Abstract
CD22 and Siglec-G are members of the Siglec (sialic-acid-binding immunoglobulin-like lectin) family of inhibitory co-receptors expressed on B cells that participate in enforcement of peripheral B cell tolerance. We have shown previously that when a B cell receptor (BCR) engages its cognate antigen on a cell surface that also express Siglec ligands, B cell Siglecs are recruited to the immunological synapse, resulting in suppression of BCR signaling and B cell apoptosis. Since all cells display sialic acids, and CD22 and Siglec-G have distinct yet overlapping specificities for sialic acid-containing glycan ligands, any cell could, in principle, invoke this tolerogenic mechanism for cell surface antigens. However, we show here that C57BL/6J mouse RBCs are essentially devoid of CD22 and Siglec-G ligands. As a consequence, RBCs that display a cell surface antigen, membrane-bound hen egg lysozyme (mHEL), strongly activate antigen-specific B cells. We reasoned that de novo introduction of CD22 ligands in RBCs should abolish B cell activation toward its cognate antigen on the surface of RBCs. Accordingly, we employed a glyco-engineering approach wherein synthetic CD22 ligands linked to lipids are inserted into the membrane of RBCs. Indeed, insertion of CD22 ligands into RBC cell surface strongly inhibited B cell activation, cytokine secretion, and proliferation. These results demonstrate that the lack of Siglec ligands on the surface of murine RBCs permits B cell responses to erythrocyte antigens, and shows that Siglec-mediated B cell tolerance is restricted to cell types that express glycan ligands for the B cell Siglecs.
Keywords: B cells, Siglecs, tolerance, erythrocyte, red blood cell, CD22, Siglec-G, sialic acid, glycans
Introduction
CD22 and Siglec-G (Siglec-10 in humans) are members of the Siglec (sialic acid-binding immunoglobulin-like lectins) immunoglobulin family, serve as inhibitory co-receptors of the B cell receptor (BCR), and play important roles in tolerance induction (1). Using polymers or liposomes decorated with both antigen and high affinity CD22 or Siglec-G ligands, we and others demonstrated that a tolerance mechanism is induced by co-ligating CD22 or Siglec-G and the BCR, which stems from inhibited BCR signaling and induction of a pro-apoptotic pathway in antigen-specific B cells (2–5).
Based on the concept that enforced ligation of CD22 or Siglec-G with the BCR leads to B cell tolerance, we have also reported that endogenous sialic acid ligands presented on cells bearing a cognate antigen for a B cell can induce a similar tolerogenic program in antigen-specific B cells through recruiting CD22 or Siglec-G into the immunological synapse (6). Since all mammalian cells have a dense array of sialic acids on their cell surface, we wondered if this mechanism of tolerance is broadly applicable to other cell types displaying a cell surface autoantigen or alloantigen, in particular erythrocytes - the most abundant cell type in the blood. Motivation for investigating whether a Siglec-dependent mechanism is at play for inducing immunological tolerance to red blood cells (RBCs) comes from several perspectives. First, several groups detected glycan ligands of CD22 on human erthrythrocytes (7–9). Second, foreign antigen immobilized on the surface of RBCs induces robust antigen-specific immunological tolerance (10–13). Work by Hubbell and co-workers showed that RBCs accumulate in hepatic immune cells, where attached antigens induce antigen-specific T cell tolerance by either deletion of antigen-specific T cells (14, 15) or generation of regulatory T cells (16). However, tolerance induction by RBCs appears to be a complex mechanism, since tolerance is not acquired in all patients receiving blood transfusions (17, 18) and tolerance to RBC antigens is abrogated under conditions of inflammation in mice (11). Moreover, in patients with sickle cell disease, the pro-inflammatory state at the time of transfusion is suggested to favor the development of alloantibodies (19). Accordingly, these results imply that mechanisms of tolerance to antigens on the surface of RBCs are not fully understood. In contrast to the effects of RBC antigens on T cells that has been investigated, less is known about the impact of antigens displayed multivalently on the surface of RBCs, which have the potential of strongly activating B cells. Interestingly, one recent report demonstrated that RBCs with a dense array of cell surface antigens are less likely to be tolerogenic in mice, leading to antibody production toward the antigen (20).
Here, we investigate the extent to which B cell Siglecs play a role in regulating B cell responses to an antigen expressed on the cell surface of RBCs. We observe that mouse RBCs are essentially devoid of CD22 and Siglec-10 glycan ligands, with the consequence being that antigens expressed on the cell surface of RBCs cannot invoke the tolerogenic mechanism mediated by Siglecs. Instead, they induce strong B cell activation both in vitro and in vivo. We further demonstrate that B cell activation to RBC membrane antigens can be suppressed by insertion of CD22 ligands into the membrane of RBCs to strongly inhibit BCR signaling.
