Supplemental digital content is available in the text.
Key Words: β-Tubulin, Fallopian tube, Secretion, BRCA, Preneoplastic condition, Ovarian cancer
Abstract
Objectives
Class V Beta tubulin isotype (βV-tubulin) was recently found to have tissue-specific expression patterns in epithelial tissues with secretory function and aberrant expression in tumors. The aims of this pilot study were (a) to examine expression of βV-tubulin in the fallopian tube epithelium (FTE) of patients who underwent salpingectomy, (b) to characterize FTE atypia in high-risk patients with BRCA mutations, and (c) to determine expression of βV-tubulin in serous ovarian neoplasms.
Methods
Immunohistochemistry, with a highly specific antibody developed in our laboratory against human βV-tubulin, was used to evaluate expression in paraffin-embedded sections of the fallopian tube (n = 82) and tumors (n = 13), from prospectively selected cases, categorized by reason for salpingectomy.
Results
βV-tubulin, when present, was expressed in secretory cells and essentially never in ciliated cells of the FTE. Histologically “normal” FTE had very rare, scattered βV-tubulin–positive cells; percentage positivity increased in cases of serous ovarian neoplasms. The highest expression was observed in FTE from patients with BRCA mutant breast cancer. Four distinct types of FTE atypia were delineated in patients with known BRCA mutations. In a few additional test cases of ovarian neoplasms, βV-tubulin was highly expressed, with the extent and intensity of staining elevated in high-grade serous carcinomas compared with serous borderline tumors.
Conclusions
In summary, βV-tubulin was localized to secretory cells of the distal FTE and its expression varied according to the clinical diagnosis. The frequency of these cells and thus expression of βV-tubulin were dramatically enriched in tissue obtained from BRCA mutant cases, which also exhibited pronounced histologic atypia indicative of early predysplastic aberrations. Furthermore, elevated expression of βV-tubulin correlated with poor differentiation status in serous ovarian neoplasms.
βV-tubulin, encoded by the TUBB6 gene, is 1 of 8 distinct isotypes of β-tubulin, each having cell- and organ-specific expression. β-Tubulin is an important therapeutic target possessing specific binding domains for chemotherapeutic agents including Taxol® and the vinca alkaloids.1 Microtubules, composed of tubulin heterodimers, are integral components of the intracellular trafficking system and exosome export, essential for the migration and invasion processes of metastasis.2,3 Technical challenges have limited progress in delineating isotype-specific functions for tubulins.4 One study documented impaired insulin secretion and slower vesicular trafficking in murine cells that express mutant Huntingtin protein, where βV-tubulin was identified as the tubulin isotype mediating the process.5
A highly specific antibody to human βV-tubulin, a poorly studied isotype of the tubulin superfamily, was generated in our laboratory and uncovered tissue-specific expression.6 βV-tubulin was present in tissue types associated with secretion, such as glandular and ductal cells, and in epithelial cells with secretory function. We also found evidence of aberrant expression in epithelial malignancies, albeit in a pilot analysis.6 Considering the probable involvement of this tubulin isotype in facilitating intracellular trafficking and secretion, and the participation of these processes in the dissemination of dysplastic cells, we evaluated the expression of βV-tubulin in the fallopian tube and in serous ovarian neoplasms.
Serous ovarian neoplasms include high-grade serous carcinomas (HGSCs), which are type 2 primarily associated with TP53 mutation, and the slower-progressing serous borderline tumors (SBTs) that are type 1 often associated with BRAF/KRAS mutation.7,8 Although the general population has a 1.3% risk of developing ovarian carcinoma by age 70 years of age, that lifetime risk rises to 39% to 60% in those with BRCA1 mutations and 11% to 27% in BRCA2 mutant carriers.9,10 Ovarian cancers were first associated with the fallopian tube epithelium (FTE), after studies examining tissue from BRCA mutant patients who underwent risk-reducing prophylactic surgery involving removal of their ovaries (oophorectomy) and fallopian tubes (salpingectomy).11–13
The close proximity of the fallopian tube with the ovary is thought to facilitate the migration of dysplastic cells of the FTE, considered an initiating event in ovarian cancer etiology.14,15 Conversely, this may also allow for intraepithelial metastases of HGSC to the fallopian tube.16 The FTE is composed of ciliated cells and nonciliated, secretory cells. An important function of the FTE is secretion of tubal fluid, which may support tumor cell dissemination; deregulation of this cellular functionality is poorly understood. It is the secretory cells of the FTE that have been identified as putative dysplastic cells of origin.17–19 Serous tubal intraepithelial carcinomas (STICs) appear to have almost all the genomic aberrations of full-blown HGSC16 and persist after neoadjuvant treatment.20 Therefore, morphological atypia in the FTE that precede STICs may merit scrutiny as putative precursor lesions. There is much yet to be deciphered about the nature of these predysplastic secretory cells of the FTE and their role in early ovarian cancer pathogenesis.
