Abstract
Background and Purpose
Serum hepatocyte growth factor (HGF) is positively associated with poor prognosis of heart failure and myocardial infarction, and it can also predict the risk of ischemic stroke in population. The goal of this study was to investigate the association between serum HGF and prognosis of ischemic stroke.
Methods
A total of 3027 acute ischemic stroke patients were included in this post hoc analysis of the China Antihypertensive Trial in Acute Ischemic Stroke. The primary outcome was composite outcome of death or major disability (modified Rankin Scale score ≥3) within 3 months.
Results
After multivariate adjustment, elevated HGF levels were associated with an increased risk of primary outcome [odds ratio, 1.50; 95% confidence interval (CI), 1.10–2.03; Ptrend=0.015] when two extreme quartiles were compared. Each standard deviation increase of log-transformed HGF was associated with 14% (95% CI: 2–27%) increased risk of primary outcome. Adding HGF quartiles to a model containing conventional risk factors improved the predictive power for primary outcome (net reclassification improvement: 17.50%, P<0.001; integrated discrimination index: 0.23%, P=0.022). The association between serum HGF and primary outcome could be modified by heparin pre-treatment (Pinteraction=0.001) and a positive linear dose-response relationship between HGF and primary outcome was observed in patients without heparin pre-treatment (Plinearity<0.001) but not in those with heparin pre-treatment.
Conclusions
Serum HGF levels were higher in the more severe stroke at baseline, and elevated HGF levels was probably associated with 3-month poor prognosis independently of stroke severity among ischemic stroke patients, especially in those without heparin pre-treatment. Further studies from other samples of ischemic stroke patients are needed to validate our findings.
Keywords: Hepatocyte growth factor, Ischemic stroke, Prognosis, Risk stratification
Introduction
Hepatocyte growth factor (HGF) is a pleiotropic cytokine which regulates growth, motility, and morphogenesis of multiple types of cells, and play important roles in tissue protection and repair of various organ1. It is reported that HGF is an endothelium-specific growth factor with proangiogenic effect and its mitogenic activity on endothelial cell growth is the most potent among various growth factors2. Dysfunction of endothelial cells is well known in cardiovascular diseases3, thus serum HGF secretion might be elevated in response to cardiovascular diseases as a counter-system against endothelial cell damage. Indeed, several epidemiological and clinical studies had indicated that elevated serum HGF was associated with increased risk of hypertension4, heart failure5, and myocardial infarction6, and predicted poor clinical outcomes in patients with heart failure7 or myocardial infarction8. In terms of stroke, serum HGF was reported to be positively associated with the incidence of ischemic stroke9, 10. However, the relationship between serum HGF and prognosis of ischemic stroke has not yet been studied. The aim of this study is to investigate the association between serum HGF and prognosis of ischemic stroke among patients from the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS).
Methods
Study participants
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author (yhzhang@suda.edu.cn). This study was conducted on the basis of the CATIS, a randomized clinical trial conducted in 26 hospitals across China. Details of the trial design and methods have been reported previously11. Briefly, from August 2009 to May 2013, a total of 4071 patients aged over 22 years who had ischemic stroke confirmed by computed tomography or magnetic resonance imaging of the brain within 48 hours of symptom onset, and with an elevated systolic blood pressure (SBP) between 140 mm Hg and 220 mm Hg were recruited. Patients with a SBP ≥220 or diastolic blood pressure (DBP) ≥120 mmHg, severe heart failure, acute myocardial infarction or unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, or resistant hypertension; those in a deep coma; and those treated with intravenous thrombolytic therapy were excluded. A further 989 patients were excluded because they refused to offer blood samples, or some collected samples were hemolyzed in storage or transport. After 3-month follow-up, 55 patients were lost and the follow-up rate was 98.2%. Finally, a total of 3027 patients were included in this analysis. Most baseline characteristics were balanced between patients who were assayed for HGF and those not assayed (Supplemental Table I), indicating that those assayed basically represented the total participants of CATIS.
The CATIS trial is registered at clinicaltrials.gov (Identifier: NCT01840072). The study was approved by the Institutional Review Boards at Soochow University in China and Tulane University in the United States. Written consent was obtained from all study participants or their immediate family members.