Materials and Methods
Animal studies
The Scripps Research Institute IACUC approved all experimental procedures involving mice. C57BL/6J-CD22−/− mice were obtained from L. Nitschke (University of Erlangen). C57BL/6J-ST6Gal-1-deficient mice (ST6Gal1−/−) were generated as described (21). C57BL/6J-MD4 and C57BL/6J-KLK4 mice were obtained from Jackson laboratories. WT C57BL/6J mice were obtained from the TSRI rodent breeding colony. All mice were maintained in pathogen-free conditions at TSRI breeding facility.
Red blood cell isolation
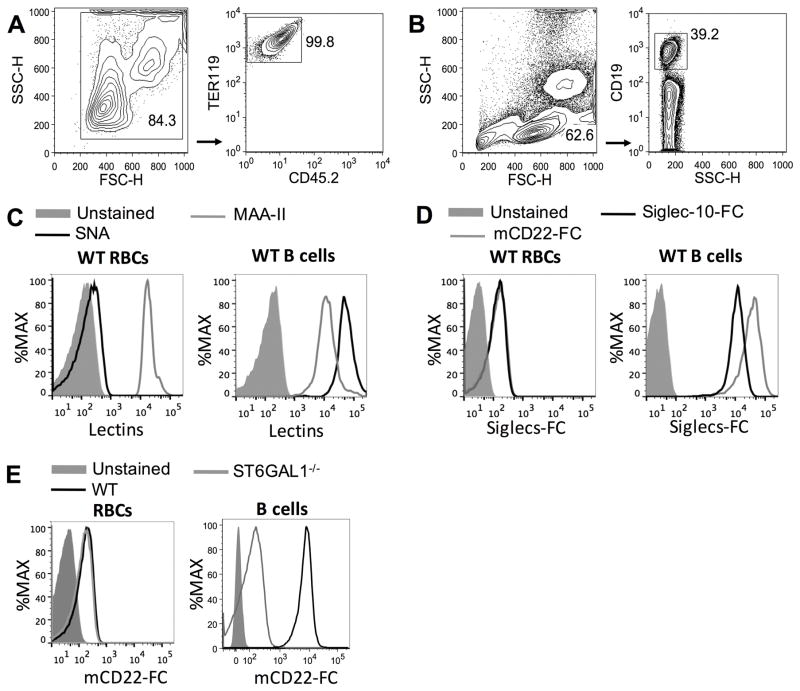
Blood was collected by cardiac puncture using a 1 mL syringe and a 22-gauge needle within 10 μL of heparin (1 U/μL). To remove leukocytes, 1 mL of blood was mixed with 1 mL of Hank’s buffered salt solution (HBSS) and gently layered on top of a 2-layer Percoll PLUS (GE Healthcare) gradient (72% and 65% in HBSS, 3 mL of each layer), and then centrifuged at 1200 xg for 35 min at 22 °C. Percoll gradients were prepared after osmolality adjustment of Percoll PLUS by adding 9 parts of Percoll PLUS to 1 part of PBS 10x. Mononuclear and polymorphonuclear cells accumulated at the top of the 65% layer and at the interface between the 72% and 65% layers, respectively, and were discarded, RBCs pelleted within the 72% layer. Cells were washed twice with HBSS and >99% of the cells were erythrocytes as determined by a CD45−Ter-119+ staining pattern by flow cytometry (FACS) (Figure 1A). FACS analysis of isolated RBCs with anti-CD41, a marker of mouse platelets, marker) showed that the RBCs did not have bound platelets (>0.03% of CD41+Ter-119+ cells, data not shown). FACS data were obtained on an LSR-II (BD Biosciences) and analyzed using FlowJo software.
Figure 1. FACS analysis reveals MAA-II binding to erythrocytes surface, but not SNA, mCD22-Fc or Siglec-10-Fc.
Isolated mouse red blood cells (RBCs) or total splenocytes were stained with SNA-FITC, biotinylated MAA-II, mCD22-Fc plus anti-human IgG1-APC or Siglec-10-Fc plus anti-human IgG1-APC. RBCs were also stained with TER 119-PE and CD45.2-FITC and splenocytes with CD19-PE. (A) Contour plots of SSC vs FSC and TER 119-PE vs CD45.2-FITC of RBCs. (B) Contour plots of SSC vs FSC and CD19-PE vs SSC-H of splenocytes. (C) Representative histograms of WT RBCs (TER 119+/CD45.2−) and WT B cells (CD19+) are showing the staining profiles for lectins SNA (black line), MAA-II (gray line) or unstained cells (filed gray lines). (D) Representative histograms of WT RBCs and WT B cells of unstained cells (filed gray lines), stained with mCD22-Fc (gray line) or Siglec-10-Fc (black line). (E) RBCs or B cells from WT (black lines) or ST6Gal1−/− (gray lines) mice were stained with mCD22-Fc; filed gray line represent unstained cells. SNA = Sambucus nigra; MAA-II = Maackia amurensis II. These experiments were independently performed 3 times in triplicate.
