Abstract
Background
Cancer-related fatigue (CRF) is a prevalent and debilitating symptom experienced by cancer survivors, yet treatment options for CRF are limited. Our study evaluated the efficacy of weekly Swedish Massage Therapy (SMT) vs. an active control condition (light touch, LT) and waitlist control (WLC) on persistent CRF in breast cancer survivors.
Methods
This early phase randomized, single-masked, 6-week investigation of SMT, LT and WLC enrolled 66 female Stage 0-III breast cancer survivors age 32–72 who had received surgery plus radiation and/or chemotherapy/chemoprevention with CRF (Brief Fatigue Inventory > 25). The primary outcome was the Multidimensional Fatigue Inventory (MFI), with the NIH PROMIS Fatigue scale secondary.
Results
Mean baseline MFI scores for 57 evaluable subjects were 62.95 for SMT, 55.00 for LT, and 60.41 for WLC. SMT resulted in a mean (sd) 6-week reduction in MFI total scores of −16.50 (6.37) (N=20), compared to −8.06 (6.50) for LT (N=20) and an increase of 5.88 (6.48) points for WLC (N=17) (treatment-by-time P<0.0001). Mean baseline PROMIS Fatigue scores were: SMT 22.25; LT 22.05 and WLC 23.24. Mean (sd) reduction in PROMIS Fatigue scores was −5.49 (2.53) points for SMT, compared to −3.24 (2.57) points for LT and −0.06 (1.88) points for WLC (treatment-by-time P=0.0008). Higher credibility, expectancy, and preference for SMT than for LT did not account for these results.
Conclusions
SMT produced clinically significant relief of CRF. This suggests that 6 weeks of a safe, widely accepted manual intervention causes a significant reduction in fatigue, a debilitating sequela for cancer survivors.
Keywords: Massage, manual therapy, fatigue, quality of life, breast cancer
Introduction
With approximately 15.5 million cancer survivors today in the United States, increased attention is being given to quality of life after cancer treatment. Cancer-related fatigue (CRF) is the most common and one of the most devastating persistent symptoms among survivors of cancer.1–3 According to the National Comprehensive Cancer Network, CRF is “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive exhaustion related to cancer or its treatment that is not proportional to recent activity”.4 CRF occurs across the spectrum of cancer types and treatments 5 and has a negative impact on mood, physical function, work performance, social interaction, family care, cognitive performance, schoolwork, and community activities.6–8 CRF is more troublesome and has a greater negative impact on quality of life than cancer-related pain, depression, or nausea1,9 and CRF can persist for months or years after cancer therapy is completed.2,10,11
Meaningful evidence-based treatment options for CRF are limited.12 A Cochrane Review found that methylphenidate was the only agent that caused a significant, albeit small, improvement in CRF13. There are a growing number of studies investigating non-pharmacologic treatments for CRF: data suggest that exercise causes a small to moderate decrease in CRF.14–19 The psychosocial interventions studied have reported small but significant reductions in fatigue.14,20,21 Overall, methodology and design challenges continue to limit the impact of this work.19,22,23
Over 50% of patients with cancer use a complementary and alternative medicine (CAM) approach for symptom management.24 One of the widely employed CAM interventions is massage therapy. Most studies investigating massage for patients with cancer focus on depression, anxiety, or pain as the outcomes of interest as supportive care for patients treated for cancer.25–34 There is one published study where CRF is one of many outcomes evaluated in breast cancer survivors and massage decreased fatigue.35 There are no published randomized controlled trials with the primary goal of examining massage as an intervention for CRF. However, as stated by the Cochrane group, there is a need for well-controlled investigations employing manualized massage interventions, standardized ratings, and appropriate potential biomarkers.13,21,27,36 This pilot feasibility and proof of concept study systematically examines the efficacy of Swedish massage therapy (SMT) for fatigue in breast cancer survivors. We hypothesized that SMT would have a clinically meaningful benefit in improving fatigue in breast cancer patients.
