Abstract
Purpose
Prostate cancer patients receiving androgen deprivation therapy (ADT) are at risk of sleep disturbance; however, the mechanisms by which ADT may affect sleep are not well understood. This study compared objective and subjective sleep disturbance in ADT recipients and controls and examined whether sleep disturbance in ADT recipients is attributable to the influence of ADT on hot flashes and nocturia.
Methods
Prostate cancer patients were assessed before or within one month after starting ADT as well as 6 months and 12 months later (n=78). Prostate cancer were treated with prostatectomy only (n=99) and men with no history of cancer (n=108) were assessed at similar intervals. Participants self-reported their sleep disturbance (Insomnia Severity Index) and interference from hot flashes (Hot Flash Related Daily Interference Scale). One hundred participants also wore actigraphs for 3 days at the 6-month assessment to measure objective sleep disturbance and reported their nocturia frequency.
Results
ADT recipients reported worse sleep disturbance, higher rates of clinically significant sleep disturbance, and greater hot flash interference than controls (ps≤.03). In cross-sectional analyses among those with actigraphy data, ADT recipients had greater objective sleep disturbance and more episodes of nocturia (ps<.01). Cross-sectional mediation analyses showed that the association between ADT and objectively- and subjectively-measured sleep disturbance was partly attributable to nocturia and hot flashes (ps<.05).
Conclusions
Results suggest that the association between ADT and sleep may be partly explained by nocturia and hot flash interference. Future studies should examine behavioral and pharmacologic interventions to address these symptoms among ADT recipients.
Keywords: prostatic neoplasms, antiandrogens, sleep, hot flashes, nocturia
Introduction
Numerous studies have shown that androgen deprivation therapy (ADT) for prostate cancer results in side effects such as hot flashes,1 muscle loss,2 fatigue,3 depression,4, 5 and cognitive impairment.6, 7 Less research has focused on the effects of ADT on sleep; however, available data suggest that sleep is a problem in this population. A recent study found that prostate cancer patients treated with ADT reported significantly worse sleep disturbance throughout the treatment course, although no significant worsening over time of sleep disturbance.8 Moreover, night sweats were associated with significantly worse sleep disturbance among ADT recipients in this study.8 Another study found that symptoms of testosterone deprivation (e.g., hot flashes, excessive perspiration) to be a significant risk factor for sleep disturbance.9 Similarly, a longitudinal study reported that sleep disturbance increased significantly after three months of ADT.10 Only one study could be found that compared ADT recipients to prostate cancer patients who did not receive ADT. This study measured self-reported sleep disturbance before starting ADT and during a 16-month follow-up period.11 ADT recipients reported significant increases in sleep disturbance in the first 6 months after starting ADT. Although not statistically significant, the rates of clinically significant sleep disturbance approximately doubled over that period. The impact of ADT on sleep disturbance was partly attributable to increases in hot flashes and urinary frequency. These results are consistent with studies of breast cancer patients in which sleep disturbance is associated with hot flashes.12–14 Nevertheless, studies of sleep in ADT recipients have been characterized by one or more methodologic limitations, including small sample sizes, reliance on only subjective measurement of sleep disturbance, the absence of a comparison group of men without cancer, and use of cross-sectional designs.
The goal of the current study was to compare objective and subjective sleep disturbance in prostate cancer patients receiving ADT compared to prostate cancer patients previously treated with surgery only as well as men without a cancer history. In addition, we examined the contribution of common side effects of prostate cancer treatment (i.e., nocturia and hot flashes) to sleep disturbance. We hypothesized that ADT recipients would demonstrate worse sleep disturbance relative to controls. We also hypothesized that ADT would be associated with worse nocturia and hot flashes and that the association between ADT and sleep disturbance would be partly attributable to the influence of ADT on nocturia and hot flashes.
