Abstract
Background and Purpose
Patient heterogeneity reduces statistical power in clinical trials of restorative therapies. Valid predictors of treatment responsiveness are needed, and several have been studied with a focus on corticospinal tract (CST) injury. We studied performance of four such measures for predicting behavioral gains in response to motor training therapy.
Methods
Patients with subacute-chronic hemiparetic stroke (n=47) received standardized arm motor therapy, and change in arm Fugl-Meyer score was calculated from baseline to 1 month post-therapy. Injury measures calculated from baseline MRI included [1] percent CST overlap with stroke, [2] CST-related atrophy (cerebral peduncle area), [3] CST integrity (fractional anisotropy) in the cerebral peduncle, and [4] CST integrity in the posterior limb of internal capsule (PLIC).
Results
Percent CST overlap with stroke, CST-related atrophy, and CST integrity did not correlate with one another, indicating that these three measures captured independent features of CST injury. Percent injury to CST significantly predicted treatment-related behavioral gains (r=−0.41, p=0.004). The other CST injury measures did not, nor did total infarct volume or baseline behavioral deficits. When directly comparing patients with mild vs. severe injury using the percent CST injury measure, the odds ratio was 15.0 (95% CI 1.54–147, p<0.005) for deriving clinically important treatment-related gains.
Conclusions
Percent CST injury is useful for predicting motor gains in response to therapy in the setting of subacute-chronic stroke. This measure can be used as an entry criterion or a stratifying variable in restorative stroke trials in order to increase statistical power, reduce sample size, and reduce the cost of such trials.
Keywords: stroke, recovery, rehabilitation, clinical trial, corticospinal tract
Subject terms: stroke, biomarker, rehabilitation, neuroimaging
Introduction
Acute perfusion therapies introduced in the initial hours post-stroke can improve patient outcomes, but there is substantial variability in patient response. Recent endovascular therapy trials addressed this by using imaging to exclude patients with large ischemic cores1. Currently, a limited fraction of patients arrives at the hospital in time to receive such interventions, largely due to the narrow time window. Restorative therapies may be able to improve clinical outcomes after stroke with a wider time window, measured in days-months. However, variability in patient responses is also a confounding factor in post-acute stroke trials that evaluate restorative therapies. Therefore, studies have examined biomarkers of stroke-related injury in an attempt to improve patient selection for trials of restorative therapies.
Many restorative therapy trials have targeted motor deficits, which are among the most common after stroke2 and which contribute substantially to post-stroke disability. The neuroanatomy of the human motor system has characteristics favorable for biomarker development, as its main white matter efferent, the corticospinal tract (CST), runs in a single bundle relatively accessible to injury assessment3. Biomarkers have the potential to inform restorative trials in several ways, such as patient selection or stratification4, 5, beyond what is provided by clinical measures6.
Several biomarkers of CST injury after stroke have been described. The current study compared four of these: (1) cerebral peduncle area, which reflects Wallerian degeneration7, 8; (2) percent CST overlap with stroke, which reflects the quantity of CST injured9–13; and (3) cerebral peduncle14, 15 as well as (4) posterior limb of internal capsule (PLIC)16–18 fractional anisotropy (FA), a diffusion tensor imaging measure that reflects the integrity or quality of CST white matter. The primary objective was to compare these CST injury measurements in patients with subacute-chronic stroke for their ability to predict response to a restorative therapy.
Methods
This study examined data from patients with hemispheric subacute-chronic stroke that participated in 1 of 2 clinical trials involving 3–4 weeks of a standardized therapy targeting arm movements. Details of the therapy are provided elsewhere11, 19. Entry criteria included radiologically confirmed stroke (ischemic or intracerebral hemorrhage), 2–24 months post-stroke, age ≥18 years, and residual arm motor deficit defined either as an Action Research Arm Test score <52 (out of 57) or affected arm completion time on the Nine Hole Peg Test ≥25% longer than with the unaffected arm. Exclusion criteria included contraindication to MRI, Mini-Mental State Exam <24 (out of 30), and a non-stroke diagnosis affecting arm function. Patients also had to have stable arm impairment at baseline prior to therapy, such that serial testing on the arm motor Fugl-Meyer scale (FM)20 spaced 1–3 weeks apart could not differ by more than 3 points. The University of California Irvine Institutional Review Board approved both studies. All patients provided written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Behavioral Assessment
Patients completed an assessment battery prior to treatment that included the Geriatric Depression Scale and National Institutes of Health Stroke Scale (NIHSS). Treatment-induced gains in motor impairment were defined as the change in FM score from the mean of the two baseline scores to 1 month post-treatment.
