Abstract
Background and Purpose
Limited data exist on the performance of the revised Framingham Stroke Risk Score (R-FSRS) and the R-FSRS in conjunction with nontraditional risk markers. We compared the R-FSRS, original FSRS (O-FSRS) and the Pooled Cohort Equation (PCE) for stroke prediction and assessed the improvement in discrimination by nontraditional risk markers.
Methods
6712 of 6814 participants of the Multi-Ethnic Study of Atherosclerosis (MESA) were included. Cox proportional hazard, area under the curve (AUC), net reclassification improvement (NRI), and integrated discrimination increment (IDI) analysis were used to assess and compare each stroke prediction risk score. Stroke was defined as fatal/non-fatal strokes (hemorrhagic or ischemic).
Results
After mean follow up of 10.7 years, 231/6712 (3.4%) strokes were adjudicated (2.7% ischemic strokes). Mean stroke risks using the R-FSRS, O-FSRS and PCE were 4.7%, 5.9% and 13.5%. The R-FSRS had the best calibration (Hosmer-Lemeshow Goodness-of- Fit, χ2 =6.55, p = 0.59). All risk scores were predictive of incident stroke. C-statistics of R-FSRS (0.716) was similar to PCE (0.716), but significantly higher than the O-FSRS (0.653, p=0.01 for comparison with R-FSRS). Adding nontraditional risk markers individually to the R-FSRS did not improve discrimination of the R-FSRS in the AUC analysis, but did improve category-less NRI and IDI for incident stroke. The addition of coronary artery calcium (CAC) to R-FSRS produced the highest category-less NRI (0.36) and IDI (0.0027). Similar results were obtained when ischemic strokes were used as the outcome.
Conclusions
The R-FSRS downgraded stroke risk but had better calibration and discriminative ability for incident stroke compared with the O-FSRS. Nontraditional risk markers modestly improved the discriminative ability of the R- FSRS, with CAC performing the best.
Subject Terms: Epidemiology, Risk Factors, Primary Prevention, Stroke
Introduction
Stroke is a leading cause of morbidity and mortality in the United States.1 Over 70% of strokes occur in those without a history of a prior stroke, emphasizing the importance of stroke primary prevention.2 Several risk scores have been developed to identify persons at high risk for future strokes and overall atherosclerotic cardiovascular disease (ASCVD).3–6 Over the last 15–20 years, stroke rates and risk factor prevalence have declined and the implementation of ASCVD/stroke prevention therapies and strategies has improved.7, 8
The Framingham Stroke Risk Profile (FSRP) combines stroke risk factors (age, sex, systolic blood pressure (SBP), use of anti-hypertensives, presence/absence of left ventricular hypertrophy (LVH) on electrocardiogram (ECG), prevalent cardiovascular disease, current smoking status, current/previous AF, and diabetes mellitus (DM)) to predict 10-year probability of stroke.3 The original FSRP (O-FSRS) is based on stroke data from the 1960s and 1970s and the application of the O-FSRP to contemporary cohorts shows overestimation of stroke risk.9, 10 A revised FSRP (R-FSRS) was developed to reflect temporal trends using updated stroke risk factors prevalence and stroke rate incidence, and may be used for examining geographic/racial differences in stroke risk and the utility of nontraditional risk markers in stroke prediction.11
Data from the Rotterdam Heart Study showed that the R-FSRS and O-FSRS had similar discriminative ability for primary stroke in Caucasians.12 Limited data exist on the performance of the R-FSRS in a multi-ethnic and ASCVD free cohort and whether nontraditional ASCVD risk markers improve the discriminative ability of the R-FSRS
We used data from the Multi Ethnic Study of Atherosclerosis (MESA) to compare the calibration and discrimination of the O-FSRS, R-FSRS, and the ASCVD Pooled Cohort Equation (PCE) for incidence strokes with 10 years of adjudicated stroke outcomes. Additionally, we assessed the ability of nontraditional risk markers (coronary artery calcium (CAC), carotid Intima-media thickness (CIMT), ankle brachial index (ABI), high sensitivity C–reactive protein (CRP) and family history of stroke to improve the discrimination of the R-FSRS.
Methods
The data that support the findings of this study are available at the NHLBI-MESA website: (www.mesa.nhlbi.org) and would be made available by the corresponding author upon reasonable request.
