Abstract
Background
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer with high recurrence rate and poor prognosis. Here we describe a novel, genetically-engineered parapoxvirus that efficiently kills TNBC.
Methods
A novel chimeric parapoxvirus (CF189) was generated via homologous recombination and identified through high-throughput screening. Cytotoxicity was assayed in vitro in four TNBC cell lines. Viral replication was examined through standard plaque assay. Orthotopic TNBC xenografts were generated by MDA-MB-468 implantation into the second and fourth mammary fat pads of athymic nude mice, and treated with virus.
Results
CF189 demonstrated dose-dependent cytotoxicity at low multiplicity of infection (MOI), with >80% cell death six days after treatment. Significant reductions in tumor size were observed two weeks after intratumoral injection at doses as low as 103 PFU compared to control (P<0.01). In addition, abscopal effect (shrinkage of non-injected remote tumors) was clearly demonstrated.
Conclusion
CF189 demonstrated efficient cytotoxicity in vitro and potent anti-tumor effect in vivo at doses as low as 103 PFU. These are data encouraging of clinical development for this highly potent agent against TNBC.
INTRODUCTION
Approximately 12–20% of one million newly diagnosed breast cancer cases worldwide each year are triple-negative,1 meaning they lack expression of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). Triple-negative breast cancer (TNBC) demonstrates poorer clinical outcomes, both due to its inherently aggressive behavior and the lack effective targeted therapies. Patients with TNBC are at higher risk of relapse and development of metastatic disease in the first five years following diagnosis than non-TNBC patients.2
Currently, the mainstay of adjuvant therapy for TNBC is cytotoxic chemotherapy, but there has been intensive research and development of targeted therapies, including studies on poly (ADP-ribose) polymerase 1 (PARP1) inhibitors, PI3K inhibitors, MEK inhibitors and programmed cell death ligand 1 (PD-L1) inhibitors.1, 3
Research and development of oncolytic viruses (OV) has increased significantly following US Food and Drug Administration approval of T-VEC (talimogene laherparepvec) as the first OV for use in humans.4 This approval was a landmark event in the fields of virology and immunotherapeutics, spurring great interest in further development of OVs for the treatments of other cancers, including TNBC. OVs demonstrate a natural tropism for cancer cells compared to normal cells, exploiting pathologic derangements in cancer cells such as altered intracellular signaling pathways and overexpression of cell surface receptors to preferentially infect cancer cells.5–7 Additionally, OV-mediated destruction of infected cells releases cytokines, tumor-associated antigens (TAA), damage-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern (PAMPs) molecules that prime both the innate and adaptive immune systems against cancer cells.6, 8–12 TNBC demonstrates increased genetic instability, increased number of neoantigens, and increased number of tumor infiltrating lymphocytes (TILs) in the microenvironment.13 This makes TNBC more immunogenic than non-triple negative disease and an appealing candidate for OV-directed therapy. Our group has previously investigated an oncolytic vaccinia virus with antitumor effect in a TNBC model.14 Here we present data on a novel chimeric parapoxvirus that is effective in TNBC models both in vitro and in vivo.
METHODS
Cell culture and cell lines
Human triple-negative breast cancer cell lines MDA-MB-231 (kindly provided by Dr. Sangkil Nam, City of Hope), MDA-MB-468 (kindly provided by Dr. John Yim, City of Hope), BT549 (Dr. Yim) and Hs578T (Dr. Yim) were cultured in RPMI 1640 (Corning, Corning NY) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. All TNBC lines were tested and verified as authentic by Genetica Cell Line Testing (Burlington, NC). African green monkey kidney fibroblasts (CV-1) and MDBK cells were obtained from American Type Culture Collection (Mannassus, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Corning, Corning NY) supplemented with 10% FBS and 1% antibiotic-antimycotic solution. All cells were grown at 37°C in a 5% CO2-humidified incubator.
Development and selection of chimeric parapoxvirus
To generate a pool of chimeric parapoxviruses, MDBK cells were co-infected with orf virus strain NZ2 (ATCC) and pseudocowpox virus strain TJS (ATCC). Infected cells were harvested at three days after infection. One hundred chimeric parapoxvirus plaques were picked from MDBK cells infected with the chimeric parapoxvirus pool. These 100 plaques were further plaque-purified two more times in MDBK cells to yield 100 clonally purified individual chimeric virus isolates, which, together with its parental viruses, were subject to high-throughput screening in the NCI-60 cell lines. The isolate CF189 demonstrated the most potent tumoricidal properties against the NCI-60 cell lines and was chosen for this study.