MRI Acquisition
Prior to therapy, patients underwent an MRI scan involving high-resolution T1-weighted imaging, T2 fluid-attenuated inversion recovery (FLAIR) imaging, and DTI. Scans were performed on a 3-Tesla Philips Achieva system (Best, the Netherlands). Investigators used a three-dimensional magnetization-prepared rapid gradient echo sequence (repetition time [TR]= 8.5 milliseconds, echo time [TE]= 3.9 milliseconds, 150 slices, voxel size= 1×1×1 mm3) to acquire structural T1-weighted images. Parameters for the remaining neuroimaging sequences were as follows: T2 FLAIR (TR= 11,000 milliseconds, TE= 125 milliseconds, 31 slices, voxel size= 0.58×0.58×5 mm3) and DTI (32 directions, b= 1000 sec/mm2, 60 slices, voxel size= 1.75×1.75×2 mm3).
Image Analysis
Analysis of neuroimaging data was performed blinded to all clinical data. Six injury measures were extracted:
-
1
Percent Injury to CST--A CST template was constructed from diffusion tensor tractography from 17 healthy, right-handed individuals as previously described10–12. Briefly, seeds were placed in the left or the right precentral gyrus and cerebral peduncle. Each binarized and Montreal Neurological Institute (MNI)-transformed tract was partitioned into 16 descending subsections. The binarized infarct mask was overlaid onto the CST template. The CST subsections were classified as injured if at least five percent10 of the section overlapped with the infarct. Rather than compute the volume of overlap between each lesion and the CST, frequently referenced in the literature as the CST lesion load, we computed a percent injury to CST value by summing the total number of injured sections, dividing this value by 16, and multiplying by 100. Percent injury to CST, as compared to CST lesion load, has a stronger correlation with behavioral state10, possibly because dividing the CST into longitudinal subsections more closely models the trajectory of axon bundles and their injury by stroke.
-
2
Cerebral Peduncle Area--For each patient’s T1-weighted image transformed to MNI space, the axial slice depicting the greatest cerebral peduncle cross-sectional area was identified. Image roll and pitch were corrected using MRIcron21 to ensure that the ipsilesional and contralesional cerebral peduncles were in the same axial plane. Cerebral peduncle masks were drawn on these T1-weighted images, remaining ventral to and avoiding the substantia nigra, and the number of voxels contained in each mask was determined in order to generate area measurements. The cerebral peduncle area was defined as the ratio of ipsilesional to contralesional cerebral peduncle area, with values <1 indicating ipsilesional peduncle atrophy.
-
3 & 4
CST Integrity in PLIC and in cerebral peduncle--Diffusion tensor images were corrected for eddy currents and head motion with a three-dimensional affine registration using the FMRIB Software Library (FSL)22. A diffusion tensor model was fit at each voxel using the DTIFIT module, and a map of FA values was generated. FA values range from 0 to 1, with higher values suggestive of a greater degree of directionality of water diffusion and structural integrity23. Ipsilesional and contralesional masks of the cerebral peduncle24 and PLIC were drawn on the T1-weighted image co-registered to the FA image. PLIC masks were drawn on three contiguous slices located midway between the appearance and disappearance of the lenticular nucleus and thalamus. Borders of the PLIC masks were the following: anterior=genu of the internal capsule, posterior=line from posterior putamen to thalamus, medial=thalamus, and lateral=globus pallidus. Ipsilesional and contralesional PLIC masks were drawn on the same slices. In cases of stroke-related damage, the ipsilesional PLIC was drawn to mirror the location and size of contralesional PLIC. Mean FA values were computed from the voxels within the masks. CST integrity was defined as the ratio of ipsilesional to contralesional FA, separately for peduncle and PLIC. Amongst the 47 patients studied, none sustained injury to the cerebral peduncle while 23 had injury to the PLIC.