Probing cells with lectins and Siglecs-Fc
Total splenocytes or isolated RBCs were re-suspended in phosphate-buffered saline (PBS) with calcium and magnesium (Gibco) and incubated with mouse Fc-block (anti-CD16/32, Biolegend) for 30 min at 4 °C, washed and stained for 30 min, 4 °C with FITC conjugated Sambucus nigra lectin (SNA-I) from elderberry bark (Vector Laboratories), biotinylated Maackia amurensis lectin II (MAA-II) (Vector Laboratories), mCD22-Fc or Siglec-10-Fc. Cells were washed twice with PBS (containing Ca+2/Mg+) and incubated with Ter-119-PE (Biolegend) and anti-human IgG1-APC (Jackson ImmunoResearch) or streptavidin-FITC. For staining of the B cells, CD19-PE (Biolegend) and anti-human IgG1-APC or streptavidin-FITC (Biolegend) were used.
Culture of Chinese hamster ovary (CHO) cells expressing mCD22-Fc or Siglec-10-FC
Expression of mCD22-Fc or Siglec-10-Fc from stably transfected CHO cells were described previously (22–24). Briefly, CHO cells were maintained in Dulbecco’s modified Eagle’s medium/F-12 medium supplemented with 10% fetal bovine serum and 500 μg/mL hygromycin B (Roche Applied Science). Supernatants of CHO cells were collected after approximately 10 days of culture, and were used in FACS assays.
Insertion of high affinity CD22 ligand into RBCs
The preparation of the high affinity CD22 ligand (6′BPANeu5Gc-PEG-DSPE) has been described previously (1, 4). 6′BPANeu5Gc-PEG-DSPE (BPANeu5Gc) or PEG-DSPE (PEG, as a control) were incubated with RBCs (1×107 cells/mL) in HBSS buffer at a concentration of 6.3 μM for one hour at 37 °C. Cells were washed twice and used immediately in the assays.
B cell isolation
Mouse IgMHEL B cells (CD19+CD45.1+IgMa+) from MD4 mice and B-cells from KLK4 mice were isolated from spleen by negative selection using magnetic beads (Miltenyi Biotec).
In Vitro B Cell Assays
CD45.1+ WT or CD22−/− splenocytes (0.4×106 cells) containing IgMHEL B cells were plated in U-bottom 96-well culture plates. B cells or RBCs isolate from CD45.2+ KLH4 mice - described as mHEL B cells and mHEL-RBCs, respectively - were added to splenocytes at different ratios and co-cultured at 37 °C, 5% of CO2 for 24 h. Dead cells were gated out with 1 μg/mL of propidium iodide (PI). The activation marker (CD86) was analyzed on PI− IgMHEL B cells (CD45.1+CD19+IgMa+) by FACS. The activation of isolated IgMHEL B cells (0.2×106 cells/well) was also evaluated for cytokine secretion following by co-culture of mHEL B cells or mHEL-RBCs at 37 °C, 5% of CO2 for 24 h. ELISA kits (Biolegend) were used to measure mouse IL-2, TNF, IL-6 and IL-10 in culture supernatant.
In vivo B cell Assays
Following RBC lysis of splenocytes from CD45.1+ MD4 mice (containing IgMHEL B cells), cells were fluorescently labeled with 1 μM Cell Trace Violet (CTV; Invitrogen) in HBSS for 7 min at room temperature. Cells were washed twice with PBS and resuspended at 5×106 cells/mL. The CTV-labeled splenocytes (1×106, 200 μl) were injected into host mice via the tail vein. The following day, PBS, 2×106 or 50×106 of mHEL-RBCs were injected via the tail vein. Host spleens were harvested on the 4th day, stained with PI and antibodies against CD45.1, CD19 and IgMa, followed by FACS analysis.
Results
Murine RBCs express low levels of CD22 ligands
Motivated to understand whether B cell tolerance though the B cell Siglecs contributes the tolerance induce to cell surface antigens we examined the levels of sialic acid-containing glycans on murine RBCs using several lectins. Specifically, we used SNA and MAA-II, which recognize sialic acids in the Siaα2-6Gal and Siaα2-3Gal linkages, respectively. These linkages of sialic acid to the underlying glycan are among the most common found on mammalian cells outside of the nervous system, and also the linkages accommodated by the B cell Siglecs, CD22 and Siglec-G, respectively (3, 25–29). Lectin binding was assessed by flow cytometry to examine staining of erythrocytes (Ter-119+) and B cells (CD19+) in parallel (Figure 1A and 1B, respectively). While SNA and MAA-II showed high binding to B cells, only MAA-II bound the RBCs (Figure 1C). These results suggest that sialic acid residues on mouse RBCs are predominantly in the Siaα2-3Gal glycosidic linkage. We next stained cells with Fc-chimera of murine CD22 (mCD22), which is specific for Neu5Gcα2-6Gal sialosides, a sialic acid produced by most mammals including mice, but not by humans that instead produce Neu5Ac (30, 31). We also used Siglec-10-Fc, the human ortholog to Siglec-G, which is specific for both Siaα2-3Gal and Siaα2-6Gal linkages (32). We find that both mCD22-Fc and Siglec-10-Fc display only weak binding to murine RBCs, yet bind to B cells at >100-fold higher levels (Figure 1D). The weak binding of mCD22-Fc to WT RBCs was indistinguishable from binding to RBCs from ST6Gal1−/− mice that lack the key sialyltransferase responsible for the biosynthesis of mCD22 ligands (Figure 1E), suggesting that weak binding over the unstained cells in Figure 1D represents background staining, or staining to non-sialic acid dependent ligands. Although non-sialic acid dependent ligands have been proposed for CD22, they were not implicated in regulation of BCR signaling (33).