Patients and Methods
Study Protocol
The study was a randomized, single-masked, three-arm clinical trial comparing six weeks of once-weekly SMT versus light touch (LT) or waitlist control (WLC) as monotherapy for CRF conducted at Emory University in Atlanta, GA. Sixty-six breast cancer survivors who had received surgery plus radiation and/or chemotherapy/chemoprevention, and had persistent CRF, were recruited by means of flyers, referrals, and web-based advertising between January of 2014 and February of 2016. All subjects signed a written informed consent prior to initiating study procedures. The study was approved by the Emory University Institutional Review Board and the Winship Cancer Institute Clinical Translational Research Committee. Protocol implementation and reporting was carried out in accordance with current recommendations for non-pharmacologic treatment trials.37 Subjects received compensation of up to $400 for participation in the study. Subjects accrued $40 for completing treatment visits 0, 1, 2, 4 and 5, and $100 for completing treatment visits 3 and 6.
Participants
Recruitment targeted women, ages 18 to 72, 3 months to 4 years post treatment for Stage 0-III breast cancer with a Brief Fatigue Inventory (BFI) score of >25.38 Ongoing chemoprevention therapy was permissible. The diagnosis of CRF was based on ICD-10 proposed criteria and required evidence from the history, physical exam, and laboratory findings that the fatigue was a consequence of cancer or cancer therapy and not primarily a consequence of comorbid physical or psychiatric disorders. The Mini-International Neuropsychiatric Interview (MINI) was employed to screen out subjects with schizophrenia, depression, generalized anxiety disorder, bipolar disorder, dementia, delirium or OCD. Subjects had satisfactory results on screening safety laboratory tests, and a urine pregnancy test and drug screen. Women of reproductive capability were enrolled, but subjects were required to utilize an effective method of contraception. Exclusion criteria (see protocol) included inability to lay supine for one hour at a time; subjects who were actively suicidal or homicidal; medical conditions felt to be clinically contributing to fatigue; medications felt to be clinically contributing to fatigue; body-mass index less than 18.5 kg/m2; use of systemic corticosteroids or other immunosuppressants within the past 6 months. In order to reduce expectancy bias, we excluded subjects who had previously used massage as a therapeutic modality, were currently receiving massages, or were using a CAM manual therapy and/or holistic therapy to treat a perceived health problem.
Randomization and Masking
Randomization lists with randomly permuted block sizes, generated by the Emory University Winship Cancer Institute Biostatistics group, indicated SMT vs. LT vs. WLC assignment of sequential subjects who were eligible and enrolled in the study at screening and returned for the first intervention session. During the course of the study, only the study coordinator, therapists and the subjects were unmasked to treatment assignment. The study PI, statistician and physicians were masked to treatment assignment.
Interventions
The treatment interventions were performed as described in Rapaport and colleagues.39 Treatment sessions occurred between 12:00 PM – 6:00 PM. At the start of each visit the study coordinator obtained information from the subject about changes in health or pregnancy status, use of prescription or over-the-counter medications, illicit substance use, and any new life events.
Interventions lasting 45 minutes were performed by licensed massage therapists from the Atlanta School of Massage, who adhered to a script that standardized their interactions with subjects and followed manualized treatment protocols for both the SMT and LT interventions. Conversation between the participant and the massage therapist was kept to a minimum. The room was dimly lit and a sound machine was used to mask unwanted noises. Each session began with the subject draped with a sheet and in a prone position on a massage table while the therapist worked slowly down the body from the shoulders to the feet. The subject then turned over to the supine position and the therapist continued the protocol from the feet back up to the shoulders and head. SMT techniques included effleurage (slow, rhythmic, continuous stroking), petrissage (slow, rhythmic kneading of underlying muscles), and tapotement (various forms of percussive touching/tapping). The same therapists performed the control LT sessions, which consisted of the light laying on of hands, in the same sequence and for the same amount of time as the SMT treatment. Quality control was maintained by reviewing the audio taped session, quarterly reliability sessions, weekly discussion of issues arising during sessions, and feedback from participants about consistency across therapists.