Data collection
Baseline data on demographic characteristics, lifestyle risk factors, medical history, clinical features, and imaging data were collected at the time of enrollment. The National Institutes of Health Stroke Scale (NIHSS) was used to evaluate stroke severity by trained neurologists at baseline12. According to the symptoms and imaging data of the patients, ischemic stroke was classified as large artery atherosclerosis (thrombotic), cardiac embolism (embolic) and small artery occlusion lacunae (lacunar)13. Three blood pressure measurements were obtained at baseline while the patient was in the supine position using a standard mercury sphygmomanometer. Routine laboratory determinations (blood glucose, blood lipids, etc.) were performed for all enrolled patients in each participating hospital at admission. Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L or triglyceride ≥2.26mmol/L or low density lipoprotein cholesterol ≥4.14 mmol/L or high density lipoprotein cholesterol <1.04mmol/L or self-reported history of physician-diagnosed dyslipidemia according to Chinese guidelines on the prevention and treatment of dyslipidemia14.
Serum HGF measurement
Blood samples were collected after at last 8 h of fasting within 24 hours of hospital admission. All blood samples were stored at −80°C until tested. We used a quantitative sandwich enzyme-linked immunosorbent assay with the Quantikine Human HGF Immunoassay kit (Catalog: SHG00; R&D Systems, Inc, Minneapolis) to test HGF levels in serum. Intra- and interassay coefficients of variation were less than 5.6% and 7.0%, respectively. Laboratory technicians who performed these measurements were blind to the clinical characteristics and outcomes of the study participants.
Outcome assessment
The primary outcome was composite outcome of death or major disability [modified Rankin Scale (mRS) score, 3–6] within 3 months. Secondary outcomes were separately those of death and major disability (mRS score of 6 and 3–5, respectively), and stroke recurrence. We also included an ordered seven-level categorical score of the mRS as a secondary outcome for neurologic functional status based on a recommendation for acute stroke trials15. Death certificates were obtained for deceased patients, and hospital data were abstracted for stroke recurrence. A trial-wide outcomes assessment committee, blinded to treatment assignment, reviewed and adjudicated subsequent outcomes based on the criteria established in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial.
Statistical Analysis
All participants were categorized into four groups according to quartiles of serum HGF concentrations. Baseline variables were compared among the four groups by analysis of variance or Kruskal-Wallis test for continuous variables and chi-squared test for categorical variables. Multivariate logistic regression analyses were performed to assess the association between HGF and clinical outcomes. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for upper quartiles of HGF with the lowest quartile as a reference. The potential covariates such as age, sex, time from onset to hospitalization, current smoking, alcohol consumption, dyslipidemia, blood glucose and SBP at baseline, history of hypertension, history of coronary heart disease, history of diabetes mellitus, family history of stroke, ischemic stroke subtypes, heparin pre-treatment, receiving immediate blood pressure reduction, and baseline NIHSS score were included in the multivariate model. The effects of serum HGF on mRS shift were analyzed using multivariable ordinal logistic regression model adjusted for the aforementioned variables. Furthermore, we calculated the continuous net reclassification index (NRI) and integrated discrimination improvement (IDI) to evaluate the predictive value of adding HGF to conventional risk factors16.
In the secondary analyses, we conducted subgroup analyses to assess the association between HGF and primary outcome. Interactions between serum HGF and subgroup variables on the primary outcome were tested in the models with interaction terms by the likelihood ratio test, adjusting for the aforementioned covariates unless the variable was used as a subgroup variable. It has been suggested that HGF concentrations are dramatically increased in response to heparin administration17. Therefore, we further evaluated the pattern and magnitude of associations between serum HGF and primary outcome using a logistic regression model with restricted cubic splines among the patients without or with heparin pre-treatment during the 24 hours before blood sampling, with three knots placed at the 10th, 50th, and 90th percentiles of HGF, and the 5% highest and lowest HGF observations were trimmed18. Two-tailed P <0.05 was considered to be statistically significant. All analyses were conducted using SAS statistical software (version 9.4, Cary, NC, USA).
Results
This study included a total of 3027 patients, including 1924 males and 1103 females, and the average age was 62 years old with a range from 25 to 95 years. A total of 1037 patients were pre-treated with heparin. Among them, 380 used unfractionated heparin, 620 used low molecular weight heparin and 37 used both unfractionated heparin and low molecular weight heparin. The participants with higher HGF tended to be older, and had higher blood glucose, baseline NIHSS score, and prevalence of heparin pre-treatment, thrombotic, and embolic than those with lower HGF, whereas the participants with higher HGF had shorter time from onset to randomization and lower prevalence of lacunar compared with those with lower HGF (Table 1).