Materials and Methods
Participants
This study constitutes secondary analyses of data from participants who were recruited as part of a longitudinal study of quality of life in prostate cancer patients treated with ADT. Details of the eligibility criteria, matching criteria, and study procedures are provided elsewhere.7 Briefly, all participants were required to: be ≥ 18 years old, be able to speak and read English, have attained at least a sixth grade education, have no history of stroke, and have not demonstrated impaired mental status based on screening with the Short Portable Mental Status Questionnaire (score ≤ 3). ADT recipients were also required to be scheduled to start ADT or have started ADT within the previous month for nonmetastatic or asymptomatic metastatic prostate cancer, be scheduled to receive ADT for ≥ 6 months, have not received treatment for any other cancers in the previous 12 months, have no history of brain cancers or previous cranial irradiation, and have no history of ADT in the previous 12 months or antiandrogen treatment in the previous 6 months. Prostate cancer patients not treated with ADT were also required to be diagnosed with nonmetastatic prostate cancer, have no history of other cancers except nonmelanoma skin cancer, have undergone only a prostatectomy for prostate cancer, have no history of recurrence since their prostatectomy, and not be receiving testosterone supplementation. Men with no history of prostate cancer were also required to have no history of cancer except nonmelanoma skin cancer and not be receiving testosterone supplementation.
ADT recipients were matched to prostate cancer patients not receiving ADT on time since diagnosis. ADT recipients were also matched to prostate cancer patients not receiving ADT as well as to men with no history of cancer based on age (within 5 years) and educational level (≤ 12 years, 13 – 16 years, ≥ 17 years).
Procedure
Assessments were completed by ADT recipients before or within one month of starting ADT as well as 6 and 12 months later. Matched controls completed assessments at similar intervals. Participants were asked to complete self-report questionnaires at each assessment. Actigraphy was added to the study later; participants were asked to wear actigraphs for 3 consecutive days at the time of the 6-month assessment. The current analyses are limited to those participants who provided subjective sleep disturbance data during the 12-month study (N = 285) and/or actigraphy data at the 6-month assessment (n = 100).
Measures
Age and race were assessed at baseline via self-report. Medical comorbidities were assessed using a self-report version of the Charlson Comorbidities Index.15 Baseline Gleason score, height, and weight were assessed via medical chart review. Baseline body mass index (BMI) was calculated using standard scoring.16
Subjective sleep disturbance was assessed in the full sample using the Insomnia Severity Index,17 a valid and reliable measure that is widely used in cancer research.18, 19 Unlike objective measures of sleep disturbance, this scale assesses subjective difficulty respondents have in falling asleep and staying asleep and the impact that their sleep disturbance has on their daily functioning. Scores range from 0 – 28, and scores ≥ 8 indicate clinically significant sleep disturbance.18
Hot flash interference was assessed in the full sample using the Hot Flash Related Daily Interference Scale,20 a 10-item measure that asks respondents to report the interference on several aspects of functioning associated with hot flashes. This valid and reliable self-report measure is widely used in prostate cancer research.1, 20–22 Scores range from 0 – 100 with higher scores indicating greater interference.
Participants wore Actiwatch Score actigraphs (Philips Respironics, Bend, OR) on their non-dominant wrist for 3 days. Actigraphy has been deemed reliable and valid for objective assessment of sleep.23 Participants were asked to record, upon awakening each morning, the previous night’s bed time, rising time, and number of times they urinated overnight during the three-day actigraphy monitoring period. Actigraphy data was used in concert with participants’ self-reported bedtime and rising time to calculate objective sleep data. Objective sleep variables included sleep efficiency, sleep onset latency, and sleep duration. Sleep efficiency is the percentage of time spent trying to sleep that is spent asleep. This value ranges from 0 – 100% with lower scores indicating greater sleep disturbance. Sleep onset latency is the amount of time patients spend trying to fall asleep and wake after sleep onset measures the amount of time spent awake after initially falling asleep at night. Higher values indicate greater sleep disturbance. Sleep duration is the amount of time spent asleep at night. Actigraphic data were scored automatically using Actiware (Philips Respironics, Bend, OR) by a trained study coordinator after establishing rest periods and non-wear time using participants’ sleep diary data.
Nocturia was assessed in daily diaries participants completed each morning upon awakening. Participants indicated the number of times they urinated during the overnight period. The number of nocturia episodes was retained as a continuous variable for analyses.
Statistical Analyses
First, descriptive statistics were calculated for demographic factors, Gleason scores, nocturia, hot flash interference, and sleep disturbance. Next, t-tests, χ2 tests, and Fisher’s exact tests were conducted to identify sociodemographic and clinical factors that differed between groups. Main effects of group as well as group-by-time interactions (i.e., group differences in change over time) in subjective sleep disturbance and hot flash interference were examined in the full sample using mixed models with PROC MIXED in SAS version 9.4 (SAS Institute, Cary, NC). Change over time in rates of clinically significant sleep disturbance was examined in the full sample using generalized estimating equation (GEE) analyses using PROC GENMOD in SAS version 9.4. Mixed models and GEE analyses allow for use of all available data at each assessment without imputing any missing data.24, 25 For these analyses, group by time interactions were examined to compare groups on change over time in outcome variables. Simple effects were examined to compare groups on outcomes at each assessment.