Here, in an immunohistochemistry-based pilot analysis, we examine the poorly studied isotype, βV-tubulin, in secretory cells of the human FTE.
MATERIALS AND METHODS
Cohort Selection
The Department of Pathology at Montefiore Medical Center (MMC) provided 77 cases for this study, and 18 cases were sourced from a tissue microarray acquired by the Weatherall Institute of Molecular Medicine.21 Tubal sections from the fimbrial end were cut from paraffin-embedded tissue of patients whose original pathology reports indicated the reasons for salpingectomy and clinical diagnosis. The menstrual cycle status of the patients was unavailable. Postsurgical tumor sections were identified from 13 cases with serous ovarian neoplasms including HGSCs (type 2) and SBTs (type 1). The study was approved by the institutional review boards of MMC and Albert Einstein College of Medicine.
Immunohistochemical Staining
Immunohistochemical (IHC) stain was performed using Envision Plus Horseradish Peroxidase system (Dako, Carpinteria, CA) as previously described.6 Briefly, rehydrated tissue sections were blocked and incubated with the relevant antibodies using standard washes. Staining was detected using the 3,3′-diaminobenzidine substrate system. Antigen retrieval was not performed. The concentration of primary antibody used was 1:1000 for βV-tubulin incubated overnight at 4°C, 1:50 for p53 (Dako, #M7001) incubated for 30 minutes, and 1:600 for Pax-8 (ProteinTech Group, #10336-1-AP) incubated for 1 hour at room temperature. Hematoxylin and eosin staining was also performed as per standard protocol. Studies were interpreted in conjunction with appropriate positive and negative controls. The patterns of immunoreactivity for Pax-8 in nonneoplastic epithelium and βV-tubulin in endothelial and stromal cells were used as internal positive controls.
Analysis of Immunostaining
After staining, which was performed blinded to case designations and patient information, analysis was carried out by 2 independent observers. The extent of immunoreactivity of βV-tubulin was estimated by pathologic evaluation of stained tumor tissue to yield semiquantitative intensity scores ranging from a scale of 0 to 3. A percentage positivity score was assigned based on the average number of positively stained cells vs unstained cells per section. This was determined by independent visual examination for number of positive cells per section. H scores were subsequently assigned from these 2 metrics and designated (−) no staining, (+) weak staining, (++) moderate staining, or (+++) strong staining.
Statistical Analysis
A nonparametric Mann-Whitney test (2-tailed) was used to compare the distribution of βV-tubulin expression in select cohorts. Analyses were performed using GraphPad Prism 6 software.
RESULTS
βV-tubulin, When Present, Is Specific to Secretory Cells of the FTE
Immunohistochemical staining using standard specific antibodies indicated the baseline distribution of the 2 FTE cell types in this cohort. As expected, FTE had an even distribution of ciliated cells, marked by acetylated α-tubulin, which localized to the ciliary membrane (Fig. 1A), and secretory cells, marked by Pax-8 that had nuclear localization (Fig. 1B). Immunohistochemistry using the βV-tubulin antibody demonstrated expression in some secretory cells and essentially none in ciliated cells of the FTE. Stromal cells of the fallopian tube and endothelial cells of blood vessels served as internal controls because they always stained positive for βV-tubulin.6
FIGURE 1.