Study Population and Data Collection
The design for the MESA has been previously published.13 MESA is a prospective population-based cohort study to investigate the prevalence, correlates, and progression of subclinical ASCVD in persons without known baseline ASCVD. The cohort includes 6,814 women and men ages 45 to 84 years recruited from six U.S. communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota). MESA included 38% white, 28% African American, 22% Hispanic, and 12% Chinese adults. Demographics, medical history, anthropometric, and laboratory data for the present study were taken from the first examination (July 2000 to August 2002). The MESA study was approved by the institutional review boards of each study site, and written informed consent was obtained from all participants.
Conventional Risk Factors
At baseline examination, traditional and additional ASCVD risk factor data was collected. Supplemental Table I show the risk factors used in the O-FSRS, R-FSRS, and PCE for future stroke risk calculation. Current smoking was defined as having smoked a cigarette in the last 30 days. Medication use was based on medication inventory. Diabetes mellitus was defined as self-reported history of diabetes mellitus, diabetes medication use or fasting glucose ≥126mg/dl. Participants who reported having diabetes mellitus should also be on anti-diabetic medications and or have fasting blood glucose of ≥ 126mg/dl to be counted as having diabetes mellitus in MESA. Resting blood pressure was measured three times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a SBP of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, and or use of antihypertensive medication. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Total and high-density lipoprotein (HDL) cholesterol were measured from blood samples obtained after a 12 hour fast. Low-density lipoprotein (LDL) cholesterol was estimated by the Friedewald equation.14
Measurement of Carotid Intima Media Thickness
Methods for measuring and interpreting CIMT were previously reported.15 The mean of the maximum intima-media thickness of the common carotid artery was used. Reproducibility was assessed by blinded replicate readings of CIMT performed by two readers. One reader reread 66 studies for a between reader correlation coefficient of 0.84 (n=66), and a second reader reread 48 studies for a correlation coefficient of 0.86. The rescan and the reread coefficients of variation were 7.07% and 3.48%.
Family history of Stroke
Family history of stroke was obtained and defined by asking participants whether any member in their immediate family (first-degree relatives: parents, siblings and children) experienced fatal or non-fatal stroke. Type of stroke (ischemic or hemorrhagic) was not asked. Additionally, the age at which the immediate family experienced a stroke was also not obtained and thus it is unclear whether experienced strokes were premature.
High-sensitivity C-reactive protein (HsCRP)
HsCRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, Illinois) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, Vermont). Analytical intra-assay coefficient of variations ranged from 2.3% to 4.4%, and inter-assay coefficient of variation ranged from 2.1% to 5.7% with a detection level of 0.18 mg/L.
Coronary Artery Calcium Score
Details of the MESA computed tomography (CT) scanning and interpretation methods have been previously reported.16 Scanning centers assessed CAC by chest CT with either a cardiac-gated electron-beam CT scanner (Chicago, Illinois; Los Angeles, California; and New York, New York field centers) or a multidetector CT system (Baltimore, Maryland; Forsyth County, North Carolina; and St Paul, Minnesota field centers). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. A radiologist or cardiologist read all CT scans at a central reading center (Los Angeles Biomedical Research Institute at Harbor–UCLA, Torrance, California). We used the mean Agatston score17 for the two scans in all analyses. Intraobserver and interobserver agreements were excellent (κ = 0.93 and κ = 0.90, respectively).
Ankle-brachial index
Details of the MESA ankle-brachial index measurement protocol have been previously published.18 SBP measurements in the bilateral brachial, dorsalis pedis, and posterior tibial arteries were obtained in the supine position using a hand-held Doppler instrument with a 5-mHz probe. To avoid potential bias from subclavian stenosis, the higher of the brachial artery pressures was used as the denominator. The ABI numerator used was the highest pressure (dorsalis pedis or posterior tibial) from that leg. Reproducibility of the ABI was evaluated using measurements of 43 participants by two technicians. The inter- and intra- reader correlation coefficients were 0.845 and 0.937 respectively with an intra- and inter- reader coefficient of variation of 5.14% and 3.27% respectively. Participants with an ABI > 1.4 were excluded.
Risk Scores
The stroke risk for each MESA participant was calculated using the published equations of the O-FSRS3, R-FSRS11, and PCE.6 For this analysis we limited PCE predicted events to stroke.
