatment. If more than 30% of the patients developed a grade 3+ hemorrhage, febrile neutropenia, grade 4 neutropenia ≥5 days, anemia, platelet count < 25,000, grade 4+ non-hematologic toxicity, and these events were considered to be likely related to treatment, enrollment to that regimen would be suspended. Adverse events were reported for all adverse events and attribution levels of possibly or greater-related. Reports also designated CPBE-randomized patients into pre and post the everolimus dosing reduction addendum (Addendum 5).
DNA sequencing analysis
Archived paraffin tumor biopsy material was requested for all patients from either primary tumors or metastases. H&E slides were reviewed by two pathologists for tumor amount and % of tumor nuclei, and then DNA was extracted by the QIAamp DSP DNA FFPE. A clinical 50-gene hotspot PCR-based Miseq next generation panel was analyzed in Mayo Medical Labs (K. Rumilla) on the extracted DNA (26). Melanoma relevant genes examined included BRAF, NRAS, C-kit, GNAQ, GNA11. Identified known mutations were coded as mutant vs wildtype. The sequenced data was then analyzed in Mayo Medical Labs and alignments visualized in Alamut. There were 114 patients with sufficient tissue for analysis.
Results
Population and Demographics
There were 148 eligible patients randomized to two treatment arms: 75 patients in carboplatin, paclitaxel, and bevacizumab, (CPB) and 73 patients in carboplatin, paclitaxel, bevacizumab, and everolimus (CPBE). Although 6 patients cancelled prior to receiving any study treatment (71 patients total in each arm), there was an intention-to-treat analysis for the primary endpoint. Patients were similar in each treatment arm (see Table 1), except arm CPB had greater percentage of male gender compared to arm CPBE (72% vs 54.8%, p = 0.03). Of patients enrolled, 38.1% had prior ipilimumab therapy, 21.2% had prior chemotherapy, and 4.1% had vemurafenib. In addition, 19.5% of patients had proven BRAF mutations. The most common primary site was the trunk at 23.3%. There were a large portion of patients enrolled in the study with uveal melanoma as primary site (17.8%). Of the 143 patients who underwent treatment, 27 were alive with a median follow up of 38.5 months (range 1.9–61.0 months). Patients received median of 5 cycles of therapy. Patient characteristics further delineated by uveal, non-uveal, and BRAF mutation status are show in Supplementary Tables 1–3.
Table 1.
Patient Characteristics.
| CPB Arm (A) N=75 | CPBE Arm (B) N=73 | Combined | p-value | ||
|---|---|---|---|---|---|
| Age | 0.62 | ||||