Cytotoxicity assays
Cells were seeded at 1000 cells/well for MDA-MB-231, BT549 and Hs578T and at 3000 cells/well for MDA-MB-468 in 96-well plates and incubated overnight. Cells were infected with 0.1, 1 and 10 MOI of each virus for MDA-MB-231 and with 0.01, 0.1 and 1 MOI for MDA-MB-468, BT549 and Hs578T. Cell viability was measured in triplicate every 24 hours for one to six days using CellTiter 96 Aqueous One solution (Promega, Madison, WI) on a spectrophotometer (Tecan Spark 10M, Mannedorf, Switzerland) at 490 nm.
Viral replication assays
Viral replication in TNBC was quantified using standard plaque assay. Cells were plated to confluence in 6-well plates in 2 ml growth media, and then infected with 0.01 MOI of each virus. Cells were harvested in triplicate for three consecutive days. MDBK cells were infected with serial dilutions of samples treated with CF189 in 24-well plates.
Orthotopic xenograft models
Twenty-six Hsd:Athymic Nude-Foxn1nu female nude mice (Envigo, Indianapolis, IN) were injected with 107 MDA-MB-468 cells with 6 mg/ml matrigel (Corning) in the second and fourth mammary fat pads at 12 weeks of age. When the tumors reached approximately 100–150 mm3 in size, mice were randomized and tumors were injected intratumorally with either PBS alone (n= 4), 103 PFU (n=6), 104 PFU (n=6) or 105 PFU (n=7) in 50 ul PBS. Variance of tumor size did not differ significantly between the groups prior to treatment. Tumor size was then measured every three days for six weeks. Tumor volume was calculated according to V (mm3) = (4/3) × (π) × (a/2)2 × (b/2), where a is the smallest diameter and b is the largest diameter. Seven days following intratumoral injection, two to four mice per group were sacrificed such that tumor and organs (lung, heart, liver, kidney, spleen, ovary, brain) were snap frozen in liquid nitrogen and used for further histopathological staining, immunohistochemical staining and viral plaque assays. For the remaining three mice, only the second mammary tumors were treated with 105 PFU in 50 ul PBS in order to observe the effect, if any, on the uninjected fourth mammary tumor. Two weeks after treatment, both tumors were harvested to determine viral titer in each.
Staining and imaging
Hematoxylin and eosin (H&E) staining of tissue sections was performed per routine protocols by our institutional pathology department, with selected slides reviewed by a veterinary pathologist at City of Hope. Tissue slides were stained for immunofluorescence with the primary antibody against ORF virus at 1:50 dilution (LSBio, ORFV086, Seattle WA). Slides were first blocked with blocking solution (TSA) for 30 minutes. Primary antibody was incubated overnight at 4°C, serially washed in Tween, then incubated with the immunofluorescent secondary antibody for 60 minutes at room temperature (AlexaFluor 546, Thermo Fischer, Waltham MA). Imaging was performed using Life Technologies EVOS FL Auto imaging system (Life Technologies, Carlsbad CA). For natural killer (NK) cell staining, heat-mediated antigen retrieval was performed using IHC-TEK Epitope Retrieval Solution (IHC World, Ellicott City, MD) following manufacturer’s protocol. The sections were incubated with rat anti-CD49b antibody (BioLegend, San Diego, CA) diluted 1:100 in TNB blocking buffer overnight in a humidified chamber at 4°C. Tumor sections were washed and incubated with HRP-conjugated goat anti-rat antibody (Abcam, Cambridge, MA) for 60 minutes at room temperature, then treated for 10 minutes with the HRP substrate 3,3′ Diaminobenzidine (DAB) (Abcam, Cambridge, MA) and counter-stained with hematoxylin. Slides were then dehydrated and imaged using the widefield light microscope Zeiss Observer Z1 (Zeiss, Jena, Germany).