Event Ascertainment
Event ascertainment procedures and the adjudication process in MESA has been published.19 Every 9–12 months from baseline examination, MESA participants (or proxies) were contacted to inquire about hospital admissions, ASCVD diagnosis, and death. Hospital and other documentation of possible stroke and deaths were obtained. Documentation was sent to at least two MESA morbidity and mortality committee members for adjudication using a standard protocol. Disagreements between adjudicators were settled by discussion until consensus was reached. Events adjudicated as stroke were classified as ischemic, hemorrhagic, or other, which includes those for whom the type of stroke is undetermined. Stroke adjudication in MESA required a focal deficit of 24 hours and was in most instances confirmed by neuroimaging. For the purposes of this study, we defined incident a stroke event as adjudicated fatal or non-fatal hemorrhagic or ischemic stroke as described by the MESA protocol (www.mesa.nhlbi.org).
Statistical Analysis
Descriptive baseline statistics of demographic, clinical, and ASCVD risk factors were reported as mean (with standard deviation) for continuous variables and percentile for categorical variables. The stroke risk associated with one standard deviation of the calculated O-FSRS, R-FSRS and the PCE were assessed using Cox proportional hazard analysis. The Cox proportional hazard analysis assessing the association between R-FSRS and O-FSRS and incident stroke was further stratified by sex, race/ethnicity and age (using 65 years as cut off). We also assessed the associations between R-FSRS and O-FSRS for ischemic strokes, hemorrhagic strokes and TIAs. Calibration of each risk score was assessed by using the Hosmer-Lemeshow Goodness-of-Fit test and by comparing the mean predicted stroke risk of each risk score in the MESA cohort with the observed stroke event rate during the follow up period. Predicted probabilities of the cohort were obtained from Cox proportional hazard models with the O-FSRS, R-FSRS, and PCE as the predictor variable and incident stroke events as the outcome of interest.
Discriminative ability of the O-FSRS, R-FSRS, and the PCE for incident stroke events was assessed using area under the curve (AUC) analysis and C–statistics. Improvement in discrimination of the O-FSRS and R-FSRS afforded by the addition of individual nontraditional ASCVD risk marker was assessed. The differences in C-statistics of the O-FSRS or R-FSRS and R-FSRS plus each nontraditional risk marker were evaluated using the method by Delong et al.20
We constructed a reclassification plot using predicted probabilities of the O-FSRS and R-FSRS to assess reclassification.21, 22 The predicted probabilities were obtained using the calculated risk scores (O-FSRS and R-FSRS) and observed stroke events in a Cox model. Category-less net reclassification index (NRI) was used to assess the improvement in discrimination afforded by the addition of nontraditional risk markers to the R-FSRS for incident stroke events. CAC, CIMT, CRP, ABI were transformed [ln (Risk marker + 1)] prior to introducing each individually into models. P value of < 0.05 was considered significant for all calculations. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and Microsoft Excel.
Results
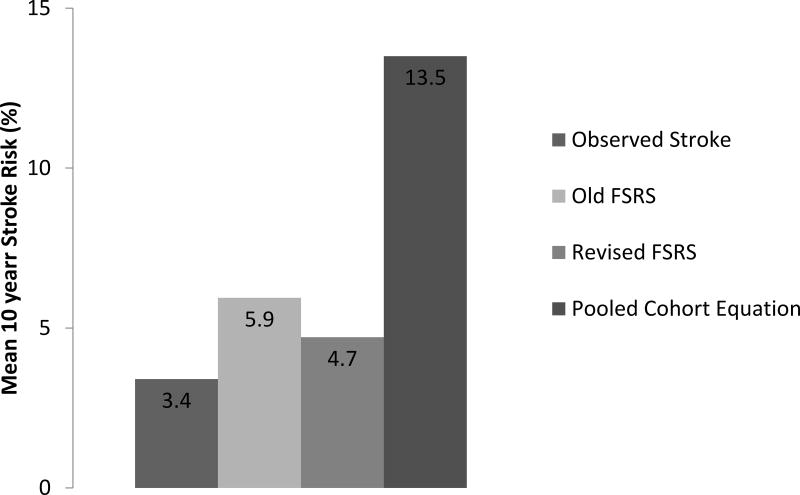
6712/6814 (98.5%) of total MESA participants had complete data and were included in our analyses. After a mean follow up of 10.7 years, 231/6712 (3.4%) participants had an adjudicated stroke (2.7% had an ischemic stroke, 0.7% hemorrhagic strokes). The mean age was 62 +/− 10 years with 53% female. 13% had diabetes, 15% were on statin therapy, 20% on aspirin therapy, and 33% on anti-hypertensives. Further demographics and distribution of the non-traditional risk markers are in Table 1. The Hosmer-Lemeshow Goodness-of-Fit test for stroke for each risk score was as follows: O-FSRS (χ2 = 34.23, p = 0.0002), R-FSRS (χ2 = 6.55, p = 0.59) and PCE (χ2 = 28.7, p=0.003). As also shown in Figure 1, the R-FSRS is better calibrated than the O-FSRS when respective mean risk prediction is compared with the observed stroke event rate. The mean +/− SD risk of the R-FSRS was significantly lower than that of the O-FSRS (p value for T-test = 0.01) and the PCE (p = 0.0001).
