Abstract
Background
Related to escalating health care costs and the questionable effectiveness of multiple interventions including lumbar facet joint interventions, cost effectiveness or cost utility analysis has become the cornerstone of evidence-based medicine influencing coverage decisions.
Methods
Cost utility of therapeutic lumbar facet joint nerve blocks in managing chronic low back pain was performed utilizing data from a randomized, double-blind, controlled trial with a 2-year follow-up, with direct payment data from 2016. Based on the data from surgical interventions, utilizing the lowest proportion of direct procedural costs of 60%, total cost utility per quality adjusted life year (QALY) was determined by multiplying the derived direct cost at 1.67.
Results
Patients in this trial on average received 5.6 ± 2.6 procedures over a period of 2 years, with average relief over a period of 2 years of 82.8 ± 29.6 weeks with 19 ± 18.77 weeks of improvement per procedure. Procedural cost for one-year improvement in quality of life showed USD $2,654.08. Estimated total costs, including indirect costs and drugs with multiplication of direct costs at 1.67, showed a cost of USD $4,432 per QALY.
Conclusions
The analysis of therapeutic lumbar facet joint nerve blocks in the treatment of chronic low back pain shows clinical effectiveness and cost utility at USD $2,654.08 for the direct costs of the procedures, and USD $4,432 for the estimated overall cost per one year of QALY, in chronic persistent low back pain non-responsive to conservative management.
Keywords: Cost-benefit analysis, Cost effectiveness, Diagnostic techniques and procedures, Low back pain, Lumbar vertebrae, Nerve block, Quality-adjusted life years (QALY), Randomized controlled trial, Therapeutic uses, Zygapophyseal joint
INTRODUCTION
Previous well-controlled studies have established facet joints, intervertebral discs, and sacroiliac joints as potential sources of low back and lower extremity pain [1,2,3]. Recent systematic reviews have revealed Level II evidence for the diagnostic validity of lumbar facet joint nerve blocks with controlled local anesthetic or placebo blocks [2,4]. Similarly, systematic reviews and comprehensive assessment by spinal interventional techniques guidelines [1,4,5] have shown Level II evidence based on best evidence synthesis for conventional radiofrequency and therapeutic lumbar facet joint nerve blocks.
In addition to clinical effectiveness, cost effectiveness or cost utility analysis studies are crucial in clinical practice and health policy related to ever-increasing health care costs [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. While the literature is replete with clinical effectiveness studies and systematic reviews [1,2,3,4,5,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], the cost effectiveness or utility analysis of interventional techniques has been limited [10,11,12,13,14,15,30].
Appropriate cost effectiveness studies have been published for caudal epidural injections derived from randomized trials [11], lumbar interlaminar epidural injections [15], percutaneous adhesiolysis [12], and spinal cord stimulation [10,14], showing procedural cost utility at USD $2,173 and USD $2,650, for caudal epidural injections and adhesiolysis, respectively. Cost effectiveness for spinal cord stimulation was shown at €5,624.
The cost effectiveness studies of physiotherapy showed it to be superior to advice alone, at a cost utility of USD $6,379 per quality adjusted life year (QALY) [31], whereas another study comparing cost effectiveness of primary care management with or without early physical therapy for acute low back pain [23] showed an incremental cost effectiveness ratio of USD $32,058 per QALY.
The cost analysis of surgical interventions demonstrated a significant benefit with direct medical costs, without including the costs of medications, of USD $18,645 (68%) with a total cost of USD $27,341 per 2 years in managing disc herniation [7], and USD $15,717 or 60% for direct costs, without medications, at a total cost per 2 years of USD $26,222 in surgical management of spinal stenosis, with USD $29,868 or 71% with a total cost of USD $42,081 for degenerative spondylolisthesis cost for 2 years [9].
Based on the above data, from Spine Patient Outcomes Research Trial (SPORT) [7,9], cost utility analysis showed USD $69,403 per QALY for disc herniation [7], USD $77,600 per QALY gained for spinal stenosis, and USD $115,600 per QALY gained for degenerative spondylolisthesis [9]. Thus, costs attributed to direct medical costs without medical therapy appear to be variable at 60% for spinal stenosis, 68% for disc herniation, and 71% for spondylolisthesis [7,9]. Extrapolating the data from highly regarded surgical intervention publications [7,9], costs for caudal epidural injections, which were calculated with direct medical costs only, will increase from USD $2,173 to USD $3,628 per QALY with the addition of 40% of costs for cost utility analysis, whereas it will increase the cost utility of percutaneous adhesiolysis from USD $2,650 to USD $4,426 per QALY [12]. In addition, cost utility analysis of lumbar interlaminar epidural injections in the treatment of lumbar disc herniation, central spinal stenosis, and axial or discogenic low back pain utilizing the extrapolated method of surgical interventions of direct cost showed an average cost of USD $3,301 per QALY [15]. Consequently, interventional techniques with preliminary analysis appear to be cost effective compared to physical therapy as well as surgical interventions.
There is a growing concern in reference to the escalating utilization of multiple interventions and health care costs in chronic pain management, including those of conservative modalities, interventional techniques, and surgery [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Recently, Dieleman et al. [56] demonstrated that low back and neck pain accounted for the third highest amount, with an estimated health care spending in 2013 of USD $87.6 billion. In this analysis of health care expenses from 1996 through 2013, USD $30.1 trillion of personal health care spending was disaggregated by 155 conditions. Health care spending increased for 143 of the 155 conditions, but spending on low back and neck pain, and on diabetes mellitus, increased the most over the 18 years, by an estimated USD $57.2 billion for low back and neck pain.
In addition, spending on emergency care and retail pharmaceuticals also increased at the fastest rates of 6.4% and 5.6% annual growth rate, which were higher than the annual rate for spending on other sectors, which also include cost attributed to low back pain. It is thus crucial to provide not only clinically effective treatments, but also meet the cost utility criteria to provide value-based, high quality care.
Even though the effectiveness of interventional techniques in managing spinal pain has been extensively debated with discordant conclusions [1,2,3,4,5,34,35,36,37,38,39,40,41,42,43,44,45,46,47,65,66,67,68,69], overall evidence has been shown to be promising in managing chronic spinal pain. Thus, facet joint interventions may be provided for patients with facet joint pain from axial or somatic low back pain, and in a small proportion of patients with post-surgery syndrome [1,2,3,4].
Manchikanti et al. [48,49,58] demonstrated significant increases in facet joint interventions in the Medicare population. They showed lumbar facet joint interventions increased at 286.2% per 100,000 beneficiaries compared to −2% for lumbar epidural injections from 2000 to 2014. Analysis of individual procedures showed increases of 567.8% for lumbar radiofrequency and 227.3% for lumbar facet joint blocks [48,49,53].