| Median | 59 | 58 | 59 | ||
| Gender | 0.03 | ||||
| Female | 21 (28.0%) | 33 (45.2%) | 54 (36.5%) | ||
| Male | 54 (72.0%) | 40 (54.8%) | 94 (63.5%) | ||
| Race | 0.22 | ||||
| White | 74 (98.7%) | 71 (97.3%) | 145 (98.0%) | ||
| Black | 1 (1.3%) | 0 (0.0%) | 1 (0.7%) | ||
| Primary Site | 0.32 | ||||
| Head | 2 (2.7%) | 7 (9.7%) | 9 (6.2%) | ||
| Neck | 1 (1.4%) | 2 (2.8%) | 3 (2.1%) | ||
| Upper Extremity | 6 (8.1%) | 8 (11.1%) | 14 (9.6%) | ||
| Lower Extremity | 17 (23.0%) | 9 (12.5%) | 26 (17.8%) | ||
| Trunk | 15 (20.3%) | 19 (26.4%) | 34 (23.3%) | ||
| Uveal | 16 (21.6%) | 10 (13.9%) | 26 (17.8%) | ||
| Mucosal | 1 (1.4%) | 1 (1.4%) | 2 (1.4%) | ||
| Anogenital | 2 (2.7%) | 0 (0.0%) | 2 (1.4%) | ||
| M stage | 0.35 | ||||
| M1a | 9 (12.0%) | 4 (5.5%) | 13 (8.8%) | ||
| M1b | 11 (14.6%) | 12 (16.4%) | 23 (15.5%) | ||
| M1c | 56 (74.7%) | 57 (78.1%) | 113 (76.4%) | ||
| Prior Chemotherapy | 0.6 | ||||
| Yes | 16 (21.6%) | 15 (20.5%) | 31 (21.1%) | ||
| No | 58 (78.4%) | 57 (78.1%) | 115 (78.2%) | ||
| Prior Immunotherapy | 0.46 | ||||
| Yes | 26 (35.1%) | 30 (41.1%) | 56 (38.1%) | ||
| No | 48 (64.9%) | 43 (58.9%) | 91 (61.9%) | ||
| Prior Angiogenesis Therapy | 0.31 | ||||
| Yes | 74 (100%) | 72 (98.6%) | 146 (99.3%) | ||
| No | 0 (0%) | 1 (1.4%) | 1 (0.7%) | ||
| Prior Ipilimumab | 0.61 | ||||
| Yes | 12 (16.0%) | 14 (19.2%) | 26 (17.6%) | ||
| No | 63 (84.0%) | 59 (80.8%) | 122 (82.4%) | ||
| Prior BRAF inhibitor | 0.67 | ||||
| Yes | 3 (4.0%) | 4 (5.5%) | 7 (4.7%) | ||
| No | 72 (96.0%) | 69 (94.5%) | 142 (95.9%) | ||
| LDH Elevated | 0.48 | ||||
| Yes | 36 (48%) | 38 (73%) | 74 (50%) | ||
| BRAF | 0.71 | ||||
| Wildtype | 41 (77.4%) | 38 (56.7%) | 79 (74.5%) | ||
| Mutated | 12 (22.6%) | 15 (48.3%) | 27 (25.4%) | ||
| C-kit | 0.59 | ||||
| Wildtype | 9 (90.0%) | 10 (100%) | 19 (95.5%) | ||
| Mutated | 1 (10.0%) | 0 (0%) | 1 (5.0%) |
CPB (Carboplatin, Paclitaxel, Bevacizumab), CPBE (Carboplatin, Paclitaxel, Bevacizumab, Everolimus)
Progression Free Survival- Primary End Point
The primary endpoint for this trial was Progression Free Survival (PFS). Time to PFS was defined as the number of days between randomization until documentation of progression (or death). With an ITT analysis, there were 142 patients (71 were able to receive treatment in each arm). The Hazard Ratio was 1.17 (95% CI=0.84–1.65, p-value=0.35), which showed no difference in PFS between the two treatment arms (Figure 2). The median PFS times were 5.6 and 5.1 months for the CPB and CPBE arms, respectively.
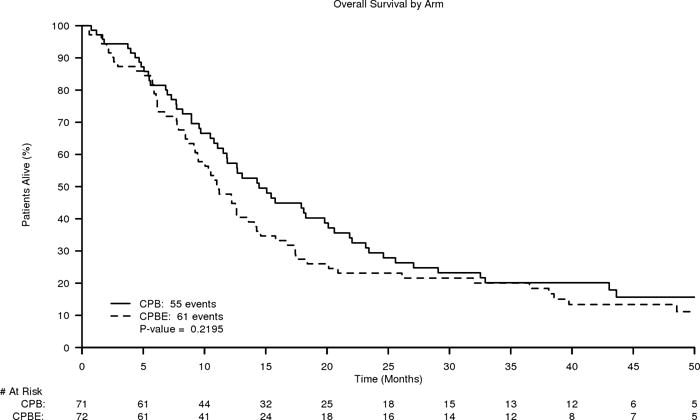
Figure 2.