Table 1.
Clinical characteristics of the study cohort (n = 6712)
| Baseline Characteristics | Mean (Median when specified) (SD or %) |
|---|---|
| Age (years) | 62.2 ± 10.2 |
| Female (%) | 52.8 |
| Race/Ethnicity (%) | |
| Whites | 32.3 |
| Chinese | 11.9 |
| African American | 27.6 |
| Hispanics | 22.2 |
| Body Mass Index (BMI) (kg/m2) | 28.3 ± 5.5 |
| Cholesterol (mg/dL) | |
| Total | 194.2 ± 35.7 |
| Low density lipoprotein (LDL) | 117.2 ± 31.4 |
| High density lipoprotein (HDL) | 51.0 ± 14.8 |
| Triglycerides | 131.6 ± 89.0 |
| Blood Pressure (mm/Hg) | |
| Systolic | 126.6 ± 21.5 |
| Diastolic | 71.9 ± 10.3 |
| Cigarette Smoking Status (%) | |
| Never | 50.3 |
| Former | 36.7 |
| Current | 13.0 |
| Diabetes Mellitus (%) | 12.7 |
| Blood Pressure Medication use (%) | 33.2 |
| Statin use (%) | 14.8 |
| Aspirin Use (%) | 20.0 |
| Original Framingham Stroke Risk Score (Original FSRS) | 5.9 ± 1.2 |
| Revised Framingham Stroke Risk Score (Revised FSRS) | 4.7 ± 5.3 |
| Pooled Cohort Equation | 13.2 ± 13.1 |
| Coronary Artery Calcium (CAC) Score (Agatston Units)¥ | |
| Median (IQR) | 0.0 (0.0 – 86.2) |
| Carotid Intima Media Thickness (CIMT) (mm) | |
| Median (IQR) | 0.8 (0.7 – 1.0) |
| High Sensitivity C Reactive Protein (CRP) (mg/L) | |
| Median (IQR) | 3.8 (0.8 – 4.3) |
| Ankle Brachial Index (ABI) | 1.1 (1.1 – 1.2) |
| Median (IQR) | |
| Family history of stroke (First degree relative) (%) | 32.6 |
| Total number of strokes | 231 (3.5%) |
IQR: Interquartile range, (Q1 to Q3, 25th to 75th percentile)
CAC range is significantly skewed given that median CAC score is 0.
Figure 1.
Comparing the mean calculated risk using the original Framingham Stroke Risk Scores (FSRS), Revised FSRS, and Pooled Cohort Equation (PCE) with observed stroke rate in the Multi Ethnic Study of Atherosclerosis (MESA).
Consistent and significant associations existed between the O-FSRS and R-FSRS and incident stroke events across each defined stratum (sex, ethnicity, age) and outcome (ischemic stroke, hemorrhagic stroke and TIA) (Supplemental Table II).