Table 1.
Baseline Characteristics of the study participants according to HGF quartiles
| Characteristics | HGF, pg/mL | ||||
|---|---|---|---|---|---|
|
| |||||
| <1242.14 | 1242.14–1577.65 | 1577.66–2115.95 | ≥2115.96 | P value | |
| Number of subjects | 756 | 757 | 758 | 756 | |
| Demographics | |||||
| Age, years | 60.93 ± 10.05 | 61.91 ± 10.03 | 62.48 ± 11.23 | 63.53 ± 11.74 | <0.001 |
| Male | 479 (63.36) | 498 (65.79) | 486 (64.12) | 461 (60.98) | 0.272 |
| Current cigarette smoking | 261 (34.52) | 275 (36.33) | 283 (37.34) | 281 (37.17) | 0.653 |
| Current alcohol drinking | 229 (30.29) | 231 (30.52) | 263 (34.70) | 218 (28.84) | 0.081 |
| Medical history | |||||
| History of hypertension | 589 (77.91) | 593 (78.34) | 592 (78.10) | 596 (78.84) | 0.975 |
| History of CHD | 71 (9.39) | 72 (9.51) | 76 (10.03) | 94 (12.43) | 0.176 |
| History of diabetes mellitus | 117 (15.48) | 122 (16.12) | 138 (18.21) | 152 (20.11) | 0.073 |
| Family history of stroke | 132 (17.46) | 143 (18.89) | 140 (18.47) | 149 (19.71) | 0.727 |
| Clinical features | |||||
| Time from onset to hospitalization, h* | 12 (5–24) | 12 (5–24) | 10 (4–24) | 8 (4–24) | <0.001 |
| Systolic BP, mmHg | 165.84 ± 16.88 | 166.14 ± 17.28 | 166.93 ± 17.27 | 166.60 ± 16.18 | 0.609 |
| Diastolic BP, mmHg | 97.15 ± 11.00 | 96.30 ± 10.77 | 97.23 ± 10.68 | 96.20 ± 11.72 | 0.137 |
| Dyslipidemia | 400 (52.91) | 427 (56.41) | 430 (56.73) | 440 (58.20) | 0.201 |
| Blood glucose, mmol/L* | 5.70 (5.00–6.80) | 5.60 (5.00–6.90) | 5.80 (5.10–7.30) | 5.96 (5.20–7.80) | <0.001 |
| Baseline NIHSS score* | 4 (2–6) | 4 (2–7) | 5 (3–8) | 6 (3–10) | <0.001 |
| Ischemic stroke subtype† | |||||
| Thrombotic | 546 (72.22) | 564 (74.50) | 588 (77.57) | 625 (82.67) | <0.001 |
| Embolic | 18 (2.38) | 33 (4.36) | 37 (4.88) | 58 (7.67) | <0.001 |
| Lacunar | 206 (27.25) | 179 (23.65) | 160 (21.11) | 87 (11.51) | <0.001 |
| Heparin pre-treatment | 219 (29.01) | 190 (25.13) | 227 (29.95) | 401 (53.04) | <0.001 |
| Receiving immediate blood pressure reduction | 393 (51.98) | 357 (47.16) | 387 (51.06) | 375 (49.60) | 0.260 |
Data were presented as mean ± standard deviation or n (%) unless otherwise noted.
Data were presented as median (interquartile range).
CHD = coronary heart disease; BP = blood pressure; HGF = hepatocyte growth factor; NIHSS = National Institute of Health Stroke Scale;
Ten patients with both thrombotic and embolic subtypes; 68 patients with thrombotic and lacunar subtypes; 4 patients with embolic and lacunar subtypes; 1 patient with all 3 subtypes.