The present investigation was undertaken in order to evaluate and determine valid and reliable cost utility information for therapeutic lumbar facet joint nerve blocks in managing chronic low back and lower extremity pain with data derived from a previously conducted double-blind, randomized controlled trial with a 2-year follow-up [70,71].
MATERIALS AND METHODS
1. Study design
A randomized, double-blind controlled trial evaluating therapeutic facet joint nerve blocks provided the basis for the current cost utility analysis [70,71]. The trial's design and methodology have been reported [70,71]. Participants in the trial had previously failed conservative management and received therapeutic facet joint injections after achieving at least 80% concordant pain relief with controlled, comparative local anesthetic blocks [1,2]. An Institutional Review Board (IRB) approved the study, which was conducted in a contemporary interventional pain management setting in the United States.
Briefly, inclusion criteria consisted of those patients with a history of chronic function-limiting low back pain of at least 6 months' duration with positive results to controlled diagnostic lumbar facet joint nerve blocks with a least 80% concordant pain relief and the ability to perform previously painful movements. For diagnostic lumbar facet joint nerve blocks (medial branch and L5 dorsal ramus blocks), the exclusion criteria included radicular pain, surgical interventions of the lumbar spine within the last 3 months, uncontrolled major depression or psychiatric disorders, heavy opioid usage of morphine equivalent of 300 mg, acute or uncontrolled medical illness, chronic severe conditions that could interfere with the interpretations of the outcome assessments, and women who were pregnant or lactating. A total of 120 patients were assigned to one of the 2 groups consisting of either a non-steroid group with local anesthetic only (Group I) or a steroid group with local anesthetic and steroid (Group II). All of the diagnostic lumbar facet joint nerve blocks were performed under-sterile conditions in the operating room under fluoroscopic guidance with injection of 0.5 ml of 1% preservative-free lidocaine, followed by 0.5 ml of 0.25% bupivacaine on a separate occasion, usually 3 to 4 weeks after the first-injection, if the results of lidocaine block were positive. Therapeutic lumbar facet joint nerve blocks were performed under fluoroscopic guidance in a sterile operating room with injection of 0.5 to 1.5 ml of mixture of clear solution with or without steroid at each level as assigned by grouping.
2. Analysis
Sixty patients were randomly assigned into each group from a total of 120 patients. Randomization was carried out in blocks of 20 patients by a computer-generated random allocation sequence. The sample size was determined as requiring 50 patients in each group. Statistical methods involved Chi-squared statistic, Fisher's exact test, paired t-test, and one-way analysis of variance. Intent-to-treat analysis was performed utilizing the last follow-up data.
All costs were assessed based on 2016 reimbursement based on their payment sources for the facility and physician services. Since reimbursement rates often change, current reimbursement was utilized.
3. Outcome measures
Pain and function were measured in the study: pain with the 11-point Numeric Rating Scale (NRS) and function with the 50-point Oswestry Disability Index (ODI). Additional measurements included employment status and opioid doses converted to morphine equivalents. Measurements for all outcomes were performed at 3, 6, 12, 18, and 24 months after treatment. Significantpain relief was described as a 50% or more reduction in the NRS score, and significant improvement in function was described as at least 40% reduction in ODI. Patients employed or unemployed on a part-time basis with limited or no employment due to pain were classified as employable.
4. Cost utility analysis
Based on their payment structure, using 2016 reimbursement data, direct procedural costs for 24 months after enrollment were assessed, including physician payments and facility expenses. Based on the most commonly quoted cost utility analysis of surgical interventions [7,9] utilizing the lowest cost for direct procedural costs without including medications, 60% was utilized. Thus, cost utility per QALY was calculated to provide realistic costs to meet the criteria of other interventions. The total cost utility was based on a multiplication of procedural costs by a factor of 1.67. This approach was based on well-regarded cost utility analyses in surgical interventions from SPORT trials [7,9] with extrapolation of costs. In these manuscripts [7,9], the authors identified direct and indirect costs. They also utilized surgical, as well as nonoperative, groups to assess the costs. Direct costs included medical and surgical costs at each time point during the follow-up period, whereas indirect costs included productivity losses, missed days of housekeeping, and unpaid caregivers, etc. Since use of medication costs were not utilized in our analysis, we eliminated those costs from direct costsand included them into indirect costs. The SPORT trials [7,9] showed a 2-year cost of managing disc herniation of USD $18,645 (68%) with a total cost of USD $27,341 without inclusion of medications, whereas for spinal stenosis and spondylolisthesis, direct costs without medication costs were USD $15,717 or USD $29,868 or total costs of USD $26,222 or USD $42,081 of which 60% were considered as direct medical expenses without medication for spinal stenosis and 71% was considered as direct expense without medication for spondylolisthesis. Quality of life improvement and cost utility was based on these costs for 2 years in these patients per QALY for disc herniation of USD $69,403 with 68% for direct medical costs without medical therapy, USD $77,600 for spinal stenosis per QALY, and USD $115,600 per QALY for degenerative spondylolisthesis with direct medical costs variable at 60% for spinal stenosis, and 71% for spondylolisthesis. Thus, utilizing the highest cost attributed as 40% for indirect expenses including medical therapy, in this analysis, 60% was utilized as direct costs without medical therapy. Consequently, it was multiplied by 1.67 to arrive at a total cost.
The present investigation utilized quality of life improvement per year (52 weeks) for 2 years (104 weeks) based on the costs of lumbar facet joint nerve blocks with primary outcomes of significant pain relief of 50% and improvement in function of 40%.
RESULTS
1. Patient flow
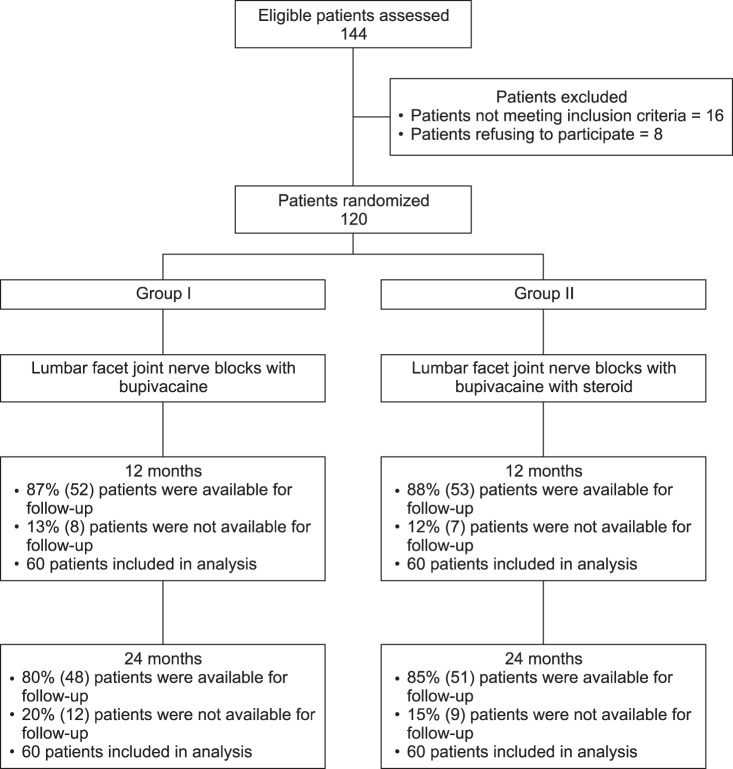
Patient flow is demonstrated in Fig. 1. Eighty percent of the patients in Group I and 85% of the patients in Group II were followed through 2 years.