RESULTS
CF189 effectively kills TNBC in vitro in a time- and dose-dependent manner
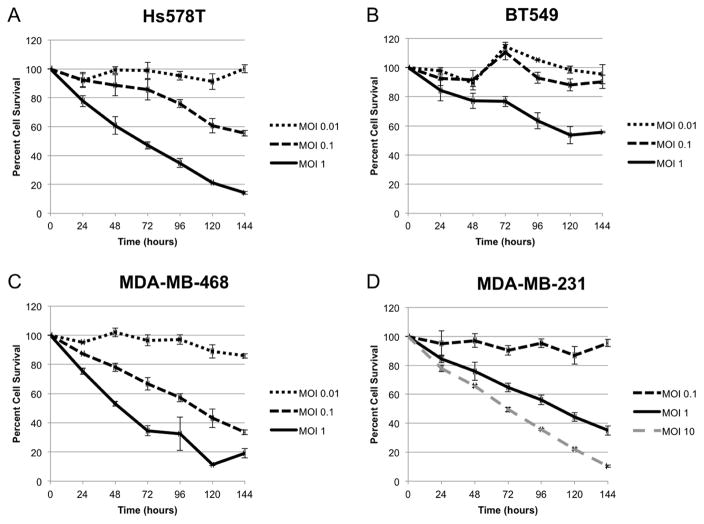
Given the heterogeneous nature of TNBC, two cell lines of metastatic origin (MDA-MB-231 and MDA-MB-468) and two cell lines of non-metastatic origin (Hs578T and BT549) were treated with CF189 at MOI ranging from 0.01 to 10 over six days. CF189 most efficiently killed Hs578T (LD50 at 96 hrs: MOI 0.396) and MDA-MB-468 (LD50, MOI 0.185) with >80% cell death at six days when treated with MOI 1 (Fig 1, A and C). Although LD50 was higher for BT549 and MDA-MB-231 (LD50, MOI 1.636 and MOI 1.712, respectively), MDA-MB-231 data shows that increasing the concentration of CF189 results in >90% cell death at MOI 10 after six days (Fig 1, D).
Fig. 1. Cytotoxic effect of CF189 in vitro is both time- and dose-dependent in triple-negative breast cancer cell lines.
(A) Hs578T. LD50, MOI 0.396 (SD 0.113), (B) BT549. LD50, MOI 1.636 (SD 0.539), (C) MDA-MB-468. LD50, MOI 0.185 (SD 0.071), (D) MDA-MB-231. LD50, MOI 1.712 (SD 1.263). LD50 (at 96 hrs), median lethal dose; MOI, multiplicity of infection; SD, standard deviation.
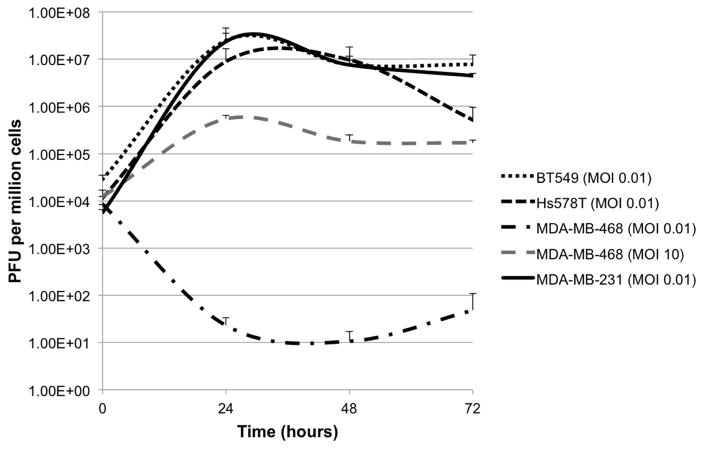
CF189 replicates in TNBC in vitro
Viral replication was assessed in all four TNBC cell lines by standard plaque assay of infected cells collected over three days. Efficient viral replication occurred in BT549, Hs578T and MDA-MB-231 at MOI 0.01, with maximal replication occurring in the 24–48 hour time period (Fig. 2). However, CF189 replication in MDA-MB-468 was poor at MOI 0.01 despite demonstrating effective cytotoxicity at low MOI. Viral replication was improved by increasing concentration to MOI 10 in the MDA-MB-468 line (Fig. 2).
Fig. 2. Replication of CF189 in triple-negative breast cancer cell lines.