RBCs with membrane antigen induce strong activation of antigen reactive B cells
The lack of Siglec ligands on RBCs is relevant to the role of Siglecs in B cell tolerance, since Siglecs are recruited to the immunological synapse if ligands are present on the antigen bearing cell, resulting in suppression of BCR signaling (1, 4, 6). To investigate whether Siglecs are involved in B cell responses to a membrane antigen on RBCs, we isolated RBCs or B cells from mice (CD45.2+) expressing membrane HEL (mHEL) (34), and examined their ability to activate B cells isolated from mice with a BCR specific for hen egg lysozyme (HEL) (IgMHEL B cells, CD45.1+) (35). Accordingly, IgMHEL B cells were co-cultured with mHEL B cells or mHEL-RBCs for 24 h, followed by analysis of CD86 expression as a marker of activation on the live (PI−) IgMHEL B cells (CD45.1+CD19+; Figure 2A). As shown in Figure 2B, mHEL-RBCs induced strong activation of IgMHEL B cells, as measured by upregulation of CD86, whereas mHEL B cells induced very weak activation of the IgMHEL B cells. The latter results are consistent with our previous reports documenting that ligands of CD22 and Siglec-G on mHEL B cells suppress BCR mediated activation of IgMHEL B cells. Conversely, the strong activation of cognate B cells by mHEL-RBCs is consistent with the absence of Siglec ligands on these cells, permitting the activation of IgMHEL B cells.
Figure 2. mHEL-RBCs induce strong activation of IgMHEL B cells.
Isolated IgMHEL B cells (0.2×106 cells) were co-culture for 24 h with medium, 0.2×107 of splenocytes-containing mHEL B cells (CD45.2+,) or 0.2×107 of isolated mHEL-RBCs. (A) Gate strategy of flow cytometry analysis is showing the contour plots of SSC vs FSC of the co-cultured cells after RBCs lysis. Dead cells were gated out with 1 μg/mL of propidium iodide (PI) and IgMHEL B cells (CD45.1+CD19+) were gated to analyze the expression of CD86. (B) Histogram of the expression of CD86. This experiment was independently performed 2 times in triplicate.
De Novo introduction of CD22 ligands into RBCs suppress B cell activation
If the absence of Siglec ligands permitted mHEL-RBCs strong activation of IgMHEL B cells, we hypothesized that insertion of CD22 ligands into the membrane of mHEL-RBCs should suppress B cell activation. We and others have demonstrated that bioactive glycolipids can be readily inserted into membranes of living cells (6, 36–39). Moreover, we have routinely used high affinity and selective CD22 ligands linked to lipids, called BPANeu5Gc-PEG-lipid (9-N-biphenylacetyl-Neu5Gcα2-6Galβ1-4GlcNAc) for formulation of liposomes, to target murine B cells in vivo (6, 40). Accordingly, BPANeu5Gc-PEG-lipid, or a corresponding control lipid without the CD22 ligand (PEG-lipid), were inserted into RBCs. Successful incorporation of ligands into the cell membrane was evaluated by staining with mCD22-Fc, SNA, and MAA-II by FACS, which clearly showed that CD22 ligands were successfully introduced into RBCs (Figure 3A). We similarly engineered mHEL-RBCs and incubated cells with and without CD22 ligands with IgMHEL B cells to test for activation. While mHEL-RBCs treated with the control PEG lipid strongly activated IgMHEL B cells in line with results described above, but mHEL-RBCs with the lipid linked CD22 ligands inserted into their membrane failed to activate IgMHEL B cells (Figure 3B). The inhibition of B cell activation in cells engineered to contain CD22 ligands was fully CD22-dependent, since these engineered cells induced strong activation of CD22−/− IgMHEL B cells (Figure 3C). To investigate this CD22-dependent inhibition in a more quantitative manner, WT or CD22−/− IgMHEL B cells were co-cultured with different numbers of mHEL-RBCs (2×103 – 4×107) (inserted graphs on Figure 3B,C). Compared to control mHEL-RBCs, insertion of CD22 ligands into mHEL-RBCs increased the number of RBCs required to achieve half-maximal activation (CD86 up-regulation) of cognate B cells by 50-fold. On the other hand, no difference in half-maximal activation between mHEL-RBCs with and without CD22 ligands was observed in CD22−/− IgMHEL B-cells, again highlighting the CD22-dependent mechanism of inhibition.