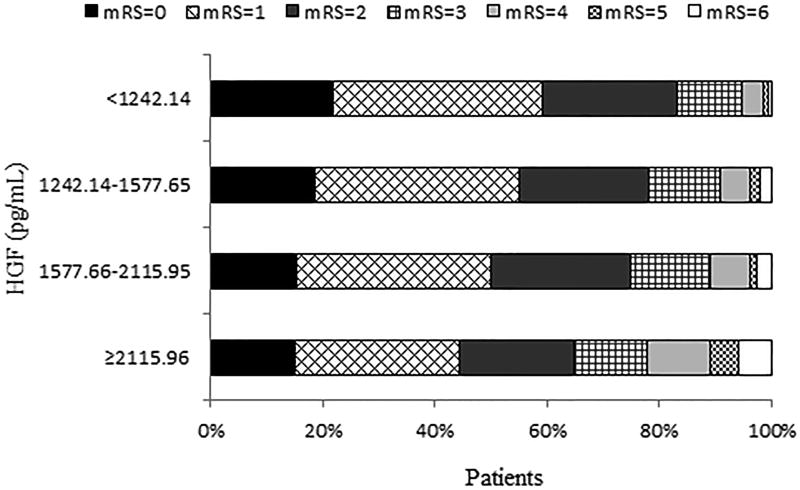
After 3-month follow-up, a total of 750 patients experienced primary outcome, among which 665 were major disability and 85 were death, and 54 patients developed stroke recurrence (Table 2). The cumulative incidence rates of primary outcome within 3 months among ischemic stroke patients in four quartiles of serum HGF (from low to high) were 16.80, 21.80, 25.20 and 35.32%, respectively. Patients in the fourth quartile of serum HGF had the highest risks of primary outcome and death. After adjustment for potential confounders, the ORs associated with highest quartile of serum HGF were 1.50 (95% CI, 1.10–2.03; Ptrend = 0.015), 1.23 (95% CI, 0.91–1.66; Ptrend = 0.215), 5.93 (95% CI, 1.76–19.97; Ptrend = 0.001), and 0.95 (95% CI, 0.41–2.17; Ptrend = 0.979) for primary outcome, major disability, death, and stroke recurrence, respectively. Similarly, on continuous analyses, each standard deviation increase of log-transformed HGF was associated with 14% (95% CI: 2–27%) and 50% (95% CI: 18–90%) increased risk of primary outcome and death, respectively, but not significantly associated with major disability and stroke recurrence. Multivariable ordinal logistic regression analyses showed a significantly worse shift in the distribution of mRS score with higher HGF quartiles in acute ischemic stroke patients (Ptrend = 0.004, Figure 1). Moreover, adding HGF quartiles to the conventional model improved the discriminatory ability for primary outcome (NRI: 17.50%; IDI: 0.23%) and death (NRI: 36.41%; IDI: 0.95%) but not for major disability and stroke recurrence (Supplemental Table II).
Table 2.
Odds ratios and 95% confidence intervals of clinical outcomes according to serum HGF quartiles in ischemic stroke patients
| HGF, pg/mL | Each standard deviation increase of log-HGF |
|||||
|---|---|---|---|---|---|---|
| <1242.14 | 1242.14–1577.65 | 1577.66–2115.95 | ≥2115.96 | Ptrend | ||
| Primary outcome: death or major disability (mRS 3–6) | ||||||
| No. of cases (%) | 127 (16.80) | 165 (21.80) | 191 (25.20) | 267 (35.32) | 750 (24.78) | |
| Model 1 | 1.00 | 1.36 (1.03–1.80) | 1.51 (1.14–1.98) | 2.43 (1.85–3.19) | <0.001 | 1.33 (1.21–1.46) |
| Model 2 | 1.00 | 1.19 (0.88–1.63) | 1.19 (0.88–1.62) | 1.50 (1.10–2.03) | 0.015 | 1.14 (1.02–1.27) |
| Secondary outcomes | ||||||
| Major disability (mRS 3–5) | ||||||
| No. of cases (%) | 123 (16.27) | 149 (19.68) | 171 (22.56) | 222 (29.37) | 665 (21.97) | |
| Model 1 | 1.00 | 1.26 (0.95–1.67) | 1.36 (1.03–1.80) | 1.91 (1.45–2.52) | <0.001 | 1.21 (1.10–1.34) |
| Model 2 | 1.00 | 1.12 (0.83–1.51) | 1.12 (0.83–1.50) | 1.23 (0.91–1.66) | 0.215 | 1.05 (0.94–1.17) |
| Death | ||||||
| No. of cases (%) | 4 (0.53) | 16 (2.11) | 20 (2.64) | 45 (5.95) | 85 (2.81) | |
| Model 1 | 1.00 | 4.06 (1.15–14.39) | 4.97 (1.45–17.09) | 11.00 (3.34–36.26) | <0.001 | 1.74 (1.41–2.13) |
| Model 2 | 1.00 | 3.23 (0.90–11.65) | 3.43 (0.98–12.05) | 5.93 (1.76–19.97) | 0.001 | 1.50 (1.18–1.90) |
| Stroke recurrence | ||||||
| No. of cases (%) | 15 (1.98) | 11 (1.45) | 12 (1.58) | 16 (2.12) | 54 (1.78) | |
| Model 1 | 1.00 | 0.80 (0.34–1.89) | 0.92 (0.41–2.08) | 0.93 (0.41–2.10) | 0.943 | 1.23 (0.93–1.62) |
| Model 2 | 1.00 | 0.81 (0.34–1.90) | 0.93 (0.41–2.10) | 0.95 (0.41–2.17) | 0.979 | 1.24 (0.94–1.64) |
Model 1, adjusted for age, sex, time from onset to hospitalization, current smoking, alcohol consumption, dyslipidemia, blood glucose and SBP at baseline, history of hypertension, history of coronary heart disease, history of diabetes mellitus, family history of stroke, ischemic stroke subtypes, heparin pre-treatment, and receiving immediate blood pressure reduction.