Efficient viral replication occurred in vitro in BT549, Hs578T and MDA-MB-231 cell lines at low multiplicity of infection (MOI 0.01). CF189 replication in MDA-MB-468 was poor at MOI 0.01. At MOI 10, CF189 replication in MDA-MB-468 remained nearly two-log lower than the other three cell lines.
Intratumoral CF189 injection effectively reduces tumor size in orthotopic TNBC xenografts without significant viral toxicity
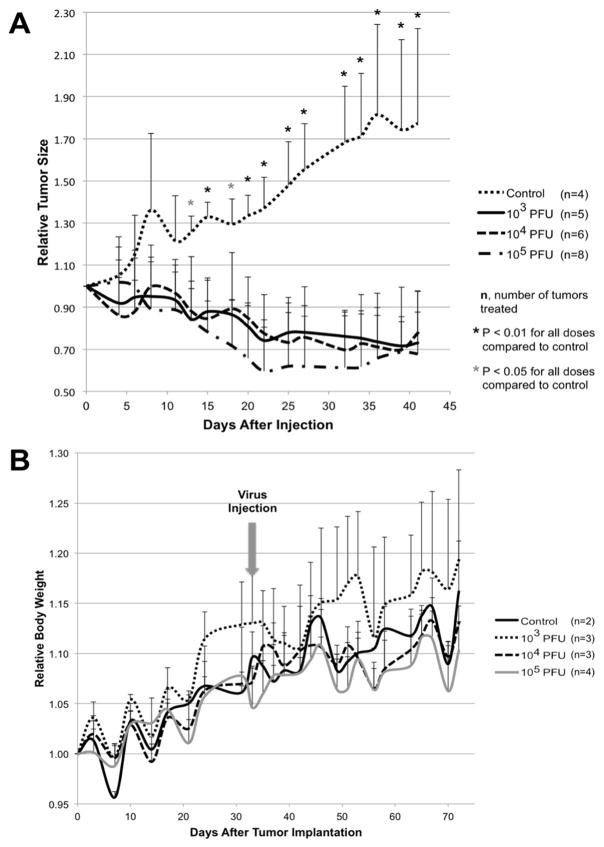
Orthotopic xenografts were created by implanting MDA-MB-468 cells into the second and fourth mammary fat pads of athymic nude mice. Both tumors received a single intratumoral injection of either PBS or CF189 (103 PFU, 104 PFU, or 105 PFU). All groups treated with CF189 demonstrated significant reduction in relative tumor size by post-treatment day 13 compared to PBS-treated controls and this treatment effect was sustained six weeks post-injection (Fig 3A). Intratumoral injections were well tolerated without notable viral toxicity in the mice, as demonstrated by lack of significant body weight reduction in treatment groups compared to controls (Fig 3B).
Fig. 3. Intratumoral injection of CF189 in MDA-MB-468 xenografts effectively reduces relative tumor size at doses as low as 103 PFU per tumor compared to control without significant toxicity.
(A) Tumors were injected with PBS (control), 103 PFU per tumor, 104 PFU per tumor or 105 PFU per tumor at an initial tumor volume of approximately 100–150 mm3. (B) No significant reductions in relative body weight were observed in nude mice treated with intratumoral CF189 injection compared to PBS-injected controls.
CF189 biodistribution at one week and six weeks post-treatment demonstrates inherent tumor-specificity in vivo
One week and six weeks after CF189 injection, two to four mice from each group were sacrificed for viral biodistribution. Viral titers of infected tumor tissue demonstrated 2-log higher CF189 titer compared to other organs at both time points, reflecting CF189’s natural tropism for cancer cells compared to normal cells (Table 1). Apart from injected tumor tissue CF189 was only detected in heart and lung tissue one week post-injection and in lung tissue six weeks post-injection, which correlates with the lack of significant systemic toxicity observed. H&E-stained tissue from heart and lung specimens in the treatment group did not demonstrated evidence of cellular injury compared to control tissues upon review by our veterinary pathologist.
Table 1.