Figure 3. Insertion of BPANeu5Gc on mHEL-RBCs impaired the activation of IgMHEL B-cell.
(A) Representative histograms of mHEL-RBCs inserted with 6′BPANeu5Gc-PEG-DSPE (BPANeu5Gc-mHEL-RBCs) or with PEG-DSPE (PEG-mHEL-RBCs) stained with mCD22-Fc plus anti-human IgG1-APC, SNA or MAA-II. (B) WT or (C) CD22−/− splenocytes-containing IgMHEL B cells (0.4×106 cells) were co-cultured with medium, 0.2×104, 0.2×105, 0.2×106, 0.2×107 or 2×107 of BPANeu5Gc-mHEL-RBCs or PEG-mHEL-RBCs for 24 h. Representative histogram of the flow cytometry analysis of CD86 expression on live IgMHEL B cells (CD45.1+CD19+) co-cultured with medium or 0.2×107 RBCs. We used the same gate strategy described in Figure 2. Inserted in this figure are graphic representation of MFI of CD86 expression on WT or CD22−/− IgMHEL B cells vs the different numbers of RBCs. These experiments were independently performed 3 times in triplicate.
Inserted CD22 ligands suppress mHEL-RBC cytokine production by B cells
B cells are traditionally recognized as antibody producers and antigen presenting cells (41). However, recent studies have shown that BCR activation also promotes release of cytokines, such as IL-2, IL-6, TNF, and IL-10 (42, 43). Therefore, we evaluated the cytokine concentration in supernatants of IgMHEL B cells co-cultured with BPANeu5Gc modified or control mHEL-RBCs for 24 h. In accordance to the results described above for CD86 expression, as a proxy to B cell activation, the concentrations of IL-2, IL-6, and TNF, but not IL-10, increased in supernatant of IgMHEL splenocytes co-cultured with control mHEL-RBCs, but not from co-cultures with mHEL-RBCs containing CD22 ligands (Figure 4).
Figure 4. BPANeu5Gc-mHEL-RBCs avoided the increased of TNF, IL-6 and IL-2 levels in the supernatant of the culture.
WT splenocytes-containing IgMHEL B cells (0.4×106 cells) were co-cultured for 24 h with medium, 0.2×104, 0.2×105, 0.2×106, 0.2×107 or 2×107 of BPANeu5Gc-mHEL-RBCs or PEG-mHEL-RBCs. Levels of IL-2, TNF, IL-10 or IL-6 were evaluated on supernatant of the culture by ELISA. This experiment was independently performed 2 times in triplicate.
Inserted CD22 ligands suppress mHEL-RBC induced B cell proliferation in vivo
Encouraged that B cell activation toward an antigen on the surface of RBCs can be inhibited by introduction of CD22 ligands into the RBCs, we sought to determine if impairing the initial events of B cell activation in vitro translates in vivo. To this end, we tested the ability of mHEL-RBCs - with or without CD22 ligands - to induce in vivo proliferation of adoptively transferred IgMHEL B cells in a host mouse. To do so, CTV-labeled CD45.1+ IgMHEL B cells were adoptively transferred into WT CD45.2+ hosts, followed by a second adoptive transfer of 2×106 (Figure 5A) or 50×106 (Figure 5B) BPANeu5Gc modified or control (PEG) mHEL-RBCs the following day. The proliferation of IgMHEL B cells, as determined by CTV dilution, was analyzed on day 4 after administering the mHEL-RBCs. IgMHEL B cells proliferated robustly in response to PEG-mHEL-RBCs, however, the mHEL-RBCs with CD22 ligands inserted failed to induce significant proliferation of the IgMHEL B cells, as demonstrated visually by the representative histograms (Figure 5A,B) and more quantitatively based on the calculated the division index (Figure 5C). Taken together, these results demonstrate that RBCs displaying a B cell antigen on their surface induce robust B cell responses, but that such strong B cell activation, cytokine production, and proliferation can be inhibited by the insertion of CD22 ligands into RBCs.
Figure 5. BPANeu5Gc-mHEL RBCs inhibited the IgMHEL B cells proliferation in vivo.
2×106 splenocytes-containing IgMHEL B cells (CD45.1+) was adoptive transferred into CD45.2+CD45.1− host mice. The following day, 2×106 (A) or 50×106 (B) BPANeu5Gc-mHEL-RBCs, PEG-mHEL-RBCs or PBS were adoptively transferred into the same host mice. Then, three days after RBCs administration (4th Day), the spleens of host mice were analyzed for HEL-reactive B cells (CD19+CD45.1+). We used the same gate strategy described in Figure 2 to analyze the proliferation of CTV-stained IgMHEL B cells. (C) Quantitative analysis of IgMHEL B cells proliferation represented by the division index, n=3. For each condition, 1×106 total splenocytes were analyzed. This experiment was independently performed 3 times in triplicate.