All analyses using objective sleep data from actigraphy and nocturia were conducted with the restricted sample of participants with those data available. In this restricted sample, ANCOVA analyses were conducted to compare ADT recipients to controls on objective sleep disturbance and nocturia while controlling for any demographic factors that differed between groups at p < .10. Lastly, two separate indirect bootstrapping analyses of multiple mediation were conducted using the PROCESS macro26 version 2.16 to determine whether nocturia or hot flash interference mediated the association between ADT and those sleep disturbance variables that differed significantly between ADT recipients and controls at p < .05. These multiple mediation analyses provide an estimate of the indirect effect of the mediator as well as a 95% confidence interval. Indirect effects whose confidence intervals do not include 0 are deemed statistically significant at α = .05. Multiple mediation analyses were conducted in the subsample with actigraphy data using data from the 6-month assessment.
Results
Sociodemographic and clinical characteristics are presented in Table 1.A ADT recipients reported more medical comorbidities and had higher Gleason scores than the prostate cancer patients not receiving ADT, were less educated than either control group, were less likely to be White than controls (ps ≤ .04). Thus, medical comorbidities score, education, and race were included as covariates in all multivariate analyses. Gleason score was not entered as a covariate because ADT is typically prescribed for more advanced disease.
Table 1.
Demographic and Clinical Characteristics of the Sample
| ADT+ (n = 78) | ADT− (n = 99) | CA− (n = 108) | ADT+ vs. ADT− | ADT+ vs. CA− | ADT+ vs. All Controls | |
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Mean (SD) | Mean (SD) | Mean (SD) | t (p) | t (p) | t (p) |
|
| ||||||
| Age, years | 68.49 (8.52) | 67.71 (7.46) | 67.80 (11.93) | 0.65 (.52) | 0.46 (.65) | 0.57 (.57) |
|
| ||||||
| Years since diagnosis | 4.01 (5.08) | 4.69 (4.53) | - | −0.91 (.37) | - | - |
|
| ||||||
| Body mass index, kg/m2 | 28.29 (4.04) | 29.30 (4.12) | 29.65 (5.19) | −1.42 (.16) | −1.73 (.09) | −1.70 (.09) |
|
| ||||||
| Comorbidity index score | 2.86 (1.11) | 2.38 (0.80) | 2.68 (1.06) | 3.17 (.002) | 1.13 (.26) | 2.43 (.02) |
|
| ||||||
| Characteristic | No. (%) | No. (%) | No. (%) | χ2 (p) | χ2 (p) | χ2 (p) |
|
| ||||||
| Education, years | 7.32 (.03) | 8.47 (.01) | 10.69 (.005) | |||
| ≤ 12 | 32 (41%) | 22 (22%) | 23 (21%) | |||
| 13 – 16 | 36 (46%) | 59 (60%) | 67 (62%) | |||
| ≥ 17 | 10 (13%) | 18 (18%) | 18 (17%) | |||
|
| ||||||
| Race | 2.42 (.12) | 4.10 (.04) | 4.68 (.03) | |||
| White | 68 (87%) | 93 (94%) | 103 (95%) | |||
| Nonwhite | 10 (13%) | 6 (6%) | 5 (5%) | |||
|
| ||||||
| Ethnicity | (.99) | (.53) | (.99) | |||
| Non-Hispanic | 74 (95%) | 95 (96%) | 101 (94%) | |||
| Hispanic | 3 (4%) | 3 (3%) | 7 (6%) | |||
| Missing | 1 (1%) | 1 (1%) | 0 (0%) | |||
|
| ||||||
| Gleason score | (< .001) | - | - | |||
| 4–6 | 12 (15%) | 31 (31%) | - | |||
| 7 | 17 (22%) | 26 (26%) | - | |||
| 8 | 18 (23%) | 2 (2%) | - | |||
| 9–10 | 8 (10%) | 0 (0%) | - | |||
| Missing | 23 (30%) | 40 (40%) | - | |||
Note. ADT+ = prostate cancer patients treated with ADT; ADT− = prostate cancer patients not treated with ADT; CA− = men with no history of prostate cancer; SD = standard deviation. p values calculated using t-tests for continuous variables and χ2 tests or Fisher’s exact tests for categorical variables. Missing values were excluded from analyses. In analyses with cell sizes ≤ 5, p values were calculated using Fisher’s exact test which yields no test statistic.