Model 2, further adjusted for baseline NIHSS score.
Figure 1.
Distribution of mRS score at 3 months according to serum HGF quartiles in ischemic stroke patients. Multivariable adjusted ORs of ordinal logistic regression analysis, 1.36 (95% CI, 1.11–1.67) for patients in the fourth quartile of HGF compared to the patients in the lowest quartile (P for trend = 0.004). Multivariable model adjusted for the same variables as model 2 in Table 2.
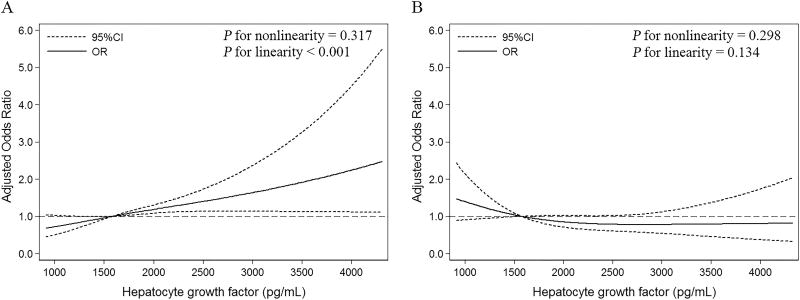
In subgroup analyses stratified by age, sex, baseline SBP, baseline NIHSS score, time from onset to hospitalization, cigarette smoking, alcohol consumption, receiving immediate blood pressure reduction medication and ischemic stroke subtypes, the modest positive associations between serum HGF and primary outcome were observed in almost all subgroups, and reached significant levels in several subgroups (Supplemental Table III). Statistical tests for interactions between serum HGF and these interested factors on primary outcome were not significant (all P > 0.05). As expected, the association between serum HGF and primary outcome could be modified by heparin pre-treatment (Pinteraction = 0.001), and serum HGF was associated with primary outcome only in patients without heparin pre-treatment but not in those with heparin pre-treatment (Supplemental Table III). As shown in Figure 2, with an increase of HGF levels, the risk of primary outcome for patients without heparin pre-treatment increased (Pnonlinearity = 0.317, Plinearity < 0.001). In contrast, we did not observe any association between serum HGF and the primary outcome in patients with heparin pre-treatment (Pnonlinearity = 0.298, Plinearity = 0.134).
Figure 2.
Adjusted ORs of primary outcome according to HGF. OR and 95 % CI derived from restricted cubic spline regression, with knots placed at the 10th, 50th, and 90th percentiles of HGF. Panels adjusted for the same variables as model 2 in Table 2. (A) Patients without heparin pre-treatment; (B) Patients with heparin pre-treatment.
Discussion
In this post hoc analysis of the CATIS trial, we found that serum HGF levels were higher in more severe stroke at baseline, and elevated HGF levels were probably associated with 3-month poor prognosis independently of stroke severity among ischemic stroke patients. We also found that adding serum HGF to a model containing conventional risk factors could improve risk prediction for the poor prognosis of ischemic stroke. In the secondary analysis, we further found that heparin pre-treatment before blood sampling could modify the association between serum HGF and primary outcome, and a positive linear dose-response relationship between HGF and poor prognosis was observed in patients without heparin pre-treatment but not in those with heparin pre-treatment.