βV-tubulin enrichment in secretory cells of the FTE. Illustrative staining delineates the 2 FTE cell types, namely, ciliated cells in panel A positive for acetylated α-tubulin and nonciliated secretory cells in panel B positive for Pax-8. Immunohistochemical staining of βV-tubulin in FTE shows negative staining in cohort 1 (C) and positive staining in isolated groups of secretory cells with aberrant morphology in cohort 2 (D), cohort 3 (E), and cohort 4 (F). The percentage of βV-tubulin–positive cells in the FTE of the various cohorts is graphically depicted in panel G. Means are indicated by + in the box-and-whisker plot. Image magnification ×60.
Differential Enrichment of βV-Tubulin Expression in BRCA Mutant vs Benign Cases
Our total sample size of 95 cases was divided into 7 subsets, labeled cohorts 1 to 7 as detailed in Table 1. This subdivision was based on the reason for salpingectomy as per pathologic annotation and BRCA mutation status. The presence and pattern of enrichment of βV-tubulin varied in a cohort-dependent manner.
TABLE 1.
Age and mutation status of patients at the time of salpingectomy

βV-tubulin was completely absent in the FTE of samples collected for contraceptive purposes at the end of pregnancy, or after an ectopic pregnancy (Fig. 1C). Although most of the epithelium was negative for βV-tubulin, rare positive secretory cells, often in small isolated groups, were observed in FTE of samples with benign conditions from cohort 2 (Fig. 1D) and also in cases with nonovarian gynecological malignancies from cohort 3 (Fig. 1E). In cases with diagnosed serous ovarian neoplasms, cohort 4, groups of βV-tubulin–positive secretory cells were present with slightly increased frequency (Fig. 1F). To put this in perspective, the actual number of positive cells was less than 0.05% in most cases from cohorts 2 and 3, with many cases having less than 10 positive cells, whereas cohort 4 had an average of approximately 1.5% positive staining for βV-tubulin (Fig. 1G). No correlation of βV-tubulin expression with age was observed. The BRCA status of the cases in cohort 4 is unknown because these patients do not currently undergo routine BRCA testing.
In contrast, BRCA mutant cases (n = 30) demonstrated significantly increased expression of βV-tubulin. Cohorts 5, 6, and 7 represent cases that were tested for BRCA mutations due to breast cancer diagnosis or familial history of breast/ovarian cancer. The mean percentage of βV-tubulin expressing secretory cells was approximately 1.6% in BRCA wild type vs a mean of ~8.3% in BRCA mutants (Fig. 1G), which is statistically significant (P = 0.0005). There was no difference in βV-tubulin expression between BRCA1 and BRCA2 mutant cases. Overall, the percentage of βV-tubulin expressing secretory cells was significantly lower in patients from cohorts 1, 2, and 3 (whose FTEs are classified as histologically “normal”) than those in cohorts 6 and 7 with known BRCA mutations (P < 0.0001).
The secretory cells that show enrichment for βV-tubulin often display subtle morphological differences from their βV-tubulin–negative counterparts (Figs. 1D, E). In some cases from cohorts 2, 3, and 4, the morphological differences include signs of atypia such as nuclear enlargement, protrusion from the epithelial layer toward the FT lumen, and discohesiveness. An increased nuclear to cytoplasmic ratio ranging from 0.5:1 to 1:1 was determined for ovarian carcinomas relative to benign cohorts. These morphological changes were never noted in the βV-tubulin–negative secretory cells.
Secretory Cell Atypias Are More Prevalent in BRCA Mutation Carriers
In FTE of patients undergoing prophylactic salpingectomy due to known BRCA mutations (cohorts 6 and 7), secretory cell atypia was more pronounced, widely prevalent, and not confined to βV-tubulin expressing secretory cells. Through close examination, we categorized these atypical phenotypes as 4 subtypes that demonstrated (a) monomorphic expansion, (b) multilayered stratification, (c) stromal intrusion, and (d) discohesive luminal extrusion. Because atypia was observed solely in the secretory cell type of the FTE, Pax-8 IHC, which marks secretory cells, was used to highlight and quantify these phenotypes. The number of cases in each cohort having these specific atypical phenotypes is presented in Table 2. Multiple atypias per case were often observed. Monomorphic expansion indicates loss of normal heterogeneous distribution of ciliated and secretory cells, in favor of continuous stretches of secretory cells only (Figs. 2A, E, I). Multilayered stratification indicates loss of normal single-layered columnar FTE in favor of multilayered organization with clusters of secretory cell outgrowths (Figs. 2B, F, J). Stromal intrusion indicates regions where clusters of secretory cells appear to invaginate toward the stroma (Figs. 2C, G, K). Discohesive luminal extrusion indicates regions where clusters of secretory cells appear beyond the plane of the adjacent epithelial cells, and to protrude toward, or detach into the FT lumen (Figs. 2D, H, L). Although subtle phenotypic aberrations were noted in BRCA untested and BRCA wild-type cases, there was a dramatic enrichment of more severe and clearly defined epithelial atypia in the BRCA mutant cohort, as evidenced by the higher incidence in cohorts 6 and 7 (Table 2).