Consistent with previous studies,1, 7 preliminary analyses were conducted to determine whether the prostate cancer patients not receiving ADT and the men with no history of prostate cancer could be combined into a single control group. The control groups did not differ on subjective sleep disturbance (i.e., Insomnia Severity Index scores) or hot flash interference at any assessment (ps ≥ .18) or on change in these variables over time (ps ≥ .61). Similarly, in the subset of participants with actigraphy data, the two control groups did not differ on objective sleep parameters or nocturia (ps ≥ .37; see Supplementary Table 1). Thus, these two control groups were combined into a single group to improve statistical power and reduce the number of statistical tests to be performed.
Differences in Sleep Disturbance
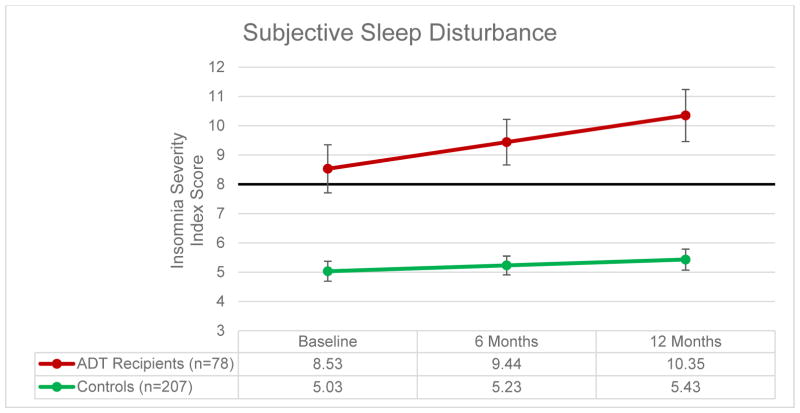
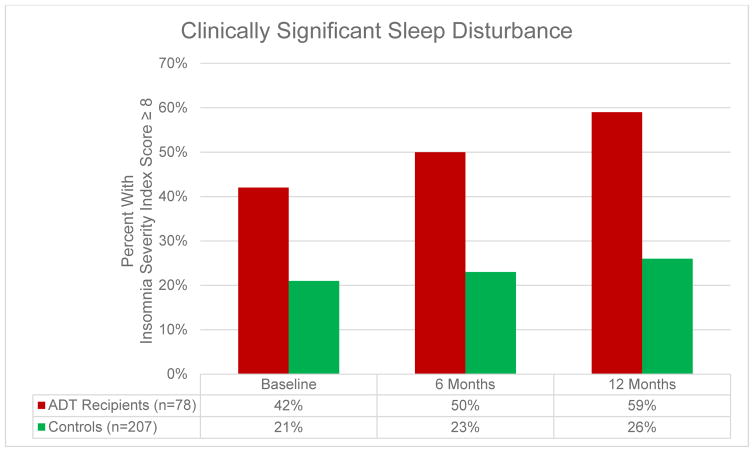
Figures 1 and 2 present data on self-reported sleep disturbance and rates of clinically-significant sleep disturbance at each assessment. Longitudinal mixed model analyses controlling for comorbidities, education, and race demonstrated that ADT recipients reported worse subjective sleep disturbance over time relative to controls (F = 4.48, p = .03) and had worse subjective sleep disturbance at each assessment (Fs ≥ 17.82, ps < .001). ADT recipients also demonstrated a trend towards higher rates of clinically significant sleep disturbance over time relative to controls (Z = −1.64, p = .10), and ADT recipients were more likely to report clinically significant sleep disturbance than controls (Zs ≥ −3.17, ps ≤ .002; see Figure 2). As reported previously,1 ADT recipients reported greater hot flash interference over time relative to controls (F = 68.14, p < .001).
Figure 1. Subjective Sleep Disturbance.
Note: Bold line indicates cutoff score for clinically significant sleep disturbance.
Figure 2.