Endothelial cells are known to secrete various antiproliferative and vasodilating factors, such as nitric oxide and natriuretic peptides19. It has been suggested that the presence of endothelial cell dysfunction with loss of substances from endothelial cells might result in the development and progression of abnormal vascular growth, such as atherosclerosis20. As we all known, endothelial dysfunction has been confirmed to be an early pathophysiological event of cardiovascular diseases3 and might be an independent predictor and prognostic factor for cardiovascular diseases21. HGF is a member of endothelium-specific growth factors and its mitogenic activity is the most potent among various growth factors2, thus serum HGF concentrations might be elevated in response to cardiovascular diseases as a counter-regulatory system against endothelial dysfunction. In fact, serum HGF has been reported to be positively associated with the risk of many cardiovascular diseases4–6, including ischemic stroke9, 10. Although several observational studies also find that elevated serum HGF is an independent predictor of poor outcomes in patients with heart failure7 or myocardial infarction8 during the follow-up, no such evidence is available for ischemic stroke.
Herein, we report that elevated serum HGF was associated with poor outcomes at 3 months after an ischemic stroke, which is concordant with the observation that higher HGF levels correlate with worse prognosis of heart failure or myocardial infarction7, 8. Moreover, serum HGF was associated with mortality but not disability at 3 months after ischemic stroke onset, suggesting that the difference of primary outcome across HGF groups is mainly driven by mortality. Previous studies had reported that serum HGF were positively related to some stroke prognostic factors, including increased age, smoking and hypertension4, 10. Interestingly, in our study, elevated HGF concentrations remained independently associated with poor prognosis when these powerful prognostic parameters were included into the multivariate models. From the findings of our study, we hypothesized that HGF might be useful in risk stratification of ischemic stroke prognosis, and could assist the selection of high-risk patients for aggressive monitoring and therapeutic interventions. However, further prospective studies from other samples of ischemic stroke patients are needed to validate our hypothesis. At first glance, these results may appear to contradict the biological function of HGF, including antiapoptotic, mitogenic, antifibrotic and proangiogenic activities1. However, this phenomenon is very similar to those previously reported for natriuretic peptides. Natriuretic peptides are known to play beneficial effects in ischemic stroke patients, but elevated levels are strong predictors of poor outcomes22. The precise mechanisms explaining the detrimental effect of elevated HGF on the stroke prognosis are not well defined, but may include inflammation23, accelerated progression of atherosclerotic lesions9, plaque neovascularization24, intraplaque hemorrhage and plaque rupture25. In addition, higher serum HGF levels may also reflect a greater degree of baseline endothelial dysfunction9 which is associated with subsequent infarct expansion26 and hemorrhagic transformation27, thus increase the risk of poor prognosis.
In intravascular thrombus-associated disorders, it was reported that release of endogenous heparin during mast cell degranulation stimulated the production and release of serum HGF28, and HGF was also called heparin-binding growth factor. It has been demonstrated that HGF levels increase markedly and rapidly after heparin administration in humans17. Therefore, we consider heparin pre-treatment before blood sampling as a confounder of the associations between HGF and poor outcomes, and the positive associations remain significant after including heparin pre-treatment into the multivariate models. Recently, one study showed a conflicting result regarding the predictive value of HGF in acute coronary syndrome patients pre-treated with intravenous heparin, namely, they found that serum HGF elevation was associated with a favorable prognosis29. Administration of heparin has already been used to evaluate the functional reserve of HGF in the organism and the capability of secreting HGF into circulation30, and exogenous supplementation of HGF by a gene transfer technique has been shown to reduce intimal hyperplasia after balloon injury31. This may explain the apparently conflicting observation. In patients without heparin pre-treatment, serum HGF is naturally HGF which produced in response to endothelial dysfunction, so higher HGF reflects a greater degree of baseline endothelial dysfunction and is associated with worse prognosis. In patients with heparin pre-treatment, serum HGF is stimulated HGF whose secretion is induced by heparin administration, so elevated HGF is associated with good prognosis through its protective effect in tissue repair29. Similarly, we found that heparin pre-treatment could modify the association between serum HGF and prognosis of ischemic stroke. In the present study, serum HGF was positively associated with poor prognosis in patients without heparin pre-treatment, whereas there was an inversely trend of the association between HGF and poor outcomes in patients with heparin pre-treatment although it did not reach statistical significance. Patients with heparin pre-treatment are only half the number of those without heparin pre-treatment, which might be underpowered to detect the significant inverse association in those pre-treated with heparin.