Fig. 1. Schematic presentation of patient flow at 2-year follow-up with therapeutic lumbar facet joint nerve blocks [70,71].
2. Demographics
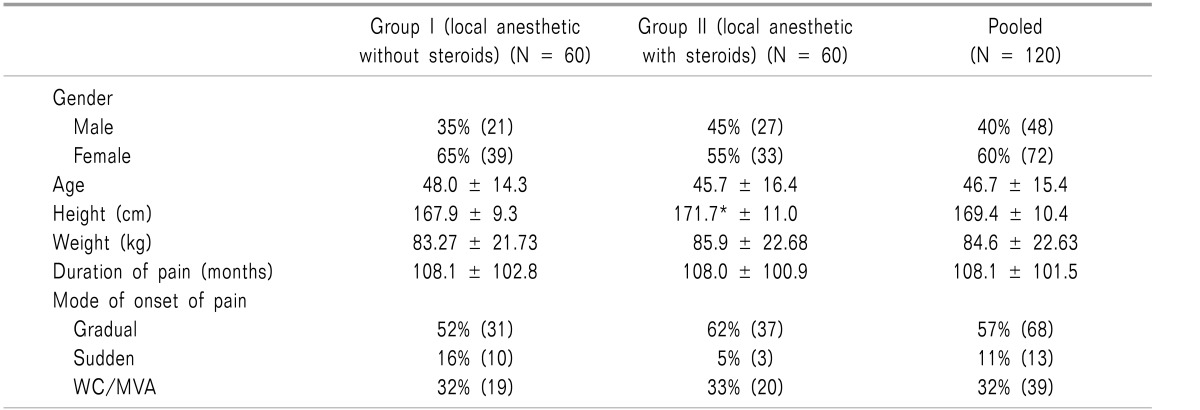
Patient demographics are demonstrated in Table 1, along with clinical characteristic data.
Table 1. Demographic Characteristics of Patients Undergoing Lumbar Facet Joint Nerve Blocks Included in Randomized Controlled Trial [70,71].
Values are mean ± SD. Group I: bupivacaine, Group II: bupivacaine and steroids. WC: workers compensation, MVA: motor vehicle injury.
*Significant difference with the group I (P < 0.05).
3. Adverse events
There were no major adverse events reported over the 2-year study period in any of the 120 participants.
4. Outcomes
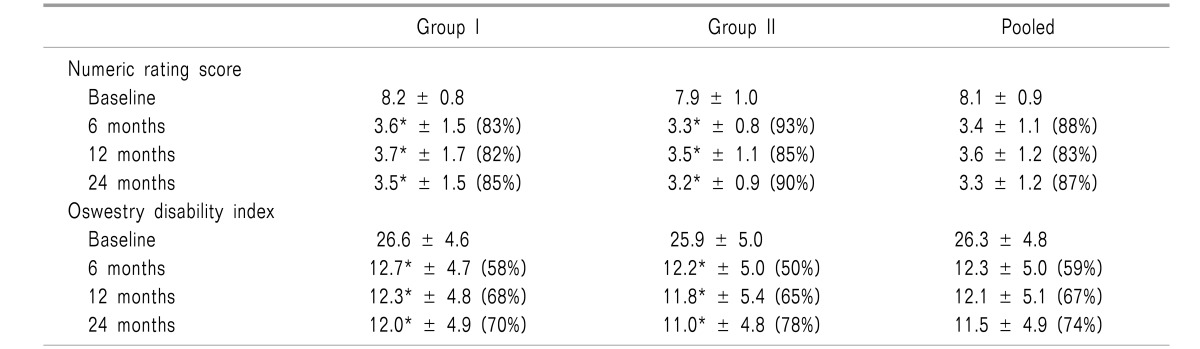
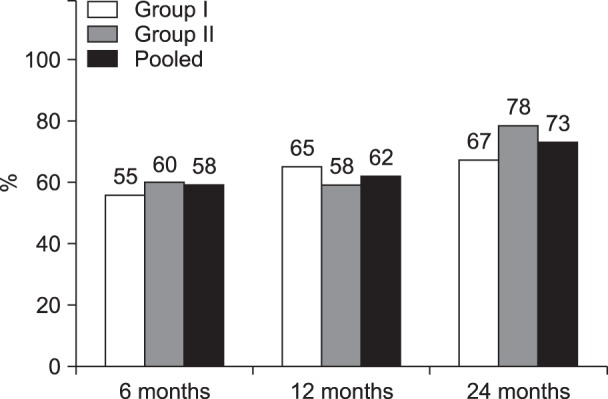
Table 2 shows comparison summaries of NRS for pain and ODI score for function. Fig. 2 demonstrates combined improvement with significant reduction in NRS of ≥ 50% and ODI of ≥ 40%.
Table 2. Pain Relief and Functional Assessment Evaluated by Oswestry Disability Index Characteristics in Randomized Controlled Trial of Lumbar Facet Joint Nerve Blocks [70,71].
(____) illustrates proportion with significant pain relief (≥ 50%) pain and 40% disability from baseline. *Significant difference with baseline values within the group (P < 0.001).
Fig. 2. Data from randomized controlled trial of lumbar facet joint nerve blocks showing proportion of patients with significant reduction in Numeric Rating Score (NRS ≥ 50% reduction from baseline) and Oswestry Disability Index (ODI ≥ 40% reduction from baseline) [69,70].