Rates of Clinically Significant Sleep Disturbance
Table 2 presents covariate-adjusted group means for objective sleep disturbance and nocturia among participants with actigraphy data at 6 months. Unadjusted group means are presented in Supplementary Table 2. After controlling for medical comorbidities, education, and race, ADT recipients experienced significantly worse wake after sleep onset and nocturia (ps < .01). No group differences were observed in sleep efficiency, sleep onset latency, or duration of sleep (ps ≥ .27).
Table 2.
Covariate-Adjusted Group Means for Objective Sleep Outcomes and Nocturia at 6 Months After Starting ADT (n = 100)
| ADT+ | Controls | ADT+ vs. Controls | ||
|---|---|---|---|---|
| M (SE) | M (SE) | F | p | |
| Sleep Efficiency | 73.22 (2.20) | 74.72 (2.17) | 0.45 | .50 |
| Wake After Sleep Onset | 65.24 (6.15) | 47.28 (6.08) | 8.30 | < .01 |
| Sleep Onset Latency | 45.43 (7.75) | 47.10 (7.66) | 0.05 | .83 |
| Sleep Duration | 417.29 (17.66) | 397.42 (17.47) | 1.23 | .27 |
| Nocturia | 3.28 (0.34) | 1.89 (0.33) | 16.77 | < .001 |
Note. ADT+ = prostate cancer patients treated with ADT; SE = standard error; F = F-statistic for the main effect of group. Covariate-adjusted means, SE values, and p values controlling for baseline medical comorbidities, education, and race were calculated using ANCOVA analyses.
Potential Mechanisms of The Association Between ADT and Subjective and Objective Sleep Disturbance
Multiple mediation analyses were conducted to determine whether nocturia and hot flashes mediated the association between receipt of ADT and greater subjective and objective sleep disturbance among those with actigraphy data (see Table 3). Nocturia significantly mediated the association between receipt of ADT with wake after sleep onset (p < .05) but not subjective sleep disturbance (p > .05) after controlling for medical comorbidities, education, and race. Hot flash interference significantly mediated the association between receipt of ADT with subjective sleep disturbance (p < .05) but not wake after sleep onset (p > .05).B
Table 3.
Results of Multiple Mediator Models Testing the Mediation Effects of Nocturia and Hot Flash Interference on Relationship Between ADT and Sleep Disturbance
| Outcome | Statistic (95% CI) | Mediator | |
|---|---|---|---|
| Nocturia | Hot Flash Interference | ||
| Wake After Sleep Onset | a paths | −1.72* (−2.44, −1.00) | −14.18* (−18.92, −9.44) |
| b paths | 8.43* (5.34, 11.51) | 0.01 (−0.46, 0.48) | |
| c′ path | −6.46 (−20.00, 7.07) | ||
| Indirect Effect | −14.50* (−26.17, −5.24) | −0.19 (−8.32, 6.53) | |
| Subjective Sleep Disturbance | a paths | −1.72* (−2.44, −1.00) | −14.18* (−18.92, −9.44) |
| b paths | 0.41 (−0.38, 1.20) | 0.22* (0.10, 0.34) | |
| c′ path | −2.51 (−5.96, 0.95) | ||
| Indirect Effect | −0.70 (−2.73, 1.07) | −3.07* (−5.47, −1.16) | |
Note. a path = associations between predictor (ADT vs. control) and mediator; b path = associations between mediator and outcome; c′ path = association between predictor and outcome that is independent of the influence of the mediator; CI = confidence interval.
p less than .05.
All estimates and CIs calculated using PROCESS macro version 2.16 with 5,000 bootstrap samples. Group membership was coded as ADT recipients = 1, Control group participants = 2. Negative indirect effects suggest that the association between receipt of ADT and worse sleep disturbance was mediated by the association between receipt of ADT and worse nocturia or hot flash interference. All analyses controlled for group differences in medical comorbidities, education, and race.