-
5
Infarct Volume--To calculate infarct volume, MRIcron21 was used to manually outline each patient's infarct on the T1-weighted MRI image, informed by the T2-FLAIR image, as described previously24. Infarct masks were binarized and spatially transformed into MNI standard stereotaxic space.
-
6
Primary Motor Cortex Injury--As described previously11, a mask of primary motor cortex gray matter (M1) was defined on a 1 mm3 T1 template, using FSL. The number of voxels overlapping between this M1 mask and each infarct in MNI space was calculated and used to measure stroke-related injury to M1.
Statistical Analysis
All statistical analyses were performed with JMP software (version 13.1, SAS, Cary, NC). Parametric statistical methods were used for measures that were normally distributed or could be transformed to a normal distribution otherwise non-parametric methods were used. Correlation coefficients were calculated to assess the relationship that cerebral peduncle area, CST integrity in cerebral peduncle, CST integrity in PLIC, and percent injury to CST had with baseline FM scores, and an adjusted alpha value of 0.0125 was used to define significance. This was repeated using treatment-related gains (change in FM score from baseline to 1 month post-therapy) as the dependent variable. The same procedures were used to evaluate specificity of these injury measures.
Results
Patients
A total of 47 patients were studied. Deficits were stable at baseline, as serial FM scores differed by only 0.3 points (p>0.20). Clinical and neuroimaging characteristics appear in Table 1. Patients were a mean of 5.5 months post-stroke, had wide-ranging arm motor deficits that ranged from mild-severe, had mean infarct volume 30.5 cc, and averaged 50.8% injury to CST.
Table 1.
Patient Clinical and Neuroimaging Characteristics
| Measurement | Value | Range |
|---|---|---|
| Age, years | 57.4 ± 14.6 | 21–86 |
| Time post-stroke, months | 5.5 ± 2.5 | 2.5–18.4 |
| NIHSS (normal=0) | 4.0 [3–5] | 0–11 |
| GDS (normal=0) | 3 [2–4] | 0–10 |
| MMSE (normal=30) | 28 [25–30] | 21–30 |
| Baseline FM (normal=66) | 35.8 ± 14.1 | 13.5–60 |
| FM change, baseline to 1 month post-treatment | 4.1 ± 3.5 | -1.5–12 |
| Infarct volume, cc | 30.5 ± 44.8 | 0.4–178 |
| Peduncle area | 0.9 ± 0.1 | 0.6–1.1 |
| CST peduncle integrity | 0.70 ± 0.20 | 0.26–1.15 |
| CST PLIC integrity | 0.75 ± 0.16 | 0.33–0.95 |
| Percent injury to CST | 50.8 ± 34.2 | 0–100 |
Values presented as mean ± SD or median [interquartile range]. The population had mild to severe deficits, with the lowest quartile of Fugl-Meyer scores ranging from 13.5–23, and the higher quartile from 47.5–60.
cc, cubic centimeter; CST, corticospinal tract; FM, arm Fugl-Meyer; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Exam; NIHSS, National Institutes of Health Stroke Scale; PLIC, posterior limb of internal capsule
Characteristics of the four CST injury measures
CST overlap with stroke, CST atrophy, and CST integrity did not correlate with one another, indicating that these three measures captured independent features of CST injury: CST peduncle integrity was unrelated to percent injury to CST (r=−0.23, p=0.12) or to peduncle area (r=−0.03, p=0.87), CST PLIC integrity was unrelated to percent injury to CST (r=−0.26, p=0.08) or to peduncle area (r=−0.01, p=0.94), and percent injury to CST was unrelated to peduncle area (r=−0.08, p=0.60). As expected, CST PLIC and peduncle integrity were related (r=0.68, p<0.0001). Three measures, CST peduncle integrity (r=0.55, p<0.0001), PLIC integrity (r=0.41, p=0.004), and percent injury to CST (r=−0.36, p=0.01), but not cerebral peduncle area (r=0.17, p=0.24), correlated significantly with motor status in subacute-chronic stroke, i.e., baseline FM score as measured at study entry. Findings with CST peduncle and PLIC integrity and percent injury to CST were specific to motor status, as these measures did not correlate with cognitive function (MMSE, p>0.44) or depression (GDS, p>0.14).