Discussion
The well-documented abundance of sialic acid on RBCs (44–48) motivated us to hypothesize that sialic acid containing ligands to B cell Siglecs may induce tolerance to antigen on the surface of RBCs in a manner similar to that we reported for antigen on the surface of B cells (6). However, to our surprise, mouse erythrocyte lack CD22 and Siglec-G/10 ligands and, consequently, induced strong B cell activation. This result is in stark contrast to cell surface antigens on lymphocytes, which do not induce B cell activation because of the presence of ligands for B cell Siglecs (6). We conclude that the specificities of B cell Siglecs (CD22 and Siglec-G) are not broad enough to recognize sialic acid containing glycans on murine erythrocytes as ligands. Thus, despite the fact that all cell types contain sialic acid-containing glycans, Siglec-mediated induction of tolerance to cell surface antigens may be restricted to cell types that express Siglec ligands, such as B and T cells, and not cell types with sialic acid-containing glycans that are not recognized as ligands, such as murine erythrocytes (6).
With regards to immune responses to antigen expressed on RBCs, previous findings have demonstrated that transfusion of membrane-bound human glycophorin A antigen (hGPA)- or mHEL-RBCs into naïve mice produced low or undetectable levels of anti-hGPA or anti-HEL antibodies with subsequent tolerance to antigen-specific challenge. However, this tolerance could be broken if RBCs were transfused together with an agonist of TLR3 (poly(I:C)), suggesting that the inflammatory response is an important component to alloantibodies appearance on blood transfusions (11). We also observed that intravenous administration of mHEL-RBCs into C57BL/6 naïve mice induced a week immune response (data not shown). The density of antigen on RBCs also could be critical to induction of antigen-specific immune response and tolerance. A recent study nicely demonstrated that while low density of antigen on RBCs drives tolerance induction, high density fails to induce robust tolerance and can even induce alloantibody responses (20). Although the mechanisms of immunological tolerance to antigens expressed on RBCs are not fully understood, several studies suggest that it is mediated through the T cell compartment (10, 12, 14–16) and also involve the engagement of the RBCs surface CD47 to the polymorphic signal regulatory protein alpha (SIRPα) on DCs (49). Moreover, activation of B cells by type 1 interferons seems to be critical to alloantibody response to RBCs antigens (50). Our results are congruent with these studies, showing that: (1) if tolerance induction does occur, it is likely not primarily at the level of B cells since they do become activated and (2) the absence of inhibition by the B cell Siglecs toward cell surface antigens on mouse RBCs may allow for alloantibody responses.
Accounting for the differential B cell response to antigens displayed on RBCs versus lymphocytes is the type of sialic acids expressed on the cell surface. Based on positive staining of RBCs with MAA, and negative staining with SNA and mCD22-Fc, our results agree with previous studies showing that instead of Siaα2-6-Gal, the preferred linkage for CD22, Siaα2-3Gal is the major form of sialic acid on mouse RBCs (44, 45, 51, 52). Interestingly, it was shown many years ago that CD22 expressed on the surface of COS-7 cells was capable of forming rosettes with human RBCs, indicating that human RBCs do express some level of CD22 ligands (7, 8). Moreover, a recently study showed that human RBCs are stained by SNA, a specific lectin that recognizes Siaα2-6-Gal, qualifying sialic acids on human RBCs as ligands for CD22 as well as for others Siglecs as demonstrated by binding on Siglec-9 (53). Thus, there are sialic acid species-specific differences between humans and mice, which determine the ability of RBCs to bind on CD22. Indeed, another study revealed that binding of human CD22 to mouse RBCs is extremely weak and negligible compared to its binding to B cells or human RBCs (9). Potentially accounting for some of these species-specific differences in sialic acid between mouse and man is that 80–90% of sialic acids on mouse RBCs are 9-O-Neu5Ac (44, 48, 54, 55). This modification to sialic acid generally blocks recognition by Siglecs (56, 57). Thus, even though mouse RBCs have an abundance of Siaα2-3Gal, which have the potential to serve as ligands for Siglec-G, binding of such glycans to Siglec-G may be impaired by the 9-O-acetylation modification. Another potential contributing factor to the lack of B cell Siglec ligands on murine RBC that the majority of sialic acids are Neu5Ac, where CD22 and Siglec-G both have a preference for Neu5Gc (48). Clearly a better understanding of the specificities of the Siglecs for natural sialioside receptors are needed to fully understand their functions though recognition of ligands on other cells.