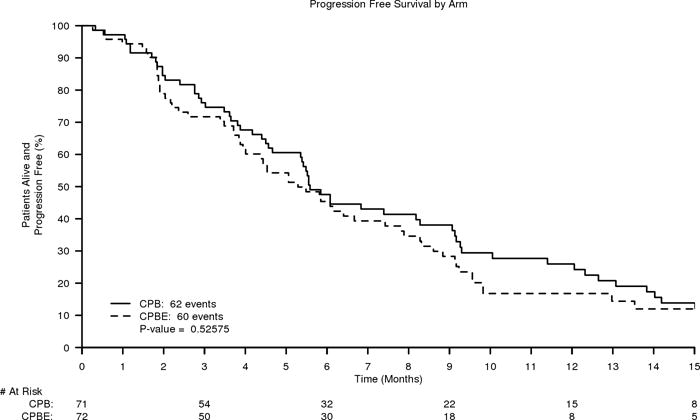
A. Kaplan-Meier curves of Progression Free Survival of CPB (carboplatin, paclitaxel, and bevacizumab) versus CPBE (carboplatin, paclitaxel, and bevacizumab plus everolimus)
B. Kaplan-Meier curves of Overall Survival of CPB (carboplatin, paclitaxel, and bevacizumab) versus CPBE (carboplatin, paclitaxel, and bevacizumab plus everolimus)
There was no significant difference in PFS for females and males. Females (n=52) had PFS 4.9 months and males (n=90) had PFS 5.6 months (p= 0.78). A large number of uveal melanoma patients were included in the study, potentially confounding the results in melanoma of cutaneous origin. Therefore a sensitivity analysis was performed excluding uveal mlenaoma patients for the prmary endpoint of PFS. Of 122 patients total, median times were 5.5 months and 5.4 months, respectively for the CPE and the CPBE arms (HR 1.12, 95% CI 0.77–1.62).
Secondary End points
For overall survival, there was no significant difference between the two treatment arms of the study. The median OS times were 14.5 and 11.2 months for arms CPB and CPBE, respectively, with HR=1.20 (CI= 0.83–1.73). See Figure 2.
In arm CPB, there were 10 confirmed responses with 2 complete and 8 partial responses. In CPBE, there was 1 complete response and 18 partial responses (p=0.22).
Drug Exposure
Overall, there was a trend towards dose reduction in the CPBE treatment rather than the CPB arm. There were less dose modifications after the addendum 5 dose modification when comparing the first 3 cycles of chemotherapy. Of 142 patients, 104 (73.2%) had at least 1 dose modification during their treatment. In arm CPB, 47/71 (66.2%) had a dose reduction compared to 57/71 (80.3%) in arm CPBE. This was not statistically significant (p- value= 0.06). IN the CPBE Arm, 18/20 (90.0%) patients had a dose reduction prior to the addendum, and 38/51 (76.5%) had a reduction after the addendum (p= 0.20). With only the first 3 rounds of chemotherapy, there was at least 1 dose modification 14/20 (70.0%) pre-addendum CPBE patients compared to 30/51 (58.8%) post addendum CPBE patients (p= 0.38). However, there was no difference in the pre and post addendum 5 on arm CPBE.
Safety Outcomes
Most common adverse events include neutropenia, leukopenia, thrombocytopenia, hypertension, fatigue, and anorexia. Grade 3+ hematologic adverse events were higher in the pre-addendum CPBE group when compared to the post-addendum group – pre-addendum arm 65.0% and post-addendum arm was 60.8%, respectively. CPBE pre Addendum 5 grade 3+ adverse event frequency was 85.0% and, despite the decrease in dose of everolimus, the post addendum frequency was 88.2%, possibly due to early dose reduction in the pre-addendum patients for hematologic toxicities. Additionally, toxicity was higher for CPBE post addendum versus CPB for Grade 3+ for all adverse events regardless of attribution. The Grade 4+ adverse event frequency for the CPBE arm post addendum was 23.5% (pre-addendum 15.0%) and CPB was 18.0%. See Table 2.
Table 2.