Discussion
This is the first study to our knowledge to compare objectively- and subjectively-measured sleep disturbance between men receiving ADT for prostate cancer and controls. Two major findings warrant discussion. First, we found that, after controlling for relevant covariates, ADT recipients spent more time awake after the onset of sleep and reported worse subjective sleep disturbance than matched controls, more episodes of nocturia, and greater hot flash interference. On average, ADT recipients began with subjective sleep disturbance that was above the cutoff for clinical significance which increased significantly over time relative to controls. ADT recipients were also more likely to report clinically significant sleep disturbance throughout the 12-month study period. This may be attributable to distress in men with the more advanced disease associated for which they were prescribed ADT.4 Nonetheless, the finding that subjective sleep disturbance significantly worsened over time relative to controls remains noteworthy. ADT recipients spent more time awake in bed while trying to sleep, reported more nocturia, and reported worse hot flash interference than men not receiving ADT. Men receiving ADT also spent more time awake in bed while trying to sleep. Findings regarding subjective sleep disturbance are consistent with those of previous studies that reported receipt of ADT is associated with worse sleep disturbance, nocturia, and hot flashes.1, 27 The finding that men receiving ADT also demonstrate worse objective wake after sleep onset is new.
The second major finding was that worse sleep disturbance experienced by ADT recipients may be partly attributable to worse nocturia and hot flashes after receipt of ADT. Specifically, we found that nocturia mediated the association between ADT and objective sleep disturbance. In addition, hot flash interference mediated the association between ADT and subjective sleep disturbance, which accounts for daytime dysfunction unlike objective measures. These findings may help explain why ADT recipients demonstrated worse wake after sleep onset but not worse sleep efficiency, sleep onset latency, or sleep duration. Consistent with previous research on this topic, nocturia28, 29 and vasomotor symptoms28 may have caused overnight awakenings after the initial onset of sleep rather than difficulty falling asleep or staying asleep. Animal models and human studies have shown that testosterone, which is drastically reduced among ADT recipients, may play a role in regulating levels of vasopressin, a key regulator of water reabsorption in the kidneys.30, 31 This study is among the first to examine the contribution of hot flashes and nocturia to sleep disturbance in men with cancer. However, these findings extend previous research demonstrating the relationship between hot flashes and sleep disturbance conducted among breast cancer survivor populations,13, 14, 32, 33 including research objectively measuring both sleep disturbance and hot flashes.34 These findings may be helpful to clinicians treating ADT recipients who complain of sleep disturbance. Because many hypnotic medications can be habit-forming, some ADT recipients may prefer treatments that reduce hot flashes or nocturia to improve sleep among prostate cancer patients treated with ADT. For example, some selective serotonin and serotonin-norepinephrine reuptake inhibitors35, 36 have shown promise in potentially reducing hot flashes among men with prostate cancer. Desmopressin, an anti-diuretic, has shown promise in reducing nocturia among older men without cancer;37 however, this drug is indicated for nocturia in adults who awaken at least twice per night to void due to nocturnal polyuria and may not apply to some of the cancer patients described here.38 In addition, cognitive-behavioral therapy for insomnia has been shown to improve sleep among cancer populations39 and may also improve vasomotor symptoms.40 Future research should examine whether treatments to address these potential mechanisms of sleep disturbance among ADT recipients may improve sleep. This study did not examine psychosocial factors that have been shown to be exacerbated by ADT as potential mediators for the association between ADT and sleep disturbance. Future studies should examine depression, anxiety, and other psychosocial factors that could contribute to better understanding of why ADT is associated with worse sleep disturbance.
Strengths of this study include a longitudinal design, use of control groups, and objective as well as subjective measurement of sleep disturbance. Some limitations of this study should also be noted. Although this study was the largest to date to examine the association between ADT and sleep disturbance, the sample size remains relatively small. The sample was homogeneous with respect to race, which limits the generalizability of these findings. Some ADT recipients had already begun ADT up to one month before their baseline assessment. Actigraphic data were calculated automatically and by a single trained study coordinator, rather than by consensus between two individuals, potentially introducing some error to actigraphic data. Because actigraphic data were only available at 6 months, we were unable to control for pre-treatment objective sleep disturbance in the analyses of objective sleep disturbance at 6 months. Future studies should incorporate longer follow-up periods in order to examine whether and when sleep disturbance recovers for ADT recipients. Future studies should also measure objective sleep for longer than the three-day period used in this study. The cross-sectional nature of the mediation analyses conducted in this study preclude any causal inference. It is also possible that participants with worse sleep disturbance were more likely to be awake to experience and recall episodes of nocturia and hot flashes. The definition of nocturia used in this study, needing to urinate during the overnight period, was used as a proxy for the accepted definition of nocturia (waking to urinate41). It is possible that episodes of nocturia referred to in this study may not have required participants to wake to urinate. Future studies should ask specifically about episodes in for which participants had to wake or employ ecological momentary assessment validated against objective sleep measurement. Nocturia is also associated with racial/ethnic background
In conclusion, ADT recipients experienced significantly worse nocturia, hot flash interference, objectively-measured wake time after sleep onset, and subjective sleep disturbance severity in the 6 months after starting ADT. Also, greater sleep disturbance was partly attributable to increases in nocturia and hot flashes after starting ADT. Future studies should explore pharmacologic and behavioral interventions that address these potential mechanisms by which sleep is worsened in ADT recipients.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by grants from the National Cancer Institute: K01 CA211789 (PI: Gonzalez) and R01 CA132803 (PI: Jacobsen). This work was also supported in part by the Survey Methods Core at Moffitt Cancer Center (P30 CA076292; PI: Sellers).