5. Cost utility analysis
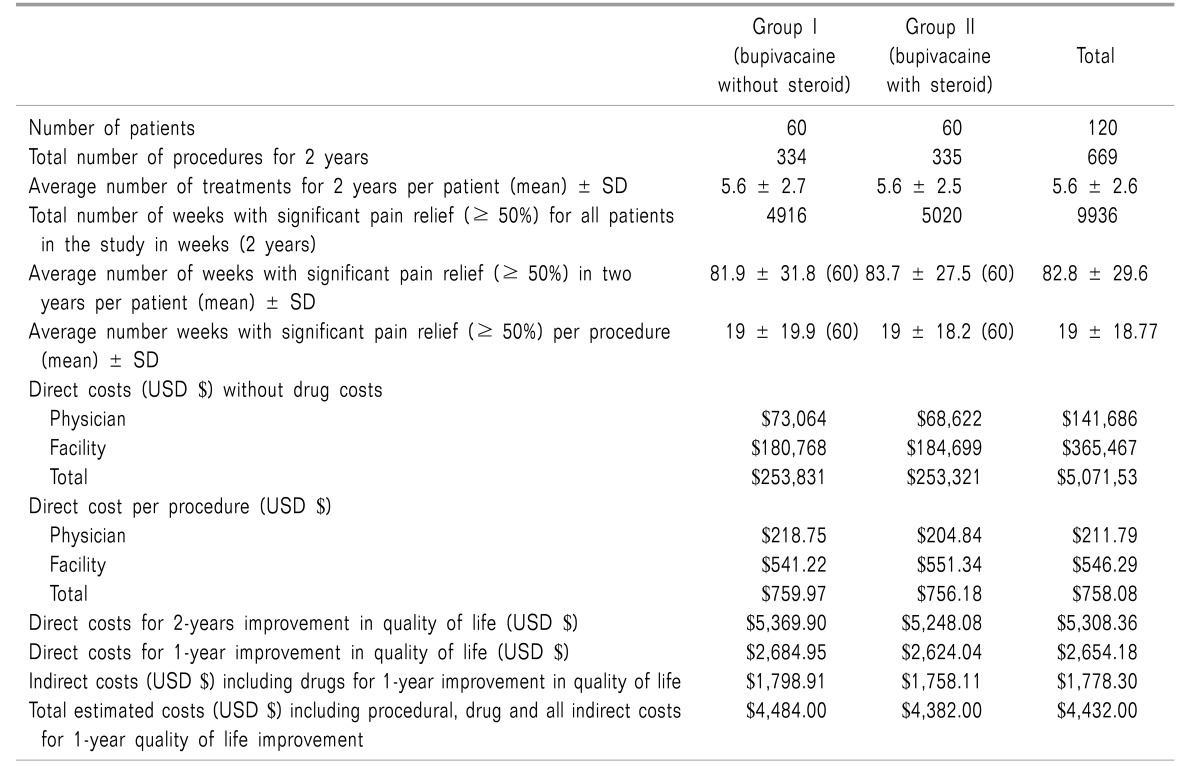
Cost utility analysis was based on the quality of life improvement and cost for procedure per QALY based on the primary outcomes of pain relief and improvement in functional status (Table 3).
Table 3. Cost Utility Analysis of Therapeutic Lumbar Facet Joint Nerve Blocks in Managing Chronic Low Back Pain.
The results showed direct costs per procedure without medical therapy of USD $758.08. Direct costs for a one-year improvement in quality of life without the inclusion of medical therapy were USD $2,654.18 with the addition of indirect costs by multiplication with 1.67 yielding USD $4,432 per QALY.
DISCUSSION
The present study reports the cost utility of lumbar facet joint nerve blocks based on a review of 120 patients who were non-responsive to conservative management, and were randomized into 2 groups either treated with local anesthetic alone or treated with local anesthetic and steroids. These patients were followed for 2 years, and using 2016 reimbursement data had a one year QALY valued at USD $2,654.18 for procedural costs and USD $4,432 with overall estimated costs. The procedures per patient were similar in the 2 groups: 5.6 ± 2.6 over 24 months. Each procedure produced a significant improvement of 19 ± 18.77 weeks; average relief overall was 82.8 ± 29.6 weeks.
These findings are in line with past cost utility analysis of randomized controlled trials of percutaneous adhesiolysis. Cost utility assessment of adhesiolysis reported direct costs of USD $2,650 and an estimated overall cost of USD $4,426 per QALY [12]. The cost utility was higher than the cost utility of caudal epidural injections with direct costs of USD $2,173 and an estimated overall cost of USD $3,628 per one year of QALY [11], and lumbar interlaminar epidural injections with overall cost of USD $3,301. Compared to caudal and lumbar epidural injections, the costs are higher despite the fact that no diagnostic interventions are included in the cost analysis. This result is probably because many of the patients with lumbar facet joint pain received bilateral facet joint nerve blocks, and all of them at 2 levels.
Cost utility was less than that of spinal cord stimulation (SCS) for failed back surgery syndrome, at £5,624 per QALY [10] or CAD at $9293 per QALY [14]. Of importance, Taylor et al. [10] utilized the United Kingdom's National Institute for Health and Care Excellence (NICE) cost effectiveness analysis [18].
In patients with nonspecific low back pain of at least the 3-month duration, with disability, an incremental cost effectiveness of USD $4,594/QALY was shown with physical therapy [72]. A favorable cost utility of USD $2,216/QALY over spinal stabilization physiotherapy was demonstrated with individual physiotherapy [73]. Physiotherapy was also more cost effective than advice alone in low back pain of the 6-week duration, at a cost utility of USD $6,379/QALY [31].
A recent study of the cost effectiveness of primary care management, with or without early physical therapy for acute low back pain [23], revealed that early physical therapy resulted in higher total one-year costs and better quality of life after one year. This assessment also showed the incremental cost effectiveness ratio was USD $32,058 (95% CI: $10,629, $151,161) per QALY. Even then, authors have concluded that early physical therapy is a cost-effective modality relative to usual primary care after one year for patients with acute, nonspecific low back pain. Further, observational research also showed that delaying referral to physical therapy is associated with increased overall health care costs and a greater risk for receiving advanced imaging or invasive procedures for low back pain [24,25,26].
Overall analysis of complementary and alternative medical treatments for cost effectiveness compared to no treatment, a placebo, physical therapy, or usual care in reducing pain immediately or at short-term after improvement, revealed significantly greater effectiveness of complementary and alternative medicine treatments [6]. Dagenais et al. [27] showed that the largest proportion of direct medical costs for low back pain was spent on physical therapy and inpatient services, with 17% for each category, followed by pharmacy (13%), and primary care (13%). They also demonstrated that those with back pain had total medical care costs that were USD $1,015 greater (USD $3,493 versus USD $2,178) than those without back pain. They also analyzed the incremental medical care costs, attributing a total of USD $26 billion with USD $11.1 billion for office based visits, USD $4.7 billion for outpatient services, USD $4.5 billion for inpatient care, USD $3.9 billion for prescription drugs, and USD $1.1 billion for emergency department visits [27].