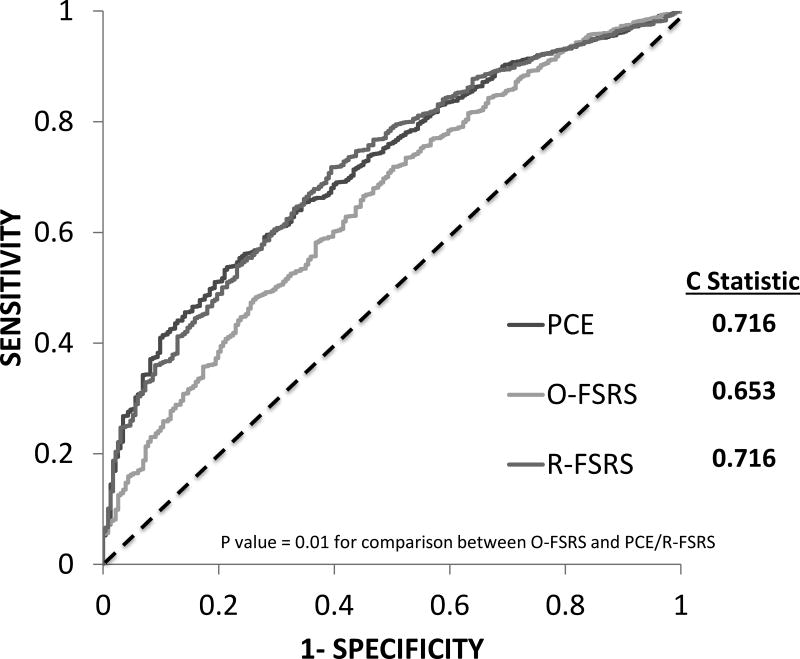
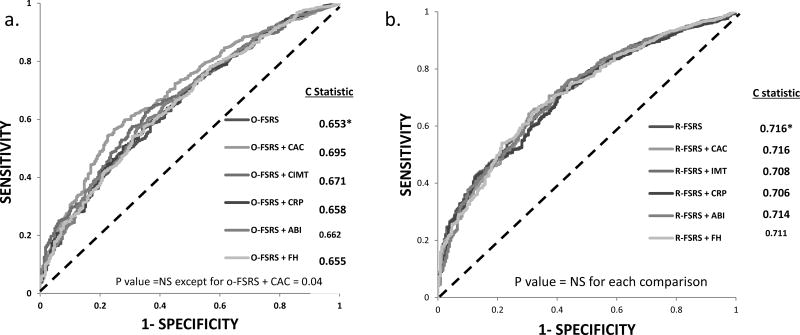
The C-statistics of the O-FSRS, R-FSRS, and PCE for stroke events were 0.653, 0.716 and 0.716, respectively (Figure 2). There was a significant difference in the C-statistics between the O-FSRS and the R-FSRS/PCE (p = 0.01 for each comparison). Figure 3 shows the improvement in discrimination afforded by the addition of CAC, CIMT, CRP, ABI and family history of stroke individually to the O-FSRS and the R-FSRS. Among the risk markers considered, only CAC modestly improved the C-statistics of the O-FSRS for future stroke events (p valve for comparison of C-statistics = 0.04) (Figure 3A). None of the risk markers improved the C statistics of the R-FSRS for future strokes (Figure 3B)
Figure 2.
Receiver operator curves (ROC) assessing the discrimination for incident stroke of the Original FSRS (O-FSRS), Revised FSRS (R-FSRS), and Pooled Cohort Equation (PCE)
Figure 3.
Receiver operator curves (ROC) assessing the improvement in discrimination afforded by the addition of coronary artery calcium (CAC), carotid intima media thickness (CIMT), high sensitivity C-reactive protein (CRP), ankle brachial index (ABI) and family history of stroke (FHS) to the (a) Original Framingham Stroke Risk Score (O-FSRS) and (b) Revised FSRS (R-FSRS) for incident stroke events in MESA. * indicates reference
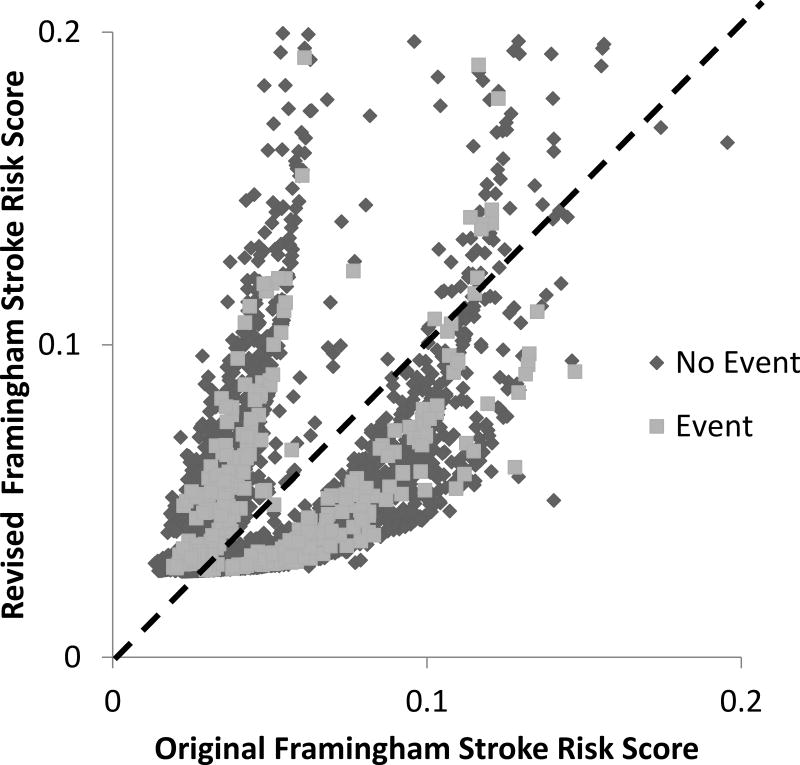
Figure 4 shows the reclassification plot comparing the improvement in reclassification of the R-FSRS (y-axis) with the O-FSRS (x-axis). A model that significantly improves reclassification will show more non-events (blue dots) below the 45-degree line and more events (red dots) above the 45-degree line. The event (red dots) and non-event (blue dots) category-less NRI when R-FSRS was compared with O-FSRS were −2.7% and 4.5% respectively. Thus, despite overestimation of stroke risk for non-event persons (Figure 4), overall the R-FSRS downgrades stroke risk in the MESA cohort. Table 2 shows the category-less NRI and IDI when CAC, CIMT, CRP, ABI and family history of stroke were added to the R-FSRS. The addition of each nontraditional risk marker showed an improvement in the overall category-less NRI and absolute IDI (Table 2). CAC showed the most improvement (NRI= 0.36, IDI=0.0027) while ABI showed the least (NRI= 0.11, IDI=0.0013) improvement.
Figure 4.