TABLE 2.
Prevalence of FT epithelial atypia in BRCA carriers vs noncarriers

FIGURE 2.

Pax8 and βV-tubulin expression highlight fallopian tube epithelial atypia in BRCA carriers. Representative IHC of hematoxylin and eosin stain is presented in panels A to D, Pax8 expression in panels E to H, and βV-tubulin in panels I to L. BRCA carriers show enrichment of atypical phenotypes in 4 categories. Blue arrows indicate stretches of monomorphic expansion, black arrows indicate multilayered stratification, red arrows indicate stromal intrusion, and green arrows indicate discohesive luminal extrusion. Image magnification ×40.
Staining with βV-tubulin in the BRCA cohort highlighted enrichment in the same 4 categories of atypia (Figs. 2I, J, K, L), although not all atypic lesions were positive for βV-tubulin. Clusters of cells with nuclei 1.5 to 2 times larger than adjacent epithelial cells were common in cohorts 6 and 7. The BRCA mutant cohort was largely negative for p53 expression, implying that the histologic remodeling and atypia documented here precede the development of “p53 signatures” in putative precursor lesions.22,23
βV-Tubulin Expression in Serous Ovarian Neoplasm May Vary With Differentiation Status
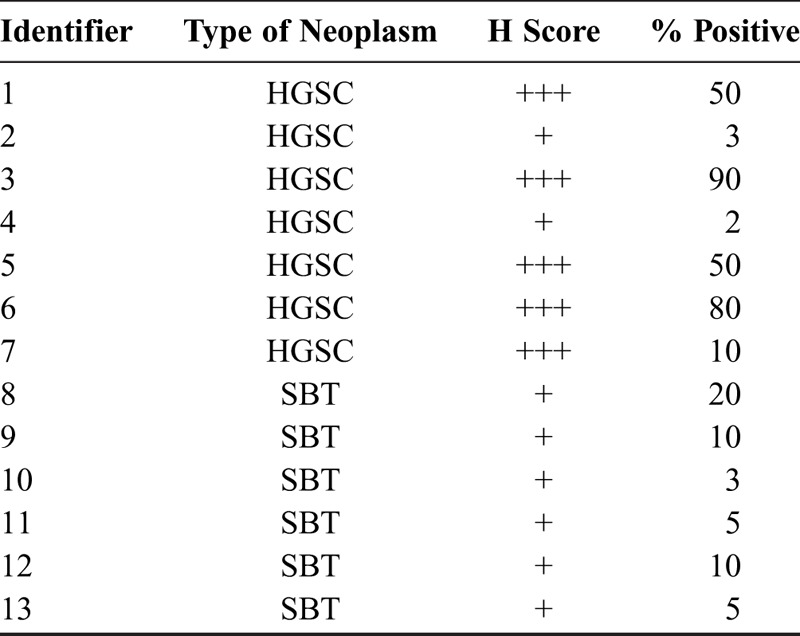
Cases with serous ovarian neoplasms, cohort 4 (n = 13), comprise both those diagnosed with HGSC and those with SBT. Clinicopathologic details such as age, International Federation of Gynecology and Obstetrics scores, and survival data, where available, are provided in Table S1, http://links.lww.com/IGC/A579. Discohesive cells that appear to detach from the tumor mass in HGSCs stain positive for βV-tubulin (Figs. 3D, E). Cases with HGSC (n = 7) exhibited more intense βV-tubulin expression that translated to a higher H score relative to SBT (n = 6; Table 3). High-grade serous ovarian cancer had intense positive staining largely diffused through the tumor section (Figs. 3B, C), whereas SBT had less intense staining in a focally clustered pattern (Figs. 3A, C). Thus, βV-tubulin isotype is enriched in more poorly differentiated neoplasms.