Viral biodistribution of CF189 in various organs one and six weeks post-treatment.
| Viral Titer at 1 week (PFU/g tissue) | Viral Titer at 6 weeks (PFU/g tissue) | |||||||
|---|---|---|---|---|---|---|---|---|
| Titer | Animals detected/total animals tested | SD | Limit of detection | Titer | Animals detected/total animals tested | SD | Limit of detection | |
| Tumor | 1.64 × 104 | 3/3 | 1.22 × 104 | 1.18 × 103 | 4/4 | 1.24 × 103 | ||
| Brain | ND | 0/3 | 2.52 × 102 | ND | 0/4 | 2.53 × 102 | ||
| Heart | 1.07 × 102 | 1/3 | 1.85 × 102 | ND | 0/4 | 4.28 × 102 | ||
| Lung | 8.96 × 102 | 3/3 | 7.23 × 102 | 3.33 × 101 | 1/4 | 6.67 × 101 | ||
| Liver | ND | 0/3 | 1.14 × 102 | ND | 0/4 | 1.12 × 102 | ||
| Spleen | ND | 0/3 | 4.87 × 102 | ND | 0/4 | 6.58 × 102 | ||
| Kidney | ND | 0/3 | 2.43 × 102 | ND | 0/4 | 2.57 × 102 | ||
| Ovary | ND | 0/3 | 2.62 × 103 | ND | 0/4 | 4.39 × 102 | ||
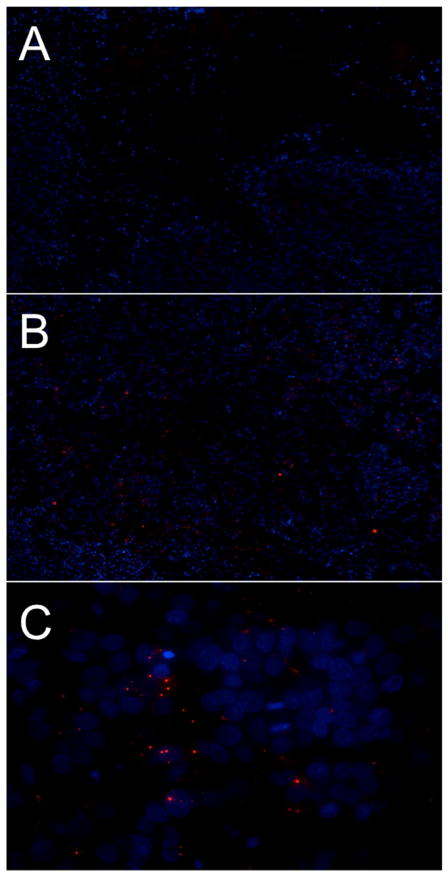
Infection and replication of CF189 in orthotopic xenograft model confirmed by immunofluorescent imaging
One week after CF189 injection, immunofluorescent detection of polyclonal antibody against orf virus demonstrated viral infection of tumor tissue treated with CF189 at 105 PFU per tumor compared to control (Fig 4, A and B). Furthermore, the pattern of antibody detection merged with DAPI counterstain is consistent with active replication in viral factories, as the orf virus antibodies (red) localize to areas just outside the nucleus (blue) (Fig 4, C).
Fig. 4. CF189 infects MDA-MB-468 tumors in vivo.
Immunofluorescent detection of polyclonal antibody against orf virus demonstrates viral infection of MDA-MB-468 xenograft tumor tissue harvested one week after intratumoral CF189 injection. (A) Control tumor, 10X, (B) Tumor from 105 PFU treatment group, 10X, (C) Tumor from 105 PFU treatment group, 60X. (ORF: red; DAPI counterstain: blue).
Virus-treated tumor tissue exhibits increased NK cell infiltration one week following CF189 treatment
Both control and treatment tumor tissue harvested one week and six weeks after CF189 were assessed for NK cell infiltration by incubation with primary antibody against CD49b, followed by a secondary HRP-linked antibody. While no NK cells were visualized in the control tumor tissue (Fig 5, A), NK cells were observed in the periphery of virus-treated tumor tissue after one week, suggesting that virus-treatment stimulated an immune-response against tumor in this time period (Fig 5, B). At 6-weeks post-treatment, there was no difference observed between the number of NK cells in treatment and control groups (Fig 5, C and D respectively).
Fig. 5. Virus-treated tumor tissue exhibits increased NK staining one week post-injection.
Both control tumor tissue (A) and tumor tissue from CF189 105 PFU treatment group (B) were stained for presence of NK cells using antibody against CD49b. No NK cells were visualized in the control tumor tissue, but many NK cells were observed in the treatment group following treatment with HRP-linked secondary antibody (arrowheads). Six weeks post-injection, there were similar numbers of NK cells present in both control (C) and virus-treated (D) tumor tissue.