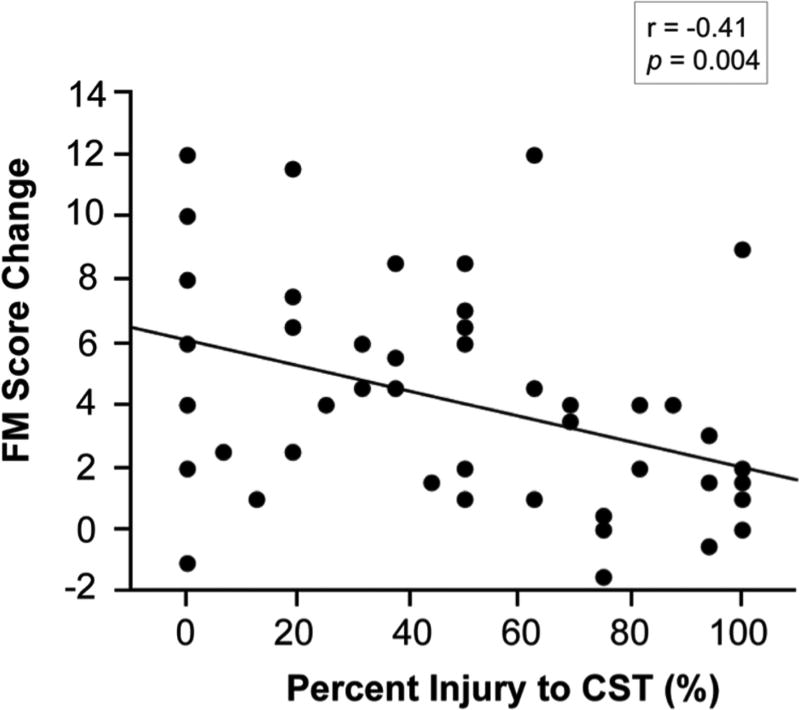
Percent injury to CST predicts treatment-related gains
From baseline to 1 month post-treatment, patients improved an average of 4.1 ± 3.5 points on the FM scale. When the four measures of CST injury were examined as predictors of these treatment-related gains, only one was significant, percent injury to CST (r=−0.41, p=0.004, Figure 1 and Table 2). Total infarct volume was not a significant predictor of treatment-related gains in arm motor status (r=−0.28, p=0.06), and neither was baseline FM score (r=0.19, p=0.21). When M1 was injured by stroke (n=23), percent injury to CST showed stronger predictive power for treatment-related arm motor gains (r=−0.44, p=0.034), and when M1 was not affected by stroke (n=24), percent injury to CST was no longer a significant predictor (r=−0.14, p=0.52). Adding measurements of CST peduncle integrity (p=0.62) or PLIC integrity (p=0.73) to percent injury to CST did not significantly improve prediction of treatment-related gains.
Figure 1.
Greater percent injury to CST significantly predicted poorer treatment-induced gains in arm motor status (n=47).
Table 2.