As shown by Dieleman et al. [56], low back pain continues to be one of the most expensive conditions in the United States with an estimated spending of USD $57.2 billion for low back and neck pain. In a cost utility analysis of value-based care in management of spinal disorders [17], great value was shown for nonoperative treatments such as graded activity increase over physical therapy and pain management, spinal manipulation over exercise, behavioral therapy and physiotherapy over advice, and finally acupuncture and exercise over usual general practitioner care. However, in a systematic review of cost utility analysis in spine care, only 45% of the cost utility studies showed estimates of less than USD $100,000 per QALY gained, whereas approximately 23% showed greater cost utility of USD $100,000 or more per QALY gain [16].
Cost utility analysis of operative interventions has shown variable estimations. The most common and expensive intervention, namely operative lumbar discectomy, showed surgical care demonstrating a significant incremental benefit and outcome advantage over nonoperative care. In assessment of the data from the Spine Patient Outcomes Research Trial (SPORT), Tosteson et al. [7] showed cost effectiveness of surgical treatment for lumbar disc herniation at USD $69,403 per QALY for the general population and USD $34,355 for the Medicare population per QALY. They also showed [9] the cost effectiveness of spinal stenosis surgeries was USD $77,600 per QALY gained, whereas, it was USD $115,600 per QALY gained for degenerative spondylolisthesis.
In modern times of escalating health care utilization and costs straining economies across the globe, value-based medicine with high quality and low cost has become the norm of public policy [54,55,56,62,63,64,74,75,76,77,78,79,80,81,82,83,84,85]. Cost utility analysis refers to a particular form of cost effectiveness analysis with measurements of outcomes in terms of QALY. The cost utility analysis allows broad comparison across differing and not necessarily comparable programs or interventions.
Consequently, use of cost utility analysis for the interventions that provide the most value to patients is essential for achieving accountable and value-based health care [74,75,76,77,78,79,80,81]. With assessment of the cost utility of an intervention, public health policy may be centered around interventions which provide the most benefit to patients as measured by patient-centered outcome measures while providing high quality care at the least expense. The outcomes to be determined in long-standing, persistent, chronic pain pose multiple challenges; however, outcomes in chronic pain may be assessed appropriately utilizing disability days saved, pain-free days, or overall improvement in quality of life [86].
Again, the measurement of quality of life, an essential part of human survival, may be measured with functional status, health status, or health related quality of life, feeling of well-being, satisfaction with care, health service utilization, and economic analysis along with improved status of medical and psychological ailments [87]. Thus, quality of life assessment, in total, is designed to evaluate the patient's ability to function in their own world, specifically in the elderly with improved physical function measures with the ability to perform daily activities of life including walking, climbing stairs, or carrying on a daily routine which they enjoy.
Consequently, we posit that this assessment has both provided appropriate cost utility analysis at a reasonable direct cost of USD $2,654 and overall cost of USD $4.432 per year of quality of life improvement. This cost is even lower than the cost utility provided by physical therapy [23] and surgical interventions [7,8,9].
The current analysis is limited because only current procedure costs were considered, and remaining costs were extrapolated at 40%. The study did not consider additional medical benefits, such as a return to work. The return to work for the patients in this study was impressive. At baseline, 39 patients were considered employable; only 27 were employed. At the end of the study, that number had increased to 38 of 39. Also, diagnostic costs were not considered. Additional limitations include that the results utilized here are from a single center assessment of 120 patients, even though it is a randomized, controlled trial and assessed long-term improvement.
The costs of the provision of interventional techniques have decreased in 2017 compared to 2016, consequently, it may even provide lower cost estimations if we utilized 2017 data. Yet, costs of diagnosis may be estimated to be at a maximum of USD $1,000 per patient with 1 or 2 controlled diagnostic blocks which were not performed in all the patients, but also provide approximately 9 weeks of significant improvement which will also add to improvement in the quality of life.
In addition, we also estimate that the overall costs reported in the current analysis might be 30% to 70% higher in a hospital setting, whereas they might be 20% to 30% lower in an office setting [88,89]. There are no cost estimations for radiofrequency neurotomy; however, considering that radiofrequency neurotomy provides approximately 6 months of relief on average, with improvement in functional status, and the costs are twice as much as therapeutic lumbar facet joint nerve blocks, the cost utility analysis probably will demonstrate similar results of costs per QALY.
Even then, it may be argued that radiofrequency neurotomy may provide considerably better and more cost effective relief which is expected to last on average about 6 months based on policy considerations with the ability to repeat the procedure after 6 months of relief [1,4,5]. Derby et al. [30] provided hypothetical cost analysis based on an 80% pain relief threshold as a criterion standard at USD $34,667; however, they have not calculated quality of life improvement.
ACKNOWLEDGEMENTS
The authors wish to thank; Tom Prigge, MA, and Laurie Swick, BS, for manuscript review; and Tonie M. Hatton and Diane E. Neihoff, transcriptionists; for their assistance in preparation of this manuscript.
Footnotes
CONFLICT OF INTEREST: Dr. Manchikanti has provided limited consulting services to Semnur Pharmaceuticals, Incorporated, which is developing non-particulate steroids.
Dr. Kaye is a speaker for Depomed, Inc.
Dr. Hirsch is a consultant for Medtronic.