Footnotes
Eleven ADT recipients were assessed on average 2–28 days after ADT (M=9). Twenty-eight ADT recipients received radiotherapy concurrently with ADT. The significance of group comparisons examining differences in subjective sleep disturbance, objective sleep disturbance, nocturia, and hot flashes did not change after excluding either the ADT recipients who were assessed after starting ADT or those who received concurrent radiotherapy. Also, the significance of mediation analyses did not change when excluding either group of participants. The 20 ADT recipients discontinuing ADT before 12 months did not differ from those continuing to receive ADT at 12 months on any mediator or outcome variable at 6 or 12 months (ps ≥ .09).
All relevant analyses were re-run after removing the sleep item in the Hot Flash Related Daily Interference Scale from the total score, yielding similar results as those reported here.
The authors have no conflicts of interest to disclose.
Author Contributions: Brian D. Gonzalez: conceptualization, methodology, formal analysis, data curation, data collection, writing original draft, review and editing, and visualization. Brent J. Small: methodology and review and editing. Mallory G. Cases: data collection, data curation, and review and editing. Noelle L. Williams: review and editing. Mayer N. Fishman: provision of study participants and review and editing. Paul B. Jacobsen: methodology, review and editing, supervision, project administration, and funding acquisition. Heather S.L. Jim: conceptualization, methodology, review and editing, supervision, project administration, and funding acquisition.
References Cited
- 1.Gonzalez BD, Jim HS, Donovan KA, et al. Course and moderators of hot flash interference during androgen deprivation therapy for prostate cancer: a matched comparison. The Journal of urology. 2015;194:690–695. doi: 10.1016/j.juro.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvao D, Taaffe D, Spry N, Joseph D, Turner D, Newton R. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer and Prostatic Diseases. 2009;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 3.Nelson AM, Gonzalez BD, Jim HS, et al. Characteristics and predictors of fatigue among men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Supportive Care in Cancer. 2016;24:4159–4166. doi: 10.1007/s00520-016-3241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psycho-Oncology. 2015;24:472–477. doi: 10.1002/pon.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh KT, Reznor G, Muralidhar V, et al. Association of androgen deprivation therapy with depression in localized prostate cancer. Journal of Clinical Oncology. 2016;34:1905–1912. doi: 10.1200/JCO.2015.64.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGinty HL, Phillips KM, Jim HS, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Supportive Care in Cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez BD, Jim HS, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. Journal of Clinical Oncology. 2015;33:2021–2027. doi: 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savard J, Ivers H, Savard MH, Morin CM. Cancer treatments and their side effects are associated with aggravation of insomnia: Results of a longitudinal study. Cancer. 2015;121:1703–1711. doi: 10.1002/cncr.29244. [DOI] [PubMed] [Google Scholar]
- 9.Savard J, Simard S, Hervouet S, Ivers H, Lacombe L, Fradet Y. Insomnia in men treated with radical prostatectomy for prostate cancer. Psychooncology. 2005;14:147–156. doi: 10.1002/pon.830. [DOI] [PubMed] [Google Scholar]
- 10.Stephens RJ, Dearnaley DP, Cowan R, et al. The quality of life of men with locally advanced prostate cancer during neoadjuvant hormone therapy: data from the Medical Research Council RT01 trial (ISRCTN 47772397) BJU Int. 2007;99:301–310. doi: 10.1111/j.1464-410X.2006.06560.x. [DOI] [PubMed] [Google Scholar]