Neuroimaging Predictors of Treatment-Related Gains
| Correlation with Treatment-related Gains | ||
|---|---|---|
| Injury Measure | r | p |
| Percent injury to CST | −0.41 | 0.004 |
| CST peduncle integrity | 0.13 | 0.38 |
| CST PLIC integrity | 0.14 | 0.34 |
| Peduncle area | 0.20 | 0.18 |
| Infarct volume | −0.28 | 0.06 |
CST, corticospinal tract; PLIC, posterior limb of internal capsule
The significance of the predictive value of this measure can be further examined by contrasting two patient subgroups that differ according to severity of percent injury to CST. In the current population, there were 14 patients with mild injury to the CST injury (infarct injured 25% or less of the CST). Their average treatment-related gain in FM score was 5.5 ± 4.0 points, and gains exceeded the minimal clinically important difference (MCID) of 4.25 points25, 26 in 50% of patients. There were 16 patients with severe injury to the CST (infarct injured 75% or more of the CST). Their average treatment-related gain in FM score was 1.8 ± 2.5 points, and gains exceeded the MCID in 6.3% of patients. When directly comparing patients with mild vs. severe CST injury, the odds ratio for deriving treatment-related gains exceeding the MCID was 15.0 (95% CI 1.54–147, p<0.005). These findings persisted when using a more restrictive MCID definition of 6.0 points, i.e., 10% of the total arm FM scale27 (odds ratio 11.2, 95% CI 1.15–110, p=0.014). Figure 2 illustrates cases of mild and severe CST injury. These findings did not persist, however, when stratifying using a behavioral measure (FM score) instead of percent injury to CST: comparing the 16 most impaired patients (baseline FM= 20.2 ± 4.7, treatment-related gain= 2.9 ± 3.1 points) to the 14 lest impaired patients (baseline FM= 53.2 ± 4.7, treatment-related FM gain= 3.1 ± 2.9 points), the odds ratio for obtaining treatment-related gains exceeding an MCID of 4.25 points was 0.42 (95% CI 0.08–2.19, p=0.30).
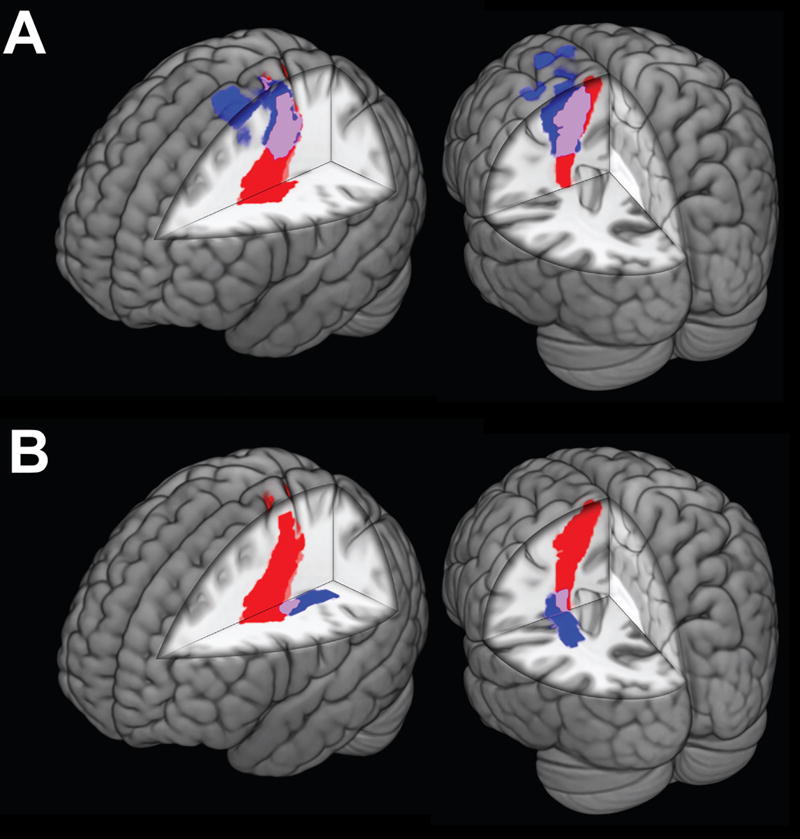
Figure 2.
Percent CST injury in two representative patients. Lesions are denoted in blue, the CST in red, and CST injury by the lesion in purple. [A] Severe CST injury: Two views of Patient 1, who had lesion volume of 7.0 cc and 93.7% CST injury. [B] Mild CST injury: Two views of Patient 2, who had lesion volume of 10.3 cc but only 18.7% CST injury. Despite similar lesion volumes, Patient 1 sustained greater percent injury to CST and had lower treatment-related gains in arm Fugl-Meyer score (1.5 points) as compared to Patient 2 (7.5 points).