FIGURE 3.

Expression of βV-tubulin correlates with differentiation status in serous ovarian neoplasms. Immunohistochemical staining for βV-tubulin reveals less intense and focal staining pattern for SBTs as seen in panel A vs more intense and diffuse staining pattern for HGSC as seen in panel B. Percentage of βV-tubulin–positive cells per tumor is graphically depicted in panel C. Means are indicated by horizontal lines in the scatter plot. Discohesiveness in βV-tubulin–positive cells of the tumor is visible in HGSC cases as seen in representative images (D and E). Image magnification ×40 (A, D, and E) and ×60 (B).
TABLE 3.
Differential βV-tubulin expression in the serous ovarian neoplasms
DISCUSSION
Our observation of βV-tubulin expression in the secretory cell type of the FTE is of interest because of its potential role in cellular secretion. In the cancer context, βV-tubulin expression in secretory cells may be significant because it is secretory cell outgrowths of the FTE that are implicated in the development of serous ovarian carcinoma.22,24 In the FTE of cases with BRCA mutations or serous ovarian neoplasms, βV-tubulin–positive cells often appeared discohesive, with protrusion toward the FT lumen, and some cells had enriched polarized expression toward the lumen. Discohesiveness was also evident in poorly differentiated HGSC that expressed high levels of βV-tubulin. These observations are relevant in light of current dogma proposing that shedding of FTE cells with migratory capabilities, followed by attachment to adjacent ovarian epithelium, is the source of ovarian cancer initiation and locoregional spread.
Furthermore, among our cohorts, we observed isolated groups of enlarged, βV-tubulin expressing secretory cells in cases with conditions such as leiomyoma (cohort 2) and in cases with nonovarian endometrial carcinoma (cohort 3), both of which are associated with a chronic inflammatory milieu.25 Significant increases in βV-tubulin expression were also evident in cases with serous ovarian neoplasms that have a strong inflammatory component, similar to endometrial cancer.26 This is in stark contrast to the absence of βV-tubulin expression in pregnancy-related salpingectomies where the inflammatory processes associated with menstruation are presumably absent. Therefore, the relationship between βV-tubulin and chronic inflammation merits further investigation.
Although FTE atypias were observed across cohorts, this study uncovered profound histologic remodeling in BRCA mutant cases. Our observations hold true for both premenopausal and postmenopausal cases within the BRCA cohort and therefore appear unrelated to age. Although similar aberrations of the FTE have previously been noted in BRCA mutant cases,27 we were able to consistently define 4 aberrant phenotypes. Of the 4, multilayered stratification was most common, followed by stretches of monomorphic expansion. Although limited areas of monomorphic stretches were relatively common in the BRCA cohorts, 2 cases had more than 80% of the FTE comprising secretory cells alone.
βV-tubulin was only expressed in regions of phenotypic atypia in BRCA mutant cases, although not all atypic regions expressed βV-tubulin. This, together with its absence in phenotypically “normal” secretory cells of BRCA untested cases, indicates an association of βV-tubulin expression with premalignant alterations in FTE.
The tissue organizational changes observed in BRCA mutant cases were evident even in the absence of ovarian carcinoma, although most patients in this cohort had confirmed breast cancer. We considered the possibility that the extent of FTE atypia may be a consequence of systemic chemotherapy from breast cancer treatment. However, both FTE atypia and aberrant βV-tubulin expression were evident at low frequency in breast cancer patients from cohort 5 who tested negative for BRCA mutation but who also received systemic chemotherapy. The possibility remains that defects in DNA-repair pathways render other tissues vulnerable to damage after exposure to systemic chemotherapy, and that this can influence the development of atypical dysplastic lesions. This hypothesis requires further evaluation in a chemo-naïve cohort.