References
- 1.Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16:S49–S283. [PubMed] [Google Scholar]
- 2.Boswell MV, Manchikanti L, Kaye AD, Bakshi S, Gharibo CG, Gupta S, et al. A best-evidence systematic appraisal of the diagnostic accuracy and utility of facet (zygapophysial) joint injections in chronic spinal pain. Pain Physician. 2015;18:E497–E533. [PubMed] [Google Scholar]
- 3.Simopoulos TT, Manchikanti L, Gupta S, Aydin SM, Kim CH, Solanki D, et al. Systematic review of the diagnostic accuracy and therapeutic effectiveness of sacroiliac joint interventions. Pain Physician. 2015;18:E713–E756. [PubMed] [Google Scholar]
- 4.Manchikanti L, Hirsch JA, Falco FJ, Boswell MV. Management of lumbar zygapophysial (facet) joint pain. World J Orthop. 2016;7:315–337. doi: 10.5312/wjo.v7.i5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manchikanti L, Kaye AD, Boswell MV, Bakshi S, Gharibo CG, Grami V, et al. A systematic review and best evidence synthesis of the effectiveness of therapeutic facet joint interventions in managing chronic spinal pain. Pain Physician. 2015;18:E535–E582. [PubMed] [Google Scholar]
- 6.Furlan AD, Yazdi F, Tsertsvadze A, Gross A, Van Tulder M, Santaguida L, et al. A systematic review and meta-analysis of efficacy, cost-effectiveness, and safety of selected complementary and alternative medicine for neck and low-back pain. Evid Based Complement Alternat Med. 2012;2012:953139. doi: 10.1155/2012/953139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosteson AN, Skinner JS, Tosteson TD, Lurie JD, Andersson GB, Berven S, et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008;33:2108–2115. doi: 10.1097/brs.0b013e318182e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malter AD, Larson EB, Urban N, Deyo RA. Costeffectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine (Phila Pa 1976) 1996;21:1048–1054. doi: 10.1097/00007632-199605010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Tosteson AN, Lurie JD, Tosteson TD, Skinner JS, Herkowitz H, Albert T, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845–853. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RS, Ryan J, O'Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26:463–469. doi: 10.1097/AJP.0b013e3181daccec. [DOI] [PubMed] [Google Scholar]
- 11.Manchikanti L, Falco FJ, Pampati V, Cash KA, Benyamin RM, Hirsch JA. Cost utility analysis of caudal epidural injections in the treatment of lumbar disc herniation, axial or discogenic low back pain, central spinal stenosis, and post lumbar surgery syndrome. Pain Physician. 2013;16:E129–E143. [PubMed] [Google Scholar]
- 12.Manchikanti L, Helm S, 2nd, Pampati V, Racz GB. Cost utility analysis of percutaneous adhesiolysis in managing pain of post-lumbar surgery syndrome and lumbar central spinal stenosis. Pain Pract. 2015;15:414–422. doi: 10.1111/papr.12195. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Rizvi S, Bishop S, Tang W. Cost impact of intrathecal polyanalgesia. Pain Med. 2013;14:1569–1584. doi: 10.1111/pme.12204. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14:1631–1649. doi: 10.1111/pme.12146. [DOI] [PubMed] [Google Scholar]
- 15.Manchikanti L, Pampati V, Benyamin RM, Hirsch JA. Cost utility analysis of lumbar interlaminar epidural injections in the treatment of lumbar disc herniation, central spinal stenosis, and axial or discogenic low back pain. Pain Physician. 2017;20:219–228. [PubMed] [Google Scholar]
- 16.Kepler CK, Wilkinson SM, Radcliff KE, Vaccaro AR, Anderson DG, Hilibrand AS, et al. Cost-utility analysis in spine care: a systematic review. Spine J. 2012;12:676–690. doi: 10.1016/j.spinee.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Indrakanti SS, Weber MH, Takemoto SK, Hu SS, Polly D, Berven SH. Value-based care in the management of spinal disorders: a systematic review of cost-utility analysis. Clin Orthop Relat Res. 2012;470:1106–1123. doi: 10.1007/s11999-011-2141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health and Clinical Excellence (GB) Guide to the methods of technology appraisal. London: National Institute for Health and Clinical Excellence; 2008. [PubMed] [Google Scholar]
- 19.Dagenais S, Roffey DM, Wai EK, Haldeman S, Caro J. Can cost utility evaluations inform decision making about interventions for low back pain? Spine J. 2009;9:944–957. doi: 10.1016/j.spinee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Hong J, Reed C, Novick D, Happich M. Costs associated with treatment of chronic low back pain: an analysis of the UK General Practice Research Database. Spine (Phila Pa 1976) 2013;38:75–82. doi: 10.1097/BRS.0b013e318276450f. [DOI] [PubMed] [Google Scholar]
- 21.Wielage R, Bansal M, Wilson K, Klein R, Happich M. Cost-effectiveness of duloxetine in chronic low back pain: a Quebec societal perspective. Spine (Phila Pa 1976) 2013;38:936–946. doi: 10.1097/BRS.0b013e31828264f9. [DOI] [PubMed] [Google Scholar]
- 22.Dagenais S, Haldeman S, Polatin PB. It is time for physicians to embrace cost-effectiveness and cost utility analysis research in the treatment of spinal pain. Spine J. 2005;5:357–360. doi: 10.1016/j.spinee.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Fritz JM, Kim M, Magel JS, Asche CV. Cost-effectiveness of primary care management with or without early physical therapy for acute low back pain: economic evaluation of a randomized clinical trial. Spine (Phila Pa 1976) 2017;42:285–290. doi: 10.1097/BRS.0000000000001729. [DOI] [PubMed] [Google Scholar]
- 24.Childs JD, Fritz JM, Wu SS, Flynn TW, Wainner RS, Robertson EK, et al. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv Res. 2015;15:150. doi: 10.1186/s12913-015-0830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz JM, Brennan GP, Hunter SJ, Magel JS. Initial management decisions after a new consultation for low back pain: implications of the usage of physical therapy for subsequent health care costs and utilization. Arch Phys Med Rehabil. 2013;94:808–816. doi: 10.1016/j.apmr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Fritz JM, Childs JD, Wainner RS, Flynn TW. Primary care referral of patients with low back pain to physical therapy: impact on future health care utilization and costs. Spine (Phila Pa 1976) 2012;37:2114–2121. doi: 10.1097/BRS.0b013e31825d32f5. [DOI] [PubMed] [Google Scholar]
- 27.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976) 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 29.Brealey S, Burton K, Coulton S, Farrin A, Garratt A, Harvey E, et al. UK Back pain Exercise And Manipulation (UK BEAM) trial--national randomised trial of physical treatments for back pain in primary care: objectives, design and interventions [ISRCTN32683578] BMC Health Serv Res. 2003;3:16. doi: 10.1186/1472-6963-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derby R, Melnik I, Lee JE, Lee SH. Cost comparisons of various diagnostic medial branch block protocols and medial branch neurotomy in a private practice setting. Pain Med. 2013;14:378–391. doi: 10.1111/pme.12026. [DOI] [PubMed] [Google Scholar]