Adverse Events by Treatment Arm (Pre- and Post Addendum)
| Adverse Event (%) | CPB | CPBE | ||
|---|---|---|---|---|
| Pre-addendum 5 | Post-Addendum 5 | |||
| Neutropenia | ||||
| Grade 3+ | 28.2 | 50 | 51 | |
| Grade 4+ | 7 | 5 | 7.8 | |
|
| ||||
| Leukopenia | ||||
| Grade 3+ | 15.5 | 35 | 19.6 | |
| Grade 4+ | 1.4 | 0 | 2 | |
|
| ||||
| Anemia | ||||
| Grade 3+ | 9.9 | 10 | 5.9 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Thrombocytopenia | ||||
| Grade 3+ | 1.4 | 10 | 3.9 | |
| Grade 4+ | 2.8 | 0 | 5.9 | |
|
| ||||
| Fatigue | ||||
| Grade 3+ | 12.7 | 15 | 17.6 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Anorexia | ||||
| Grade 3+ | 2.8 | 10 | 5.9 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Hypertension | ||||
| Grade 3+ | 12.7 | 20 | 21.6 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Nausea | ||||
| Grade 3+ | 4.2 | 0 | 11.8 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Vomiting | ||||
| Grade 3+ | 2.8 | 0 | 5.9 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Thromboembolism | ||||
| Grade 3+ | 2.8 | 10 | 3.9 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Sensory Neuropathy | Grade 3+ | 2.8 | 0 | 2 |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Diarrhea | ||||
| Grade 3+ | 5.6 | 5 | 5.9 | |
| Grade 4+ | 0 | 0 | 2 | |
|
| ||||
| Generalized Muscle Weakness | ||||
| Grade 3+ | 2.8 | 5 | 3.9 | |
| Grade 4+ | 0 | 0 | 0 | |
|
| ||||
| Abdominal Pain | ||||
| Grade 3+ | 7 | 0 | 11.8 | |
| Grade 4+ | 0 | 0 | 0 | |
Uveal melanoma
There were 25 evaluable uveal melanoma patients in this study, which is relatively a large amount for this rarer disease. There were also 2 mucosal melanoma patients. The median PFS for the uveal/mucosal group in arm CPB was 5.6 months compared to 5.6 months in all other disease groups (p-value=0.68). In arm CPBE, the median PFS was 4.5 months for the uveal/mucosal patients compared to and 5.5 for patients with any other disease site (p-value=0.25). If the treatment arm is ignored, and only uveal melanoma included (N=25) then the median PFS is 5.4 months for uveal vs 5.5 for all others (p=0.53, Supplementary Figure 1). The was one partial response in the CPB arm among uveal melanoma patients (1/16, 6%). There were no responses in the CPBE arm ((0/10, 0%).
BRAF status Analysis
BRAF status was analyzed in 114 patients. We compared outcomes in each arm for patients with known BRAF mutant tumors vs known wildtype. There was no difference in PFS or OS in the CPE arm. (Supplementary Figure 2) However, for the CPBE arm, BRAF mutant patients had a superior PFS to wildtype patients (median 6.0 vs 3.9 months, p-value=0.039). There was not survival benefit however in the wildtype group (median survival 11.2 vs 9.5, p-value=0.793).
Extraordinary patients
There were 2 patients on the CPBE arm with exceptional outcomes. One patient, a 30 year old female with metastases to the lung, was on treatment for 35 cycles. The patient is still alive after 52.5 months of follow-up. The patient had received prior immunotherapy (GM-CSF) and prior radiation therapy. This patient had a confirmed CR before going off treatment due to disease progression. BRAF was not tested, and no tissue was available for analysis.
Another patient, a 61 year old female with liver metastases, was on treatment for 30 cycles. The patient is still alive after 41.4 months of follow-up. This patient was BRAF wildtype. She received prior immunotherapy (ipilimumab) and had a confirmed PR on CPBE before going off treatment due to disease progression.
Because of these notable outcomes, a subset analysis of patients who received ipilimumab or any immunotherapy prior to enrollment was performed. Unfortunately, it demonstrated no notable differences in outcome from other patients. (Data not shown).
Discussion
Our study showed no improvement in progression free survival with the addition of everolimus to carboplatin, paclitaxel, and bevacizumab. Reasons for this may include inactivity of the agent in combination and the inability to give full dose of everolimus due to toxicity, which was predominantly cytopenias. Other studies have also demonstrated the difficulty in maintaining dose strength of everolimus in combination with chemotherapies that cause cytopenias.22 The overall survival curve appears to trend toward a better outcome for CPB patients, although it does not reach statistical significance. The lack of meaningful difference in PFS between arms suggests that treatment did not notably impact this outcome, and therefore the underlying reason for any difference is uncertain, although the lower toxicity in the CPB arm may influence these curves.