The atypical βV-tubulin cells seem to develop at a stage before the postulated progression from p53 mutational signatures to STICs and to invasive ovarian cancer. Most FTEs in our study had negative p53 staining and a scattered pattern of Ki-67 expression (data not shown), suggesting that these frequently evaluated molecular changes occur subsequent to the enrichment of βV-tubulin expression documented here. However, we are cautious about correlating p53 expression with mutational status because several studies have indicated that it is controversial, requires careful interpretation, and should be complemented by other approaches.23,28
There is a need to refine and expand the existing compendium of IHC markers available for accurate detection of dysplastic lesions in FTE, and also to help delineate the molecular etiology of the disease. Thus, in addition to its putative role in cellular secretion, βV-tubulin merits further investigation as an IHC-based surrogate for FTE secretory cell atypia that precedes pathologic evidence of dysplasia. More research is required to address whether expression of βV-tubulin has a causative association with tumorigenesis or reflects increased protein trafficking to support the proinflammatory secretion typical of this disease.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr Gary Goldberg for assistance with accessing the MMC cancer database and Camille Black, who identified and retrieved tissue blocks. They also gratefully acknowledge the contribution of Dr Ahmed Ashour Ahmed and Fiona Chen for technical support and discussions.
Footnotes
Sources of support: These studies were supported by the Breast Cancer Research Foundation, the National Foundation for Cancer Research, National Cancer Institute Grant CA077263, and the Albert Einstein Cancer Center Support Grant of the National Institutes of Health, under award number P30CA013330.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no conflicts of interests.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.Orr GA, Verdier-Pinard P, McDaid H, et al. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnaeker EM, Ossig R, Ludwig T, et al. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: prerequisite in human melanoma cell invasion. Cancer Res. 2004;64:8924–8931. [DOI] [PubMed] [Google Scholar]
- 3.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. [DOI] [PubMed] [Google Scholar]
- 4.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith R, Bacos K, Fedele V, et al. Mutant huntingtin interacts with {beta}-tubulin and disrupts vesicular transport and insulin secretion. Hum Mol Genet. 2009;18:3942–3954. [DOI] [PubMed] [Google Scholar]
- 6.Chao SK, Wang Y, Verdier-Pinard P, et al. Characterization of a human βV-tubulin antibody and expression of this isotype in normal and malignant human tissue. Cytoskeleton (Hoboken). 2012;69:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vang R, Shih Ie M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62:44–58. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SH, Shaw P. BRCA and early events in the development of serous ovarian cancer. Front Oncol. 2014;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. [DOI] [PubMed] [Google Scholar]
- 12.Folkins AK, Jarboe EA, Roh MH, et al. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009;113:391–396. [DOI] [PubMed] [Google Scholar]
- 13.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. [DOI] [PubMed] [Google Scholar]
- 14.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108:7547–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piek JM, van Diest PJ, Zweemer RP, et al. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844. [DOI] [PubMed] [Google Scholar]
- 16.Eckert MA, Pan S, Hernandez KM, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. [DOI] [PubMed] [Google Scholar]
- 18.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. [DOI] [PubMed] [Google Scholar]
- 19.Shaw PA, Rouzbahman M, Pizer ES, et al. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–1138. [DOI] [PubMed] [Google Scholar]
- 20.Colon E, Carlson JW. Evaluation of the fallopian tubes after neoadjuvant chemotherapy: persistence of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2014;33:463–469. [DOI] [PubMed] [Google Scholar]
- 21.Hellner K, Miranda F, Fotso Chedom D, et al. Premalignant SOX2 overexpression in the fallopian tubes of ovarian cancer patients: discovery and validation studies. EBioMedicine. 2016;10:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehra K, Mehrad M, Ning G, et al. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci (Elite Ed). 2011;3:625–634. [DOI] [PubMed] [Google Scholar]
- 23.Lassus H, Butzow R. The classification of p53 immunohistochemical staining results and patient outcome in ovarian cancer. Br J Cancer. 2007;96:1621–1622 author reply 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen EY, Mehra K, Mehrad M, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace AE, Gibson DA, Saunders PT, et al. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010;206:141–157. [DOI] [PubMed] [Google Scholar]
- 26.Hung RJ, Ulrich CM, Goode EL, et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Ning Y, Abushahin N, et al. Secretory cell expansion with aging: risk for pelvic serous carcinogenesis. Gynecol Oncol. 2013;131:555–560. [DOI] [PubMed] [Google Scholar]
- 28.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