- 31.Rivero-Arias O, Gray A, Frost H, Lamb SE, Stewart-Brown S. Cost-utility analysis of physiotherapy treatment compared with physiotherapy advice in low back pain. Spine. 2006;31:1381–1387. doi: 10.1097/01.brs.0000218486.13659.d5. [DOI] [PubMed] [Google Scholar]
- 32.Critchley DJ, Ratcliffe J, Noonan S, Jones RH, Hurley MV. Effectiveness and cost-effectiveness of three types of physiotherapy used to reduce chronic low back pain disability: a pragmatic randomized trial with economic evaluation. Spine (Phila Pa 1976) 2007;32:1474–1481. doi: 10.1097/BRS.0b013e318067dc26. [DOI] [PubMed] [Google Scholar]
- 33.Manchikanti L, Benyamin RM, Falco FJ, Kaye AD, Hirsch JA. Do epidural injections provide short- and long-term relief for lumbar disc herniation? A systematic review. Clin Orthop Relat Res. 2015;473:1940–1956. doi: 10.1007/s11999-014-3490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manchikanti L, Nampiaparampil DE, Manchikanti KN, Falco FJ, Singh V, Benyamin RM, et al. Comparison of the efficacy of saline, local anesthetics, and steroids in epidural and facet joint injections for the management of spinal pain: a systematic review of randomized controlled trials. Surg Neurol Int. 2015;6:S194–S235. doi: 10.4103/2152-7806.156598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manchikanti L, Manchikanti KN, Gharibo CG, Kaye AD. Efficacy of percutaneous adhesiolysis in the treatment of lumbar post surgery syndrome. Anesth Pain Med. 2016;6:e26172. doi: 10.5812/aapm.26172v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchikanti L, Knezevic NN, Boswell MV, Kaye AD, Hirsch JA. Epidural injections for lumbar radiculopathy and spinal stenosis: a comparative systematic review and metaanalysis. Pain Physician. 2016;19:E365–E410. [PubMed] [Google Scholar]
- 37.Kaye AD, Manchikanti L, Abdi S, Atluri S, Bakshi S, Benyamin R, et al. Efficacy of epidural injections in managing chronic spinal pain: a best evidence synthesis. Pain Physician. 2015;18:E939–E1004. [PubMed] [Google Scholar]
- 38.Manchikanti L, Pampati V, Benyamin RM, Boswell MV. Analysis of efficacy differences between caudal and lumbar interlaminar epidural injections in chronic lumbar axial discogenic pain: local anesthetic alone vs. local combined with steroids. Int J Med Sci. 2015;12:214–222. doi: 10.7150/ijms.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJ. A randomized, double-blind controlled trial of lumbar interlaminar epidural injections in central spinal stenosis: 2-year follow-up. Pain Physician. 2015;18:79–92. [PubMed] [Google Scholar]
- 40.Helm S, 2nd, Racz GB, Gerdesmeyer L, Justiz R, Hayek SM, Kaplan ED, et al. Percutaneous and endoscopic adhesiolysis in managing low back and lower extremity pain: a systematic review and meta-analysis. Pain Physician. 2016;19:E245–E282. [PubMed] [Google Scholar]
- 41.Manchikanti L, Nampiaparampil DE, Candido KD, Bakshi S, Grider JS, Falco FJ, et al. Do cervical epidural injections provide long-term relief in neck and upper extremity pain? A systematic review. Pain Physician. 2015;18:39–60. [PubMed] [Google Scholar]
- 42.Manchikanti L, Singh V, Pampati V, Falco FJ, Hirsch JA. Comparison of the efficacy of caudal, interlaminar, and transforaminal epidural injections in managing lumbar disc herniation: is one method superior to the other? Korean J Pain. 2015;28:11–21. doi: 10.3344/kjp.2015.28.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manchikanti L, Hirsch JA, Kaye AD, Boswell MV. Cervical zygapophysial (facet) joint pain: effectiveness of interventional management strategies. Postgrad Med. 2016;128:54–68. doi: 10.1080/00325481.2016.1105092. [DOI] [PubMed] [Google Scholar]
- 44.Manchikanti L, Kaye AD, Manchikanti K, Boswell M, Pampati V, Hirsch J. Efficacy of epidural injections in the treatment of lumbar central spinal stenosis: a systematic review. Anesth Pain Med. 2015;5:e23139. doi: 10.5812/aapm.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, et al. Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician. 2016;19:E33–E54. [PubMed] [Google Scholar]
- 46.Manchikanti L, Staats PS, Nampiaparampil DE, Hirsch JA. What is the role of epidural injections in the treatment of lumbar discogenic pain: a systematic review of comparative analysis with fusion. Korean J Pain. 2015;28:75–87. doi: 10.3344/kjp.2015.28.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou R, Hashimoto R, Friedly J, Fu R, Dana T, Sullivan S, et al. Pain management injection therapies for low back pain. Rockville (MD): Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 48.Manchikanti L, Pampati V, Hirsch JA. Utilization of interventional techniques in managing chronic pain in medicare population from 2000 to 2014: an analysis of patterns of utilization. Pain Physician. 2016;19:E531–E546. [PubMed] [Google Scholar]
- 49.Manchikanti L, Hirsch JA, Pampati V, Boswell MV. Utilization of facet joint and sacroiliac joint interventions in medicare population from 2000 to 2014: explosive growth continues! Curr Pain Headache Rep. 2016;20:58. doi: 10.1007/s11916-016-0588-2. [DOI] [PubMed] [Google Scholar]
- 50.Manchikanti L, Pampati V, Hirsch JA. Retrospective cohort study of usage patterns of epidural injections for spinal pain in the US fee-for-service medicare population from 2000 to 2014. BMJ Open. 2016;6:e013042. doi: 10.1136/bmjopen-2016-013042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 52.Manchikanti L, Kaye AM, Kaye AD. Current state of opioid therapy and abuse. Curr Pain Headache Rep. 2016;20:34. doi: 10.1007/s11916-016-0564-x. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch JA, Chandra RV, Pampati V, Barr JD, Brook AL, Manchikanti L. Analysis of vertebral augmentation practice patterns: a 2016 update. J Neurointerv Surg. 2016 doi: 10.1136/neurintsurg-2016-012767. [in press] [DOI] [PubMed] [Google Scholar]
- 54.Manchikanti L, Kaye AM, Knezevic NN, McAnally H, Slavin K, Trescot AM, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American society of interventional pain physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3–S92. [PubMed] [Google Scholar]
- 55.Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997–2006. Spine (Phila Pa 1976) 2009;34:2077–2084. doi: 10.1097/BRS.0b013e3181b1fad1. [DOI] [PubMed] [Google Scholar]
- 56.Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, et al. US spending on personal health care and public health, 1996–2013. JAMA. 2016;316:2627–2646. doi: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 2013;38:916–926. doi: 10.1097/BRS.0b013e3182833e7c. [DOI] [PubMed] [Google Scholar]
- 58.Manchikanti L, Pampati V, Falco FJ, Hirsch JA. An updated assessment of utilization of interventional pain management techniques in the Medicare population: 2000–2013. Pain Physician. 2015;18:E115–E127. [PubMed] [Google Scholar]