Reclassification plot of the predicted probabilities of the Original and Revised Framingham Stroke Risk Score for incident stroke events in MESA. (Both axes truncated at 0.2 to eliminate outliers)
Table 2.
Category-less Net Reclassification Improvement (NRI) and Integrated Discrimination Index (IDI) with the addition of subclinical atherosclerotic cardiovascular disease (ASCVD) markers to the Revised Framingham Stroke Risk Score (FSRS) for incident stroke.
| N | Revised FSRS > Revised FSRS + Marker (Downward) |
Revised FSRS < Revised FSRS + Marker (Upward) |
NRI | Total NRI | Absolute IDI |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Revised FSRS + CAC | ||||||
| Event | 231 | 98 | 133 | 0.15 | 0.36 | 0.0028 |
| Non-Event | 6481 | 3928 | 2553 | 0.21 | ||
|
| ||||||
| Revised FSRS + CIMT | ||||||
| Event | 231 | 127 | 104 | −0.10 | 0.22 | 0.0013 |
| Non-Event | 6481 | 3996 | 2485 | 0.23 | ||
|
| ||||||
| Revised FSRS + CRP | ||||||
| Event | 231 | 110 | 121 | 0.05 | 0.25 | 0.0020 |
| Non -Event | 6481 | 3881 | 2600 | 0.20 | ||
|
| ||||||
| Revised FSRS + ABI | ||||||
| Event | 231 | 131 | 100 | −0.13 | 0.05 | 0.0010 |
| Non-Event | 6481 | 3803 | 2678 | 0.17 | ||
|
| ||||||
| Revised FSRS + FH | ||||||
| Event | 231 | 141 | 90 | −0.22 | 0.13 | 0.0014 |
| Non-Event | 6481 | 4383 | 2098 | 0.35 | ||
In our secondary analysis, both O-FSRS and R-FSRS were predictive of ischemic stroke and hemorrhagic stroke (supplemental Table II). The C statistics for PCE, R-FSRS and O-FSRS for ischemic strokes were 0.725, 0.727 and 0.653 respectively (P value for comparison = 0.02). The C statistics when CAC, CIMT, CRP, ABI and FH were individually added to the O-FSRS for ischemic stroke prediction were; 0.702, 0.674, 0.663, 0.660, 0.656 respectively with only the addition of CAC being statistically significant (P value =0.01). The C statistics when CAC, CIMT, CRP, ABI and FH were individually added to R-FSRS for ischemic stroke prediction were 0.722, 0.717, 0.721, 0.730 and 0.720 (P value for NS for each comparison with the C-statistics for R-FSRS alone). The category-less NRI and IDI using ischemic strokes as the outcome were similar to that of all strokes in Table 2 (data not shown). Thus, the primary outcome (stroke) results appear to have been driven by ischemic stroke. The number of adjudicated hemorrhagic strokes (49 events) was too few and therefore we were underpowered for such as analysis.