While a negative study, there are some interesting findings in our analysis. First, two patients on the everolimus combination arm performed exceptionally well, receiving over 30 cycles of therapy. Both had received prior immunotherapy, with ipilimumab and GM-CSF, respectively. However, subset analysis of prior ipilimumab and immunotherapy exposure showed no difference in outcome compared with all other subjects. In addition, the overall progression-free survival exceeding 5 months in both arms suggests some anti-tumor activity of the carboplatin, paclitaxel, and bevacizumab combination. A prior randomized phase II of the combination revealed a progression free survival of 4.4 vs 2.7 (HR 0.52) months for carboplatin and paclitaxel alone, although the likely underpowered trial did not reach statistical significance.23
Of particular interest is the relatively large number of uveal melanoma patients. There were a relatively large number enrolled, likely due to other trial options and intercurrent approvals (Supplementary Figure 3) for cutaneous melanoma including BRAF inihibitors and immunotherapy, which likely also impacted the frequency of BRAF mutant patients enrolled. Uveal melanoma patients had similar efficacy outcomes to patient with melanoma of cutaneous origin, with PFS over 5 months regardless of arm (95% CI: 3.8–9.1 months). This is in distinction to the recent report of anti-PD1 therapy with a median PFS of 2.6 months (95% CI 2.4–2.8), with an overall survival of 7.6 months.24 Similarly, a phase III trial was launched for the MEK inhibitor selumetinib for uveal melanoma vs chemotherapy given an initial increase in median PFS from 7 to 16 weeks.25 However, the phase III was discontinued earlier this year due to failure to meet endpoints. In addition, a randomized phase II study of MEK inhibitor trametinib with and without Akt inhibitor GSK2141795 was negative for the combination, with median PFS below 16 weeks in both arms. A recent study of tumor-infiltrating lymphocytes (TIL) therapy in uveal melanoma reported a response rate over 30%, however, progression-free survival was not reported.26 Although comparisons between studies are difficult, the combination of carboplatin, paclitaxel, and bevacizumab appears a reasonable option for patients with uveal melanoma given the absence of activity of alternatives.
In our study, patients with BRAF mutations had improved PFS in the CPBE arm compared to CPB arm (6.0 vs 3.9 months respectively). There is some rationale as to BRAF mutant melanomas having an activated mTOR pathway, with the latter representing a resistance mechanism to targeted therapy. For instance, in an in vitro study, the combination of BRAF inhibitors plus mTOR inhibition in vitro enhanced cell growth inhibition while decreasing expression of proteins involved in mTOR activation, thus overcoming the resistance of mutated cells to BRAF inhibition.27 In brain tumors, mTOR activation has been shown to be increased in BRAF mutant tumors, as measured through expression of pS6.28 In a phase I clinical trial of vemurafenib and everolimus, 65% of patients with BRAF mutant advanced cancers responded to this combination chemotherapy, while also being safe and well-tolerated29, although this is comparable to response rates of vemurafenib alone. Therefore, our results suggesting an improved PFS with the addition of everolimus in the BRAF mutant patients are of interest, and merit further study, although the benefit appears mostly due to underperfomance of CPBE in the wildtype patients. (Supplementary Figure 2).
Further use of chemotherapy combinations for melanoma will likely decrease over time as immunotherapy options increase in efficacy. However, until all metastatic melanoma patients achieve durable responses with immunotherapy or targeted therapy, cytotoxic chemotherapy will likely remain an option for some refractory patients.
Conclusion
The addition of everolimus to carboplatin, paclitaxel, and bevacizumab increased toxicity, but did not increase efficacy for metastatic melanoma.