In the biochemical complementation strategy described herein, we inserted lipid-linked glycan ligands into the membrane of RBCs to inhibit B cell activation and proliferation. We had employed this methodology in our previous work investigating cell surface antigens on lymphocytes, where we showed that first destroying CD22 ligands, by treating the mHEL B cells with neuraminidase, followed by reintroducing lipid-linked CD22 ligands fully restored CD22-dependent inhibition of B cell activation (6). The modified RBCs support our previous findings that CD22 ligands presented on the same surface as antigen have a profound effect in inhibiting B cell activation (4, 6). We suggest that such engineered cells could, in principle, be used therapeutically to prevent alloantibody responses, which is relevant in the context that a significant percentage of patients receiving blood transfusions mount alloantibody response (17, 18) – a finding that is recapitulated in mice (20, 58). In attempting to induce tolerance with the engineered RBCs, B cell responses were indeed blunted as expected from the in vitro studies, but we found that the lipid-linked CD22 ligands were lost from the surface of the cells, which did not induce lasting tolerance (data not shown). Further studies are needed to determine whether exploiting the function of CD22 as an inhibitory receptor as application in this area.
In summary, the results presented here demonstrate that lack of CD22 or Siglec-G ligands on RBCs prevent recruitment of Siglecs to the BCR of B cells that recognize cell antigens (e.g. mHEL) resulting in activation/proliferation of the B cells. Although we had previously hypothesized that Siglecs would mediate B cell tolerance to membrane antigens on any cell (6), the data provided here show that this is not the case for murine erythrocytes, and the extent to which it applies to other cells will depend on the recognition of their sialic acid containing glycans by B cell Siglecs.
Acknowledgments
We would like to thank Britni Arlian for technical assistance.
Footnotes
NIAID (AI099141 & R01 AI050143 to JCP), and CNPq (449282/2014-7 to FS; FS is a CNPq-PQ fellow).
References
- 1.Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. J Immunol. 2013;191:1724–1731. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, Nemazee D. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, Paulson JC. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123:3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanoue A, Batista FD, Stewart M, Neuberger MS. Interaction of CD22 with alpha2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur J Immunol. 2002;32:348–355. doi: 10.1002/1521-4141(200202)32:2<348::AID-IMMU348>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Macauley MS, Paulson JC. Siglecs induce tolerance to cell surface antigens by BIM-dependent deletion of the antigen-reactive B cells. J Immunol. 2014;193:4312–4321. doi: 10.4049/jimmunol.1401723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamenkovic I, Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74–77. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 8.Engel P, Nojima Y, Rothstein D, Zhou LJ, Wilson GL, Kehrl JH, Tedder TF. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993;150:4719–4732. [PubMed] [Google Scholar]
- 9.Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 10.Cremel M, Guerin N, Horand F, Banz A, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013;443:39–49. doi: 10.1016/j.ijpharm.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Smith NH, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE. Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs. Blood. 2012;119:1566–1569. doi: 10.1182/blood-2011-09-382655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson KE, Hendrickson JE, Cadwell CM, Iwakoshi NN, Zimring JC. Partial tolerance of autoreactive B and T cells to erythrocyte-specific self-antigens in mice. Haematologica. 2012;97:1836–1844. doi: 10.3324/haematol.2012.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson KE, Lin E, Hendrickson JE, Lukacher AE, Zimring JC. Regulation of primary alloantibody response through antecedent exposure to a microbial T-cell epitope. Blood. 2010;115:3989–3996. doi: 10.1182/blood-2009-08-238568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontos S, I, Kourtis C, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013;110:E60–68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci Adv. 2015;1:e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci Rep. 2015;5:15907. doi: 10.1038/srep15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Mater Med Pol. 1990;22:21–25. [PubMed] [Google Scholar]
- 18.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 19.Fasano RM, Booth GS, Miles M, Du L, Koyama T, Meier ER, Luban NL. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 20.Arthur CM, Patel SR, Smith NH, Bennett A, Kamili NA, Mener A, Gerner-Smidt C, Sullivan HC, Hale JS, Wieland A, Youngblood B, Zimring JC, Hendrickson JE, Stowell SR. Antigen Density Dictates Immune Responsiveness following Red Blood Cell Transfusion. J Immunol. 2017;198:2671–2680. doi: 10.4049/jimmunol.1601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macauley MS, Kawasaki N, Peng W, Wang SH, He Y, Arlian BM, McBride R, Kannagi R, Khoo KH, Paulson JC. Unmasking of CD22 Co-receptor on Germinal Center B-cells Occurs by Alternative Mechanisms in Mouse and Man. J Biol Chem. 2015;290:30066–30077. doi: 10.1074/jbc.M115.691337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One. 2012;7:e39039. doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, Nicoll G, Floyd H, Mattei MG, Moore P, Liu D, Crocker PR. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355:489–497. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya N, I, Goldstein J, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 27.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 28.Knibbs RN, I, Goldstein J, Ratcliffe RM, Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991;266:83–88. [PubMed] [Google Scholar]