Our study has some strengths. First, this is the first study to investigate the association between serum HGF and clinical outcomes in acute ischemic stroke patients. Second, this is an observational study based on the patients from CATIS, a randomized clinical trial with strict quality controls in data collection and outcome assessment, and a relatively large sample size enables us perform analysis with high statistical power. Some limitations should be discussed here. First, this study is a post hoc analysis of the CATIS trial and a selection bias may exist. However, baseline characteristics of participants in this study were similar to those from the China National Stroke Registry32, suggesting that the selection bias may be minimal. Second, serum HGF concentrations were tested only once at baseline. Therefore, we don't know how the levels of HGF change with differences in baseline characteristics and further studies are needed to investigate this. We also have no data to examine the association between HGF changes and prognosis of acute ischemic stroke although HGF levels are reported to be relatively stable over several years10. Third, the number of patients with heparin pre-treatment which account for 1/3 of total participants is relatively few, which might be underpowered to detect the significant inverse association in those pre-treated with heparin. Fourth, we did not consider the lesion volume when we examined the association of HGF with prognosis. However, lesion volume is highly correlated with NIHSS score33 and we have adjusted baseline NIHSS score in the analysis. Finally, relatively few number of stroke recurrence (maybe because some fatal stroke recurrences were classified into death events) were observed during 3 month follow-up of relatively short time, which might limited our power to detect significant association between serum HGF and stroke recurrence. Further long-term follow-up studies are required to verify these relationships.
Conclusions
In summary, we found that serum HGF levels were higher in more severe stroke at baseline, and elevated HGF levels were probably associated with 3-month poor prognosis independently of stroke severity among ischemic stroke patients, especially in those without heparin pre-treatment. Further prospective studies from other samples of ischemic stroke patients are needed to validate our findings.
Supplementary Material
Acknowledgments
We thank the study participants and their relatives and the clinical staff at all participating hospitals for their support and contribution to this project.
Sources of funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81773522, 81320108026] and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China; and by the National Institute of General Medical Sciences of the National Institutes of Health [grant number P20GM109036].
Footnotes
Disclosures
None.
References
- 1.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of hgf/met in the cardiovascular system. Clinical science. 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura Y, Morishita R, Higaki J, Kida I, Aoki M, Moriguchi A, et al. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: Additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J Hypertens. 1996;14:1067–1072. doi: 10.1097/00004872-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? Journal of the American College of Cardiology. 2005;45:1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Morishita R, Nakamura S, Aoki M, Moriguchi A, Matsumoto K, et al. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension. 1996;28:409–413. doi: 10.1161/01.hyp.28.3.409. [DOI] [PubMed] [Google Scholar]
- 5.Ueno S, Ikeda U, Hojo Y, Arakawa H, Nonaka M, Yamamoto K, et al. Serum hepatocyte growth factor levels are increased in patients with congestive heart failure. Journal of cardiac failure. 2001;7:329–334. doi: 10.1054/jcaf.2001.27686. [DOI] [PubMed] [Google Scholar]
- 6.Matsumori A, Furukawa Y, Hashimoto T, Ono K, Shioi T, Okada M, et al. Increased circulating hepatocyte growth factor in the early stage of acute myocardial infarction. Biochemical and biophysical research communications. 1996;221:391–395. doi: 10.1006/bbrc.1996.0606. [DOI] [PubMed] [Google Scholar]
- 7.Lamblin N, Susen S, Dagorn J, Mouquet F, Jude B, Van Belle E, et al. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. American heart journal. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Susen S, Sautiere K, Mouquet F, Cuilleret F, Chmait A, McFadden EP, et al. Serum hepatocyte growth factor levels predict long-term clinical outcome after percutaneous coronary revascularization. European heart journal. 2005;26:2387–2395. doi: 10.1093/eurheartj/ehi436. [DOI] [PubMed] [Google Scholar]