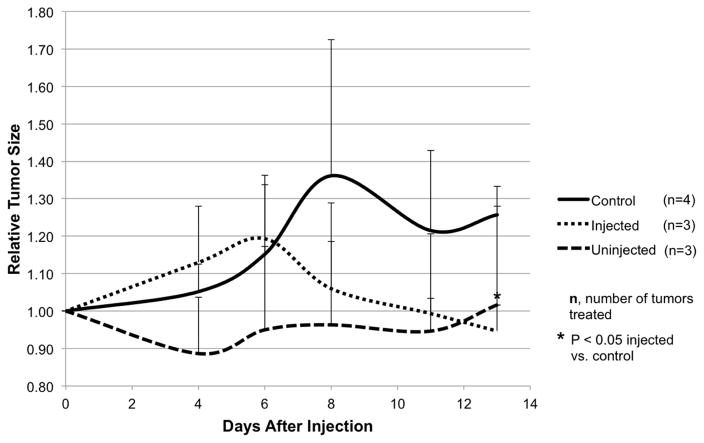
Intratumoral CF189 injection produces tumoristatic effect on distant uninjected tumors
Three animals were treated with CF189 at 105 PFU in only the second mammary tumor, while the fourth mammary tumor remained untreated. Tumor size was measured every three days in comparison to PBS-treated controls and viral titers were quantified by standard plaque assay at the end of two weeks. While relative tumor size increased initially in the injected tumor group (likely due to the trauma of the injection itself), tumor size decreased after day six with significant reduction compared to control by day 13 (p<0.05). Relative tumor size of the uninjected tumor group remained stable despite never being directly treated with CF189 (Fig 6). Viral titers at the end of two weeks showed the average titer of injected tumors was 3.37 × 103 PFU/g tissue compared to the average of uninjected tumors at 1.15 × 103 PFU/g tissue.
Fig. 6. Intratumoral CF189 injection produces tumoristatic effect on a distant uninjected tumor.
Second mammary tumors produced in MDA-MB-468 xenografts were treated with a single intratumoral injection of CF189 at 105 PFU, while the fourth mammary tumors were not injected. Control tumors were injected with PBS. Tumor size was measured approximately every three days.
DISCUSSION
Our data demonstrates that a novel chimeric parapoxvirus, CF189, has in vitro cytotoxic effects in four different TNBC cell lines, both of metastatic and non-metastatic origin. Single intratumoral injection of CF189 in athymic nude mice TNBC xenografts significantly reduced relative tumor size without signs of obvious toxicity. Furthermore, the virus was also detected at non-injected distant tumor sites with size stabilization of those tumors. Thus, CF189 demonstrates the ability to travel systemically and target distant sites of disease, which may have applications for neoadjuvant therapy and also in metastatic settings. Given that TNBC tends to exhibit more aggressive behavior than receptor-positive disease and lacks well-characterized molecular targets for therapy, an oncolytic virus with natural tropism for TNBC cells with the ability to potentially target occult disease is an exciting prospect in the treatment of this challenging disease entity.
Of note, in vivo reduction of tumor size was observed with CF189 doses as low as 103 PFU per tumor. This suggests that the antitumor effect is unlikely a result of direct oncolysis from viral replication, as CF189 demonstrated poor in vitro replication. Genetic sequencing of CF189 reveals close relationship to one of its parent viruses, the parapoxvirus orf virus (ORFV). Previous studies of ORFV have shown that ORFV treatment induces a strong immunomodulatory effect, particularly with regard to natural killer (NK) cell activation.15, 16 Even treatment with ultraviolet (UV)-inactivated ORFV elicited similar, although weaker, response, suggesting that the virus itself likely harbors an antigenic structural component that is capable of inducing an immune response independent of its actual replication. Our staining results support the hypothesis that CF189 stimulates the innate immune system against tumor cells, providing an explanation of the effect seen at low dose and its poor replication in vitro. At the one-week time point NK cells are observed at the periphery of the treated tumor tissue, but not in the control tumors. Of interest, at the 6-week time point, NK cells are noted in both the control and treatment tumors in similar density. This suggests that perhaps even in untreated mice, the immune system has some degree of response against tumor over time, and the initial effect of the single CF189 treatment has diminished. This correlates with the observation that although tumor size is significantly reduced compared to control with this reduction in size stabilized over the duration of the study, continued reduction in tumor size does not continue beyond the third post-injection week. In future works, this period would serve as an interesting time point to experiment with repeat dosing of CF189 in order to determine whether there is continued response can be induced toward complete tumor resolution.