Supplementary Material
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 andU10CA180882 (to the Alliance for Clinical Trials in Oncology), CA025224, U10CA180790, UG1CA189825, and UG1CA189863. Also supported in part by funds from Novartis and Genentech/Roche. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Author Contributions:
Conceptualization: R McWilliams, S Markovic
Methodology: J Allred, R McWilliams
Formal analysis: J Allred
Investigation: R McWilliams, S Markovic
Data curation: J Allred
Writing – original draft: R McWilliams, J Allred, J Slostad
Writing – review and editing: R McWilliams, J Allred, J Slostad, R Katipamula, R Dronca, K Rumilla, L Erickson, A Bryce, R Joseph, L Kottschade, D King, J Leitch, S Markovic
Visualization: J Allred, R McWilliams, J Slostad
Supervision: S Markovic, R McWilliams
Project administration: R McWilliams
Funding acquisition: R McWilliams
Conflicts of Interest: There are no conflicts of interest directly related to the subject matter of the manuscript. Authors with disclosed unrelated conflicts include: McWilliams (Merrimack, Ipsen), Dr. Joseph (BMS, Merck, Incyte, Exelexis, Eisai)
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New England Journal of Medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of Clinical Oncology. 2015;33(25):2780–88. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. The New England journal of medicine. 2015;372(1):30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. The New England journal of medicine. 2014;371(20):1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. Journal of Clinical Oncology. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Soiffer RJ, Clark J, Finkelstein DM, Haluska FG. Phase II study of paclitaxel and carboplatin for malignant melanoma. American Journal of Clinical Oncology. 2002;25(3):283–6. doi: 10.1097/00000421-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Zimpfer-Rechner C, Hofmann U, Figl R, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG) Melanoma Research. 2003;13(5):531–6. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106(2):375–82. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 11.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a Phase III, Randomized, Placebo-Controlled Study of Sorafenib in Combination With Carboplatin and Paclitaxel As Second-Line Treatment in Patients With Unresectable Stage III or Stage IV Melanoma. J Clin Oncol. 2009;27(17):2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 12.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials [see comment] Journal of Clinical Oncology. 2008;26(4):527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 13.Guidelines NCCN. Melanoma. Available from URL: www.nccn.org [accessed December 18, 2016.
- 14.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115(1):119–27. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased Serum Concentration of Angiogenic Factors in Malignant Melanoma Patients Correlates With Tumor Progression and Survival. J Clin Oncol. 2001;19(2):577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 16.Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine Causes Transcriptional Up-Regulation of Interleukin 8 and Vascular Endothelial Growth Factor in Melanoma Cells: A Possible Escape Mechanism from Chemotherapy1. Molecular Cancer Therapeutics. 2003;2(8):753–63. [PubMed] [Google Scholar]
- 17.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–86. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 18.Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent Induction of Lymphangiogenesis, Angiogenesis, and Macrophage Recruitment by Vascular Endothelial Growth Factor-C in Melanoma. Am J Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 2007;71(8):771–77. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Kishimoto T, Kamata S, Otsuka M, Miyazaki M, Ishikura H. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. [see comment] Cancer Science. 2007;98(5):726–33. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma. Cancer. 2010;116(17):4122–29. doi: 10.1002/cncr.25320. [DOI] [PubMed] [Google Scholar]
- 22.Costello BA, Borad MJ, Qi Y, et al. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Investigational new drugs. 2014;32(4):710–6. doi: 10.1007/s10637-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: A Randomized Phase II Study Evaluating the Activity of Bevacizumab in Combination With Carboplatin Plus Paclitaxel in Patients With Previously Untreated Advanced Melanoma. Journal of Clinical Oncology. 2012;30(1):34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–53. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvajal RD, Sosman JA, Quevedo J, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA. 2014;311(23):2397–405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandran SS, Somerville RPT, Yang JC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. The Lancet Oncology. 2017;18(6):792–802. doi: 10.1016/S1470-2045(17)30251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by <em>NRAS</em> or <em>MEK</em> Mutations. Molecular Cancer Therapeutics. 2012;11(4):909–20. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 28.Prabowo AS, Iyer AM, Veersema TJ, et al. BRAF V600E Mutation Is Associated with mTOR Signaling Activation in Glioneuronal Tumors. Brain Pathology. 2014;24(1):52–66. doi: 10.1111/bpa.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen S, Khawaja MR-u-H, Khatua S, et al. Co-targeting BRAF with mTOR inhibition in solid tumors harboring BRAF mutations: A phase I study. Journal of Clinical Oncology. 2016;34(15_suppl):2517–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