- 11.Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology. 2013;22:1381–1388. doi: 10.1002/pon.3150. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 13.Bardwell WA, Profant J, Casden DR, et al. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psychooncology. 2008;17:9–18. doi: 10.1002/pon.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savard J, Davidson JR, Ivers H, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004;27:513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. Journal of chronic diseases. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. SLEEP. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 19.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. Journal of Clinical Oncology. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. Journal of pain and symptom management. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 21.Beer TM, Benavides M, Emmons SL, et al. Acupuncture for hot flashes in patients with prostate cancer. Urology. 2010;76:1182–1188. doi: 10.1016/j.urology.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulloa EW, Salup R, Patterson SG, Jacobsen PB. Relationship between hot flashes and distress in men receiving androgen deprivation therapy for prostate cancer. Psycho-Oncology. 2009;18:598–605. doi: 10.1002/pon.1427. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. SLEEP. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 24.Diggle P. Analysis of Longitudinal Data. 2. Oxford University Press; 2002. [Google Scholar]
- 25.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. Doing data analysis with the multilevel model for change; pp. 75–123. [Google Scholar]
- 26.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- 27.Gay HA, Michalski JM, Hamstra DA, et al. Neoadjuvant androgen deprivation therapy leads to immediate impairment of vitality/hormonal and sexual quality of life: Results of a multicenter prospective study. Urology. 2013;82:1363–1369. doi: 10.1016/j.urology.2013.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanisch LJ, Gooneratne NS, Soin K, Gehrman PR, Vaughn DJ, Coyne JC. Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur J Cancer Care (Engl) 2011;20:549–554. doi: 10.1111/j.1365-2354.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feigenberg SJ, Hanlon AL, Horwitz EM, Uzzo RG, Eisenberg D, Pollack A. Long-term androgen deprivation increases Grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. International Journal of Radiation Oncology Biology Physics. 2005;62:397–405. doi: 10.1016/j.ijrobp.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Pavo I, Varga C, Szücs M, et al. Effects of testosterone on the rat renal medullary vasopressin receptor concentration and the antidiuretic response. Life sciences. 1995;56:1215–1222. doi: 10.1016/0024-3205(95)00061-a. [DOI] [PubMed] [Google Scholar]
- 31.Liao C-H, Chiang H-S, Yu H-J. Serum testosterone levels significantly correlate with nocturia in men aged 40–79 years. Urology. 2011;78:631–635. doi: 10.1016/j.urology.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31:591–5598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]
- 33.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 34.Savard MH, Savard J, Caplette-Gingras A, Ivers H, Bastien C. Relationship between objectively recorded hot flashes and sleep disturbances among breast cancer patients: investigating hot flash characteristics other than frequency. Menopause. 2013;20:997–1005. doi: 10.1097/GME.0b013e3182885e31. [DOI] [PubMed] [Google Scholar]
- 35.Naoe M, Ogawa Y, Shichijo T, Fuji K, Fukagai T, Yoshida H. Pilot evaluation of selective serotonin reuptake inhibitor antidepressants in hot flash patients under androgen-deprivation therapy for prostate cancer. Prostate Cancer and Prostatic Diseases. 2006;9:275–278. doi: 10.1038/sj.pcan.4500891. [DOI] [PubMed] [Google Scholar]
- 36.Irani J, Salomon L, Oba R, Bouchard P, Mottet N. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial. The lancet oncology. 2010;11:147–154. doi: 10.1016/S1470-2045(09)70338-9. [DOI] [PubMed] [Google Scholar]
- 37.Weiss JP, Blaivas JG, Bliwise DL, et al. The evaluation and treatment of nocturia: a consensus statement. BJU International. 2011;108:6–21. doi: 10.1111/j.1464-410X.2011.10175.x. [DOI] [PubMed] [Google Scholar]
- 38.Fralick M, Kesselheim AS. FDA Approval of Desmopressin for Nocturia. JAMA. 2017;317:2059–2060. doi: 10.1001/jama.2017.4316. [DOI] [PubMed] [Google Scholar]
- 39.Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–1124. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savard M-H, Savard J, Trudel-Fitzgerald C, Ivers H, Quesnel C. Changes in self-reported hot flashes and their association with concurrent changes in insomnia symptoms among women with breast cancer. Menopause. 2011;18:985–993. doi: 10.1097/gme.0b013e31820db6a1. [DOI] [PubMed] [Google Scholar]
- 41.van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardisation of terminology in nocturia: Report from the Standardisation Sub-Committee of the International Continence Society. Neurourology and urodynamics. 2002;21:179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.