In comparison to other OVs, ORFV has not been well-studied in the context of clinical therapeutics development. Much of what is known with regard to its natural disease history has been gleaned from veterinary medicine. However, there are several properties of ORFV that may be advantageous for developing it as an OV for the treatment of human cancers. Firstly, ORVF infection does not cause serious diseases in humans.17 Secondly, ORFV infection induces a potent immune system stimulation (Th1 dominated) and even inactivated viral particles retain the ability to induce an immune response.18, 19 Thirdly, neutralizing antibody is rare and reinfection can occur despite antibody production against ORFV. This means that repeated doses can potentially be given to the same patient,16 a logistical improvement over other OVs in clinical development that require serotype switching or second doses of antigenically unique viruses for continued response. Lastly, clinical response with the use of lower viral titers of ORFV is another practical improvement for OV development, as OV production is difficult, cost- and time-intensive and typically requires titers in the range of 106 to 109 PFU per injection.
In summary, CF189 is a novel wild-type chimeric parapoxvirus effective against TNBC both in vitro and in vivo. With targeted therapies lacking for TNBC treatment, CF189 represents a promising avenue for immunotherapeutics in this field. In terms of future directions, we plan further preclinical testing in other xenograft models, as well as genetic modifications to the wild-type virus to enhance tumor selectivity and overall potency.
Acknowledgments
Funding sources: Research reported in this publication included work performed in the High Throughput Screening Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: none
Presentations: presented at the 12th Annual Academic Surgical Congress, February 8, 2017, Las Vegas, NV.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12(2):106–16. doi: 10.7497/j.issn.2095-3941.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curigliano G, Goldhirsch A. The triple-negative subtype: new ideas for the poorest prognosis breast cancer. J Natl Cancer Inst Monogr. 2011;2011(43):108–10. doi: 10.1093/jncimonographs/lgr038. [DOI] [PubMed] [Google Scholar]
- 3.Bianchini G, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andtbacka RCM, Li A, Shilkrut M, Ross MI. Phase 2, multicenter, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma. J Clin Oncol. 2015;33:TPS9094. [Google Scholar]
- 5.Anderson BD, et al. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–26. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006;103(12):4640–5. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benencia F, et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther. 2005;12(5):789–802. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Gauvrit A, et al. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68(12):4882–92. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 10.Guillerme JB, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19(5):1147–58. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 11.Haen SP, Rammensee HG. The repertoire of human tumor-associated epitopes--identification and selection of antigens and their application in clinical trials. Curr Opin Immunol. 2013;25(2):277–83. doi: 10.1016/j.coi.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–75. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migali C, et al. Strategies to modulate the immune system in breast cancer: checkpoint inhibitors and beyond. Ther Adv Med Oncol. 2016;8(5):360–74. doi: 10.1177/1758834016658423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gholami S, et al. A novel vaccinia virus with dual oncolytic and antiangiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014;148(3):489–99. doi: 10.1007/s10549-014-3180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiebig HH, et al. Inactivated orf virus (Parapoxvirus ovis) induces antitumoral activity in transplantable tumor models. Anticancer Res. 2011;31(12):4185–90. [PubMed] [Google Scholar]
- 16.Rintoul JL, et al. ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic. Mol Ther. 2012;20(6):1148–57. doi: 10.1038/mt.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson AJ, Petersen GV. Orf virus infection of workers in the meat industry. N Z Med J. 1983;96(725):81–5. [PubMed] [Google Scholar]
- 18.Fachinger V, et al. Poxvirus-induced immunostimulating effects on porcine leukocytes. J Virol. 2000;74(17):7943–51. doi: 10.1128/jvi.74.17.7943-7951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friebe A, et al. Characterization of immunostimulatory components of orf virus (parapoxvirus ovis) J Gen Virol. 2011;92(Pt 7):1571–84. doi: 10.1099/vir.0.028894-0. [DOI] [PubMed] [Google Scholar]