Discussion
In a diverse population of patients with hemiparesis in the subacute-chronic phase post-stroke, three types of CST injury measures capturing both quantity and quality of CST injury, i.e., percent injury, atrophy, and integrity, were examined and found to be independent of each other. The main finding of the current analysis is that percent injury to CST was the only measure to significantly predict treatment-related gains. When directly comparing patients with mild vs. severe CST injury using the percent injury to CST measure, the odds ratio was 15.0 for deriving a treatment-related gain that exceeded the MCID. Just as recent successful endovascular therapy trials used imaging to exclude patients with large ischemic cores1, current findings suggest that restorative trials may similarly choose to use imaging to exclude patients with severe CST injury in order to maximize the likelihood of detecting a treatment effect.
The percent injury to the CST caused by stroke predicted treatment-related gains in arm motor status (Figure 1). This confirms the predictive value of the same measure as described in two prior, independent populations where the interventions were either robotic training10 or brain stimulation12, and emphasizes the superior performance of percent CST injury relative to CST integrity and peduncle area in terms of predictive value in the subacute-chronic population (Table 2). Percent injury to the CST describes the fraction of descending CST fibers critically injured by stroke10. Abundant evidence indicates that therapies that improve motor status in the chronic stroke state generally do so by promoting plasticity in the cortex and other brain structures, and that the CST is a critical efferent pathway by which these changes are expressed28–30. Current findings suggest that treatment-induced changes in brain function are most likely to produce behavioral gains when CST injury is limited, and that percent CST injury is a bigger factor than total infarct volume for predicting treatment response in this population. The findings further indicate that this dependence on CST is greater when M1 sustains stroke-related injury. The fact that the odds ratio is 15.0 for achieving treatment-induced arm motor gains that exceed the MCID when comparing mild vs. severe percent CST injury suggests that percent injury to CST may have utility as an entry criterion in clinical trials of restorative therapies in subacute-chronic stroke, as this measure enables study design to identify those patients who have a higher likelihood of achieving clinically important gains. Baseline behavior did not provide this predictive power, though most restorative trials in chronic stroke currently rely on behavioral criteria for study entry.
None of the other measures of CST injury studied (peduncle integrity, PLIC integrity, or peduncle area) was a significant predictor of treatment-related arm motor gains. This might in part reflect the white matter bundles assessed by each measure. The CST template utilized in computing percent injury to CST contained fibers originating from M1 only. However, FA values in the cerebral peduncle reflect integrity of fibers originating from cortex throughout the brain, and FA values in PLIC reflect integrity of both afferent and efferent fibers. Gains from a motor therapy that targets ipsilesional M1 may be better predicted by specifically measuring injury to those fibers issued by M1 rather than by measuring injury to a heterogeneous collection of fiber tracts.
CST integrity did not predict treatment-related gains in the current cohort. The constellation of findings with CST integrity--this measure correlated with baseline motor status but not with treatment-induced motor gains--echo results of a prior study of 22 patients with chronic stroke undergoing 2 weeks of constraint-induced movement therapy31. CST integrity is based on FA, which describes directionality of proton diffusion32, and characterizes white matter integrity given that FA values are influenced by the microstructural properties of white matter, e.g., myelination, axon density and diameter, and fiber organization/orientation33. Serial assessment of this measure may have value as a surrogate marker of treatment effects after stroke, as studies in healthy rodents have found training-related increases in FA within corpus callosum34 and white matter subjacent to M135, and studies in rodents with an experimental infarct have found increases in FA within the ischemic boundary zone following neural progenitor cell therapy36. The peduncle area measure is calculated based on tissue loss and reflects Wallerian degeneration8. Peduncular area did not correlate with degree of motor deficits in the current cohort, though this measure did in a prior study by Warabi et al7 of 89 patients with chronic hemiplegic stroke. These divergent findings might be attributable to differences in the population studied or methods used to calculate peduncle area. Together, the findings suggest that the capacity to improve motor status in the subacute-chronic stroke state is more dependent on extent to which CST undergoes critical injury rather than extent to which CST shows reduced integrity or Wallerian degeneration, at least in response to the current interventions.