- 9.Rajpathak SN, Wang T, Wassertheil-Smoller S, Strickler HD, Kaplan RC, McGinn AP, et al. Hepatocyte growth factor and the risk of ischemic stroke developing among postmenopausal women: Results from the women's health initiative. Stroke; a journal of cerebral circulation. 2010;41:857–862. doi: 10.1161/STROKEAHA.109.567719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell EJ, Larson NB, Decker PA, Pankow JS, Tsai MY, Hanson NQ, et al. Hepatocyte growth factor is positively associated with risk of stroke: The mesa (multi-ethnic study of atherosclerosis) Stroke; a journal of cerebral circulation. 2016;47:2689–2694. doi: 10.1161/STROKEAHA.116.014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: The catis randomized clinical trial. Jama. 2014;311:479–489. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 12.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke; a journal of cerebral circulation. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke; a journal of cerebral circulation. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 15.Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke; a journal of cerebral circulation. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 16.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Statistics in medicine. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 17.Salbach PB, Bruckmann M, Turovets O, Kreuzer J, Kubler W, Walter-Sack I. Heparin-mediated selective release of hepatocyte growth factor in humans. British journal of clinical pharmacology. 2000;50:221–226. doi: 10.1111/j.1365-2125.2000.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 19.Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990;346:69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- 20.Dzau VJ, Gibbons GH. Endothelium and growth factors in vascular remodeling of hypertension. Hypertension. 1991;18:Iii115–121. doi: 10.1161/01.hyp.18.5_suppl.iii115. [DOI] [PubMed] [Google Scholar]
- 21.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. International journal of cardiology. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Makikallio AM, Makikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, et al. Natriuretic peptides and mortality after stroke. Stroke; a journal of cerebral circulation. 2005;36:1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 23.Beilmann M, Vande Woude GF, Dienes HP, Schirmacher P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood. 2000;95:3964–3969. [PubMed] [Google Scholar]
- 24.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, Sai Y, et al. Hepatocyte growth factor and c-met expression in pericytes: Implications for atherosclerotic plaque development. The Journal of pathology. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 25.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagita Y, Kitagawa K, Sasaki T, Terasaki Y, Todo K, Omura-Matsuoka E, et al. Rho-kinase activation in endothelial cells contributes to expansion of infarction after focal cerebral ischemia. Journal of neuroscience research. 2007;85:2460–2469. doi: 10.1002/jnr.21375. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: Mechanisms, models, and biomarkers. Molecular neurobiology. 2015;52:1572–1579. doi: 10.1007/s12035-014-8952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita M, Miyamoto T, Ohashi N, Sasayama S, Matsumori A. Thrombosis increases circulatory hepatocyte growth factor by degranulation of mast cells. Circulation. 2002;106:3133–3138. doi: 10.1161/01.cir.0000039344.98537.be. [DOI] [PubMed] [Google Scholar]
- 29.Heeschen C, Dimmeler S, Hamm CW, Boersma E, Zeiher AM, Simoons ML. Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003;107:524–530. doi: 10.1161/01.cir.0000048183.37648.1a. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda S, Goto Y, Sumida H, Noguchi T, Baba T, Miyazaki S, et al. Angiotensin-converting enzyme inhibition restores hepatocyte growth factor production in patients with congestive heart failure. Hypertension. 1999;33:1374–1378. doi: 10.1161/01.hyp.33.6.1374. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi K, Nakamura S, Morishita R, Moriguchi A, Aoki M, Matsumoto K, et al. In vivo transfer of human hepatocyte growth factor gene accelerates re-endothelialization and inhibits neointimal formation after balloon injury in rat model. Gene Ther. 2000;7:1664–1671. doi: 10.1038/sj.gt.3301284. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y, Wang X, Matsushita K, Wang C, Zhao X, Hu B, et al. Associations between estimated glomerular filtration rate and stroke outcomes in diabetic versus nondiabetic patients. Stroke; a journal of cerebral circulation. 2014;45:2887–2893. doi: 10.1161/STROKEAHA.114.005380. [DOI] [PubMed] [Google Scholar]
- 33.Tong DC, Yenari MA, Albers GW, O'Brien M, Marks MP, Moseley ME. Correlation of perfusion- and diffusion-weighted mri with nihss score in acute (<6.5 hour) ischemic stroke. Neurology. 1998;50:864–870. doi: 10.1212/wnl.50.4.864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.