Discussion
This study compared the calibration and discriminative ability of the R-FSRS, O-FSRS, and PCE for incident stroke and the improvement in discrimination afforded by the addition of nontraditional risk markers to the R-FSRS in a multi-ethnic U.S. cohort free of baseline clinical ASCVD. We showed that the R-FSRS had the best calibration for stroke events compared with the O-FSRS or PCE. Despite the R-FSRS showing a modest but significantly higher discriminative ability (C-statistic) compared with the O-FSRS, it downgrades stroke risk in this low risk cohort (Figure 4). In our area under the curve analyses, none of the nontraditional risk markers improved the discrimination of the R-FSRS for future stroke prediction. However, in the category-less NRI and IDI analyses, all the nontraditional risk markers (CAC, CIMT, CRP, ABI and FH) modestly improved discrimination of the R-FSRS, with CAC being the most useful (category-less NRI = 0.36) and ABI the least (category-less NRI = 0.05). To our knowledge, this is the first study to assess the improvement in discrimination afforded by the addition of nontraditional risk markers to the R-FSRS for future strokes.
Of the three stroke risk prediction scores available to clinicians for assessing stroke risk in the general U.S. population, the O-FSRS and R-FSRS were developed in a Caucasian population (Framingham Heart Study)3,11, validated in other cohorts10–12 and are recommended for stroke prediction.3,10–12 The PCE was not primarily derived for stroke risk prediction, but rather for a broader primary ASCVD outcome, which includes fatal and non-fatal strokes.6 A dilemma exists for clinicians, even if one assumes that the R-FSRS replaces the O-FSRS, as to which risk score to use in clinical practice for assessing primary stroke risk. In the present analysis, the PCE and R-FSRS have similar discriminative ability (C-statistics), and the R-FSRS has better calibration. While the PCE overestimates stroke risk, the R-FSRS appear to downgrade stroke risk compared with the O-FSRS. Thus, based on our analysis, the R-FSRS appears to be superior to the PCE/O-FSRS for primary stroke prediction. The present study supports the use of the R-FSRS as the preferred primary stroke risk prediction tool for general clinical practice in the US population.
It should be noted that the category-less NRI and IDI do not take into consideration calculated stroke risk cut offs/ranges that may warrant therapy and which modifiable risk factors to target to minimize this risk. Thus, although our data showed a modest improvement in discrimination when nontraditional risk markers are added to the R-FSRS for primary stroke prediction, the absence of R-FSRS risk cut offs for the initiation of therapy limits our ability to infer whether the upward/downward reclassifications observed in our models when the nontraditional risk markers were added to the R-FSRS would have resulted in any clinically meaningful change in risk categories and hence a change in clinical decision making / therapy. The lack of established clinically meaningful cut off of R-FSRS risk also makes it difficult, if not impossible, to determine the CAC levels or ranges associated with either upward/downward reclassification of individuals in our study similar to what is being established for ASCVD risk assessment.23 Our study calls for more research to establish clinically meaning R-FSRS risk cut points/ranges associated with low, intermediate and high 10 year risk of primary strokes to help guide therapeutic considerations in the U.S. population similar to that established for ASCVD prevention.6
Despite the large sample size, multi-ethnic nature of our cohort, long follow up and adjudicated stroke events used for this analysis, our study has significant limitations. First, our study is an observational study and therefore our results may be due to residual confounding. We did not account for treatment of risk factors in our participants with medications (such as antihypertensive medications, statins etc.) known to reduce stroke risk at baseline and during the follow up period. Medication use may have resulted in a reduction in the observed stroke events during the follow up period and may have affected our results. We also assessed the utility of the three stroke risk prediction tools (PCE, O-FSRS and R-FSRS) available in the U.S. in this cohort. However, unlike the O-FSRS and the R-FSRS, the PCE was developed to predict a composite outcome of fatal and non-fatal coronary heart diseases events and strokes. This may have resulted in the PCE having the worse calibration in our study. However, the discriminative ability of the PCE was similar to that of the R-FSRS for future stroke.