- 59.Hirsch JA, Leslie-Mazwi TM, Patel AB, Rabinov JD, Gonzalez RG, Barr RM, et al. MACRA: background, opportunities and challenges for the neurointerventional specialist. J Neurointerv Surg. 2016;8:868–874. doi: 10.1136/neurintsurg-2015-011952. [DOI] [PubMed] [Google Scholar]
- 60.Manchikanti L, Staats PS, Boswell MV, Hirsch JA. Analysis of the carrot and stick policy of repeal of the sustainable growth rate formula: the good, the bad, and the ugly. Pain Physician. 2015;18:E273–E292. [PubMed] [Google Scholar]
- 61.Hirsch JA, Leslie-Mazwi TM, Nicola GN, Bhargavan-Chatfield M, Seidenwurm DJ, Silva E, et al. PQRS and the MACRA: value-based payments have moved from concept to reality. AJNR Am J Neuroradiol. 2016;37:2195–2200. doi: 10.3174/ajnr.A4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manchikanti L, Hammer M, Benyamin RM, Hirsch JA. Physician quality reporting system (PQRS) for interventional pain management practices: challenges and opportunities. Pain Physician. 2016;19:E15–E32. [PubMed] [Google Scholar]
- 63.Manchikanti L, Helm Ii S, Benyamin RM, Hirsch JA. Merit-based incentive payment system (MIPS): harsh choices for interventional pain management physicians. Pain Physician. 2016;19:E917–E934. [PubMed] [Google Scholar]
- 64.Manchikanti L, Helm Ii S, Calodney AK, Hirsch JA. Merit-based incentive payment system: meaningful changes in the final rule brings cautious optimism. Pain Physician. 2017;20:E1–E12. [PubMed] [Google Scholar]
- 65.Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and metaanalysis. Ann Intern Med. 2012;157:865–877. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 66.Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371:11–21. doi: 10.1056/NEJMoa1313265. [DOI] [PubMed] [Google Scholar]
- 67.Manchikanti L, Candido KD, Kaye AD, Boswell MV, Benyamin RM, Falco FJ, et al. Randomized trial of epidural injections for spinal stenosis published in the New England Journal of Medicine: further confusion without clarification. Pain Physician. 2014;17:E475–E488. [PubMed] [Google Scholar]
- 68.Chou R, Hashimoto R, Friedly J, Fu R, Bougatsos C, Dana T, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:373–381. doi: 10.7326/M15-0934. [DOI] [PubMed] [Google Scholar]
- 69.Boswell MV, Manchikanti L. Appropriate design, methodological quality assessment, and clinically relevant outcomes are essential to determine the therapeutic role of epidural injections for low back pain and radiculopathy. Evid Based Med. 2016;21:89. doi: 10.1136/eb-2015-110310. [DOI] [PubMed] [Google Scholar]
- 70.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, doubleblind controlled trial: Clinical Trial NCT00355914. Pain Physician. 2008;11:121–132. [PubMed] [Google Scholar]
- 71.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Evaluation of lumbar facet joint nerve blocks in managing chronic low back pain: a randomized, double-blind, controlled trial with a 2-year follow-up. Int J Med Sci. 2010;7:124–135. doi: 10.7150/ijms.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitehurst DG, Lewis M, Yao GL, Bryan S, Raftery JP, Mullis R, et al. A brief pain management program compared with physical therapy for low back pain: results from an economic analysis alongside a randomized clinical trial. Arthritis Rheum. 2007;57:466–473. doi: 10.1002/art.22606. [DOI] [PubMed] [Google Scholar]
- 73.Johnson RE, Jones GT, Wiles NJ, Chaddock C, Potter RG, Roberts C, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1578–1585. doi: 10.1097/BRS.0b013e318074f890. [DOI] [PubMed] [Google Scholar]
- 74.Manchikanti L, Hirsch JA. Repeal and replace of affordable care: a complex, but not an impossible task. Pain Physician. 2016;19:E1109–E1113. [PubMed] [Google Scholar]
- 75.Manchikanti L, Helm Ii S, Benyamin RM, Hirsch JA. A critical analysis of obamacare: affordable care or insurance for many and coverage for few? Pain Physician. 2017;20:111–138. [PubMed] [Google Scholar]
- 76.Manchikanti L, Hirsch JA. Obamacare 2012: prognosis unclear for interventional pain management. Pain Physician. 2012;15:E629–E640. [PubMed] [Google Scholar]
- 77.Hirsch JA, Barr RM, McGinty G, Nicola GN, Schaefer PW, Silva E, 3rd, et al. Affordable care 2014: a tale of two boards. J Neurointerv Surg. 2014;6:718–720. doi: 10.1136/neurintsurg-2014-011322. [DOI] [PubMed] [Google Scholar]
- 78.Manchikanti L, Hirsch JA. Patient protection and affordable care act of 2010: a primer for neurointerventionalists. J Neurointerv Surg. 2012;4:141–146. doi: 10.1136/neurintsurg-2011-010036. [DOI] [PubMed] [Google Scholar]
- 79.Hirsch JA, Leslie-Mazwi TM, Barr RM, McGinty G, Nicola GN, Patel AB, et al. The Burwell roadmap. J Neurointerv Surg. 2016;8:544–546. doi: 10.1136/neurintsurg-2015-011706. [DOI] [PubMed] [Google Scholar]
- 80.Porter ME, Teisberg EO. Redefining health care: creating value-based competition on results. Boston (MA): Harvard Business School Press; 2006. [Google Scholar]
- 81.Manchikanti L, Helm Ii S, Singh V, Hirsch JA. Accountable interventional pain management: a collaboration among practitioners, patients, payers, and government. Pain Physician. 2013;16:E635–E670. [PubMed] [Google Scholar]
- 82.Manchikanti L, Falco FJ, Benyamin RM, Gharibo CG, Candido KD, Hirsch JA. Epidural steroid injections safety recommendations by the Multi-Society Pain Workgroup (MPW): more regulations without evidence or clarification. Pain Physician. 2014;17:E575–E588. [PubMed] [Google Scholar]
- 83.Manchikanti L, Hirsch JA. Neurological complications associated with epidural steroid injections. Curr Pain Headache Rep. 2015;19:482. doi: 10.1007/s11916-015-0482-3. [DOI] [PubMed] [Google Scholar]
- 84.Manchikanti L, Benyamin RM. Key safety considerations when administering epidural steroid injections. Pain Manag. 2015;5:261–272. doi: 10.2217/pmt.15.17. [DOI] [PubMed] [Google Scholar]
- 85.Manchikanti L, Falco FJ. Safeguards to prevent neurologic complications after epidural steroid injections: analysis of evidence and lack of applicability of controversial policies. Pain Physician. 2015;18:E129–E138. [PubMed] [Google Scholar]
- 86.Goossens ME, Evers SM, Vlaeyen JW, Rutten-van Mõlken MP, van der Linden SM. Principles of economic evaluation for interventions of chronic musculoskeletal pain. Eur J Pain. 1999;3:343–353. doi: 10.1053/eujp.1999.0140. [DOI] [PubMed] [Google Scholar]
- 87.Hopwood M. Pain management. Edited by Abram SE. Philadelphia (PA): Churchill-Livingstone; 1998. Outcomes assessment in pain management; pp. 14.1–14.11. [Google Scholar]
- 88.Manchikanti L, Kaye AD, Hirsch JA. Proposed medicare physician payment schedule for 2017: impact on interventional pain management practices. Pain Physician. 2016;19:E935–E955. [PubMed] [Google Scholar]
- 89.Manchikanti L, Singh V, Hirsch JA. Facility payments for interventional pain management procedures: impact of proposed rules. Pain Physician. 2016;19:E957–E984. [PubMed] [Google Scholar]