- 29.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 31.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 33.Santos L, Draves KE, Boton M, Grewal PK, Marth JD, Clark EA. Dendritic cell-dependent inhibition of B cell proliferation requires CD22. J Immunol. 2008;180:4561–4569. doi: 10.4049/jimmunol.180.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 35.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 36.Markwell MA, Svennerholm L, Paulson JC. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci U S A. 1981;78:5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng W, de Vries RP, Grant OC, Thompson AJ, McBride R, Tsogtbaatar B, Lee PS, Razi N, Wilson IA, Woods RJ, Paulson JC. Recent H3N2 Viruses Have Evolved Specificity for Extended, Branched Human-type Receptors, Conferring Potential for Increased Avidity. Cell Host Microbe. 2017;21:23–34. doi: 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods EC, Yee NA, Shen J, Bertozzi CR. Glycocalyx Engineering with a Recycling Glycopolymer that Increases Cell Survival In Vivo. Angew Chem Int Ed Engl. 2015;54:15782–15788. doi: 10.1002/anie.201508783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes. J Am Chem Soc. 2008;130:5947–5953. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper MD. The early history of B cells. Nat Rev Immunol. 2015;15:191–197. doi: 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- 42.Nova-Lamperti E, Fanelli G, Becker PD, Chana P, Elgueta R, Dodd PC, Lord GM, Lombardi G, Hernandez-Fuentes MP. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+T-cell responses. Sci Rep. 2016;6:20044. doi: 10.1038/srep20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 44.Sarris AH, Palade GE. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979;254:6724–6731. [PubMed] [Google Scholar]
- 45.Arimitsu N, Akimitsu N, Kotani N, Takasaki S, Kina T, Hamamoto H, Kamura K, Sekimizu K. Glycophorin A requirement for expression of O-linked antigens on the erythrocyte membrane. Genes Cells. 2003;8:769–777. doi: 10.1046/j.1365-2443.2003.00674.x. [DOI] [PubMed] [Google Scholar]
- 46.Eylar EH, Madoff MA, Brody OV, Oncley JL. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962;237:1992–2000. [PubMed] [Google Scholar]
- 47.Bulai T, Bratosin D, Pons A, Montreuil J, Zanetta JP. Diversity of the human erythrocyte membrane sialic acids in relation with blood groups. FEBS Lett. 2003;534:185–189. doi: 10.1016/s0014-5793(02)03838-3. [DOI] [PubMed] [Google Scholar]
- 48.Varki A, Kornfeld S. An autosomal dominant gene regulates the extent of 9-O-acetylation of murine erythrocyte sialic acids. A probable explanation for the variation in capacity to activate the human alternate complement pathway. J Exp Med. 1980;152:532–544. doi: 10.1084/jem.152.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi T, Li J, Chen H, Wu J, An J, Xu Y, Hu Y, Lowell CA, Cyster JG. Splenic Dendritic Cells Survey Red Blood Cells for Missing Self-CD47 to Trigger Adaptive Immune Responses. Immunity. 2015;43:764–775. doi: 10.1016/j.immuni.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibb DR, Liu J, Santhanakrishnan M, Natarajan P, Madrid DJ, Patel S, Eisenbarth SC, Tormey CA, Stowell SR, Iwasaki A, Hendrickson JE. B cells require Type 1 interferon to produce alloantibodies to transfused KEL-expressing red blood cells in mice. Transfusion. 2017 doi: 10.1111/trf.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinkman-Van der Linden EC, Sonnenburg JL, Varki A. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal Biochem. 2002;303:98–104. doi: 10.1006/abio.2001.5539. [DOI] [PubMed] [Google Scholar]
- 52.Kelm S, Schauer R, Manuguerra JC, Gross HJ, Crocker PR. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconj J. 1994;11:576–585. doi: 10.1007/BF00731309. [DOI] [PubMed] [Google Scholar]
- 53.Lizcano A, Secundino I, Dohrmann S, Corriden R, Rohena C, Diaz S, Ghosh P, Deng L, Nizet V, Varki A. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood. 2017;129:3100–3110. doi: 10.1182/blood-2016-11-751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuter G, Vliegenthart JF, Wember M, Schauer R, Howard RJ. Identification of 9-O-acetyl-N-acetylneuraminic acid on the surface of BALB/c mouse erythrocytes. Biochem Biophys Res Commun. 1980;94:567–572. doi: 10.1016/0006-291x(80)91269-3. [DOI] [PubMed] [Google Scholar]
- 55.Howard RJ, Seeley DC, Jr, Kao V, Wember M, Schauer R. Sialic acid analysis and tritium-labelling of sialoglycoproteins of mouse erythrocytes infected with Plasmodium berghei. Parasitology. 1986;92(Pt 3):545–557. doi: 10.1017/s0031182000065434. [DOI] [PubMed] [Google Scholar]
- 56.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 57.Sjoberg ER, Powell LD, Klein A, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta can be masked by 9-O-acetylation of sialic acids. J Cell Biol. 1994;126:549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hendrickson JE, Eisenbarth SC, Tormey CA. Red blood cell alloimmunization: new findings at the bench and new recommendations for the bedside. Curr Opin Hematol. 2016 doi: 10.1097/MOH.0000000000000277. [DOI] [PubMed] [Google Scholar]