Thirdly, the MESA cohort by design had no history of cardiovascular disease at baseline and since the O-FSRS and R-FSRS were derived and validated in cohorts with and without history of cardiovascular disease, this may have affected the performance of these 2 risk scores. The PCE on the other hand was developed and validated in cohorts without history of cardiovascular disease. Thus, the PCE may have an “unfair” advantage over the O-FSRS and R-FSRS when compared in a cohort such as MESA. Nonetheless, our analysis comparing the discriminative ability of the O-FSRS and R-FSRS for future stroke should not be affected by this since both have history of cardiovascular disease as a component. In addition, since all these risk scores have stroke as a component of their composite outcome (PCE) or as their primary outcome (O-FSRS and R-FSRS), were developed to predict future stroke in populations without history of cardiovascular disease (PCE) or populations with and without history of cardiovascular disease (O-FSRS and R-FSRS), there is validity in comparing future stroke prediction of these three risk scores in a primary prevention population such as MESA. Finally, our results may not be applicable to other race/ethnic groups and other populations with characteristics dissimilar to that of the MESA cohort.
Conclusion
In this multiethnic cohort free of clinical ASCVD at baseline, the R-FSRS has better discriminative ability and calibration for incident stroke compared with the O-FSRS. Nontraditional risk markers modestly improve the discriminative ability of the R-FSRS, with CAC providing the greatest improvement. Further studies are needed to define R-FSRS risk levels/ categories that warrant therapeutic treatment for primary stroke prevention similar to that available for the primary ASCVD prevention.
Supplementary Material
Acknowledgments
We thank Georgia Saylor at Wake Forest Baptist Health for her statistical help. The authors also thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of MESA investigators/institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-TR-000040 and UL1-TR-001079 from National Center of Competence in Research (NCCR).
Footnotes
Disclosures: None
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parmar P, Krishnamurthi R, Ikram MA, Hofman A, Mirza SS, Varakin Y, et al. and Stroke Riskometer TMCWG. The Stroke Riskometer(TM) App: validation of a data collection tool and stroke risk predictor. Int J Stroke. 2015;10:231–44. doi: 10.1111/ijs.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 1996;27:1479–86. doi: 10.1161/01.str.27.9.1479. [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. American journal of epidemiology. 2004;160:259–69. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 7.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–46. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 8.Ovbiagele B, Schwamm LH, Smith EE, Hernandez AF, Olson DM, Pan W, et al. Recent nationwide trends in discharge statin treatment of hospitalized patients with stroke. Stroke; a journal of cerebral circulation. 2010;41:1508–13. doi: 10.1161/STROKEAHA.109.573618. [DOI] [PubMed] [Google Scholar]
- 9.Bineau S, Dufouil C, Helmer C, Ritchie K, Empana JP, Ducimetiere P, et al. Framingham stroke risk function in a large population-based cohort of elderly people: the 3C study. Stroke; a journal of cerebral circulation. 2009;40:1564–70. doi: 10.1161/STROKEAHA.108.532325. [DOI] [PubMed] [Google Scholar]
- 10.McClure LA, Kleindorfer DO, Kissela BM, Cushman M, Soliman EZ, Howard G. Assessing the performance of the Framingham Stroke Risk Score in the reasons for geographic and racial differences in stroke cohort. Stroke; a journal of cerebral circulation. 2014;45:1716–20. doi: 10.1161/STROKEAHA.114.004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufouil C, Beiser A, McLure LA, Wolf PA, Tzourio C, Howard VJ, et al. Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation. 2017;135:1145–1159. doi: 10.1161/CIRCULATIONAHA.115.021275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos D, Ikram MA, Leening MJG, Ikram MK. The Revised Framingham Stroke Risk Profile in a Primary Prevention Population: The Rotterdam Study. Circulation. 2017;135:2207–2209. doi: 10.1161/CIRCULATIONAHA.117.028429. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke; a journal of cerebral circulation. 2011;42:3017–21. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M., Jr Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015 Oct 13;66:1657–68. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.