Together, the findings across CST injury measures introduce questions important to the development of recovery biomarkers after stroke37. Percent injury to CST best predicted treatment-related gains, but CST peduncle integrity best explained baseline motor status. These were independent measurements, as CST integrity did not correlate with percent CST injury. The findings suggest a distinction between quantity of remaining tract (percent injury) and quality of remaining tract (CST integrity). A threshold of injury that limits treatment gains may be more easily definable for quantity of injury38. This may be less true for CST integrity, as conceivably axons may be able to conduct signals, and so support gains, across a wide range of FA reductions.
There are several strengths to the current study. Three independent types of CST injury were directly compared. Percent CST injury, which emerged as the sole measure predicting treatment-related motor gains, can be obtained using any technique that images an infarct, and so this approach could theoretically be extended to studies that used a CT scan to image the infarct. Current results emphasize the specificity of the percent injury to CST measure, that is, it correlates with behavioral deficits in motor but not other systems, and furthermore support a modality-specific approach to calculating injury after stroke39 given that a motor system injury measure (percent CST injury) but not a global injury measure (total infarct volume) predicted treatment-related motor gains (Table 2). Performance of the CST measure predicting treatment-related gains did not vary with infarct topography, as an interaction term for infarct depth (cortical vs. subcortical) was not significant (p=0.64). There are also several weaknesses. Current analyses were focused only on injury measures, though measurement of both neural injury and neural function may more robustly predict motor outcomes after stroke11, 40. The current study excluded patients with stroke in the brainstem given the complexities of CST neuroanatomy in this region. The findings may not extrapolate directly to acute stroke, where differences in the underlying neurobiology41 necessitate separate evaluation of biomarkers. The current focus was also on neuroimaging-based CST injury biomarkers, but physiological measures such as those obtained using transcranial magnetic stimulation, useful in predicting motor outcomes in the acute stroke setting16, 40, may also have utility in the chronic stroke context and warrant further study in this setting. Injury was measured only to that portion of the CST descending from M1, which we have previously found to be the most important predictor of treatment gains in the chronic stroke setting10, and future studies can examine the performance of injury measures to additional brain structures42, which could potentially boost the predictive power of percent CST injury.
The findings from the current analysis may prove useful in clinical trials of restorative therapies that choose to incorporate a measure of CST injury for the purposes of optimizing patient selection and stratification. Trial design might increase the chances of detecting a treatment effect by excluding patients with severe CST injury, who might be offered enrollment in trials studying alternative interventions that are not predicted to provide poor treatment gains.
Acknowledgments
None
Sources of Funding
This work received support from the National Institutes of Health (K24HD074722, T32AR047752, and K99HD091375) and the University of California, Irvine Institute for Clinical and Translational Science (UL1-TR000153).
Footnotes
Disclosures
JC, GT, and EQ report no conflicts of interest. SC has served as a consultant for Dart Neuroscience, MicroTransponder, and Roche.
References
- 1.Goyal M, Hill MD, Saver JL, Fisher M. Challenges and opportunities of endovascular stroke therapy. Ann Neurol. 2016;79:11–17. doi: 10.1002/ana.24528. [DOI] [PubMed] [Google Scholar]
- 2.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke. 2002;33:2718–2721. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 3.Kim B, Winstein C. Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair. 2016 doi: 10.1177/1545968316662708. [DOI] [PubMed] [Google Scholar]
- 4.Milot MH, Cramer SC. Biomarkers of recovery after stroke. Curr Opin Neurol. 2008;21:654–659. doi: 10.1097/WCO.0b013e3283186f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke E, Cramer SC. Biomarkers and predictors of restorative therapy effects after stroke. Curr Neurol Neurosci Rep. 2013;13:329. doi: 10.1007/s11910-012-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warabi T, Inoue K, Noda H, Murakami S. Recovery of voluntary movement in hemiplegic patients. Correlation with degenerative shrinkage of the cerebral peduncles in CT images. Brain. 1990;113:177–189. doi: 10.1093/brain/113.1.177. [DOI] [PubMed] [Google Scholar]
- 8.Mark VW, Taub E, Perkins C, Gauthier LV, Uswatte G, Ogorek J. Poststroke cerebral peduncular atrophy correlates with a measure of corticospinal tract injury in the cerebral hemisphere. Am J Neuroradiol. 2008;29:354–358. doi: 10.3174/ajnr.A0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77:132–145. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77:1076–1083. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78:860–870. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughty C, Wang J, Feng W, Hackney D, Pani E, Schlaug G. Detection and predictive value of fractional anisotropy changes of the corticospinal tract in the acute phase of a stroke. Stroke. 2016;47:1520–1526. doi: 10.1161/STROKEAHA.115.012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groisser BN, Copen WA, Singhal AB, Hirai KK, Schaechter JD. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair. 2014;28:751–760. doi: 10.1177/1545968314521896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015;78:848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- 17.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43:2248–2251. doi: 10.1161/STROKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138:2359–2369. doi: 10.1093/brain/awv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27:732–741. doi: 10.1177/1545968313491000. [DOI] [PubMed] [Google Scholar]
- 21.MRIcron Tutorial for Drawing Regions of Interest. MRIcron Index– McCausland Center For Brain Imaging. [Accessed August 1, 2016]; http://www.people.cas.sc.edu/rorden/mricon/stats.html.
- 22.FMRIB's Diffusion Toolbox. FSL - FslWiki - Oxford Centre for Functional MRI of the Brain. [Accessed August 1, 2016]; http://www.fmrib.ox.ac.uk/fsl/fslwiki/FDT.
- 23.Tuor UI, Morgunov M, Sule M, Qiao M, Clark D, Rushforth D, et al. Cellular correlates of longitudinal diffusion tensor imaging of axonal degeneration following hypoxic-ischemic cerebral infarction in neonatal rats. Neuroimage Clin. 2014;6:32–42. doi: 10.1016/j.nicl.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke E, Dodakian L, See J, McKenzie A, Riley JD, Le V, et al. A multimodal approach to understanding motor impairment and disability after stroke. J Neurol. 2014;261:1178–1186. doi: 10.1007/s00415-014-7341-8. [DOI] [PubMed] [Google Scholar]
- 25.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity fugl-meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 26.Bushnell C, Bettger JP, Cockroft KM, Cramer SC, Edelen MO, Hanley D, et al. Chronic stroke outcome measures for motor function intervention trials: Expert panel recommendations. Circ Cardiovasc Qual Outcomes. 2015;8:S163–169. doi: 10.1161/CIRCOUTCOMES.115.002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the action research arm test and the fugl-meyer assessment scale in chronic stroke patients. J Rehabil Med. 2001;33:110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 28.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 29.Oujamaa L, Relave I, Froger J, Mottet D, Pelissier JY. Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med. 2009;52:269–293. doi: 10.1016/j.rehab.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Baker SN, Zaaimi B, Fisher KM, Edgley SA, Soteropoulos DS. Pathways mediating functional recovery. Prog Brain Res. 2015;218:389–412. doi: 10.1016/bs.pbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Sterr A, Dean PJ, Szameitat AJ, Conforto AB, Shen S. Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair. 2014;28:335–343. doi: 10.1177/1545968313510972. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor mr imaging and fiber tractography: Theoretic underpinnings. Am J Neuroradiol. 2008;29:632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 34.Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion mri of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6:e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, et al. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int J Stroke. 2017;12:480–493. doi: 10.1177/1747493017714176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young W. Spinal cord regeneration. Science. 1996;273:451. doi: 10.1126/science.273.5274.451. [DOI] [PubMed] [Google Scholar]
- 39.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38:1393–1395. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 40.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The prep algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135:2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael ST. Brain excitability in stroke: The yin and yang of stroke progression. Arch Neurol. 2012;69:161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rondina JM, Park CH, Ward NS. Brain regions important for recovery after severe post-stroke upper limb paresis. J Neurol Neurosurg Psychiatry. 2017;88:737–743. doi: 10.1136/jnnp-2016-315030. [DOI] [PMC free article] [PubMed] [Google Scholar]