Baseline and Outcome Assessments
Prior to starting the first intervention session (baseline), subjects in the two active groups completed the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q40), the Multidimensional Fatigue Inventory (MFI), and the PROMIS Fatigue Short Form 7a (PROMIS41–43). Following the interventions at visits 3 and 6, subjects also completed the MFI, PROMIS and Q-LES-Q. Subjects randomized to the WLC group completed all of these assessments only at the start (baseline) and end of the 6-week non-intervention period.
The primary outcome measure for this study was the MFI, a brief (20-item) self-report instrument that assesses 5 dimensions of fatigue during the past 7 days: general fatigue, physical fatigue, mental fatigue, reduced activity, and reduced motivation.44–46 Item responses range from 1 (“yes that is true”) to 5 (“no that is not true”). Within each subscale (corresponding the 5 dimensions of fatigue), 2 items (which have values reversed for scoring) are worded in the direction of fatigue and 2 items are worded in the direction of activity/energy, resulting in a possible fatigue score ranging from 20 to 100. A minimal clinically important difference in cancer fatigue during radiation treatment has been established for the MFI as 2 points per subscale (or 10 points on the total MFI score).47
The PROMIS Fatigue Short Form 7a scale consists of 7 items that are self-rated on a frequency scale from 1 (“never”) to 7 (“always”) for the past 7 days. Only item 7 is worded in the direction of energy, so response values printed on the form for that item are reversed (5 = ”never” and 1 = “always”). The resulting fatigue score has a possible range from 7 to 35.
The Q-LES-Q consists of 16 items self-rated from 1 to 5 indicating very poor, poor, fair, good, or very good life enjoyment/satisfaction in that area during the past 7 days. An overall score is based on items 1–14, excluding item 15 (satisfaction with medications) and item 16 (overall life satisfaction and contentment during the past week). Rather than using a simple sum of items 1–14 for a possible score ranging from 14 to 70, the authors recommend expressing the sum of item 1–14 responses as a percentage of the maximum possible based on the number of items answered; this results in a more interpretable score with a possible range from 0 to 100 percent. Community norms for Q-LES-Q items and total scores have been published based on a large sample of subjects (N=527), for sub-groups that vary in terms of lifetime and current history of psychiatric disorders.48
Credibility, Expectancy, and Preference Assessments
Likert scales (Condition Preference/Expectancy Scales - CPES), derived from previously published scales, were administered to subjects after the two biologically active intervention conditions49,50 had been described to them using a script (Appendix 4 of the Supplementary Material) at the screening visit, prior to randomization.51–53 Subjects indicated on a Condition Preference Scale whether and by how much they preferred SMT or LT for treating their symptoms.
At the first treatment visit, after being informed of their treatment assignment, the coordinator again read treatment descriptions and the participant filled out the Credibility/Expectancy Questionnaire (CEQ).54 The CEQ assesses the subjects’ opinion about the credibility of the assigned treatment to be effective, and their expectation that the intervention will improve their symptoms.54
Statistical Analysis
Analyses comparing the three treatment conditions were based on a modified intent-to-treat (MITT) sample of 57 evaluable subjects who had at least one post-baseline visit. Comparisons of the groups on baseline characteristics were made by ANOVA for continuous measures and by chi-squared and Fisher’s Exact Tests for categorical variables. Mixed model repeated measures (MMRM) analysis was carried out to test within and between group differences in change from baseline to the end of week 6, on MFI, PROMIS, and Q-LES-Q scores. Models included subjects as a random effect, and treatment group and visit number as fixed effects. Terms for baseline scores were not included in the model because groups did not differ at P<0.10 on any baseline assessment. An auto-regressive covariance structure was used because it provided the best fit to the data. Standardized treatment effect sizes and detailed parameters are provided for MFI, PROMIS Fatigue, and Q-LES-Q outcome measures, as a guide for designing adequately powered future studies. The effect size (ES) for each pair of treatment groups (SMT vs. WLC, LT vs. WLC, and SMT vs. LT) was computed based on the difference in group mean change from baseline to week 6 divided by the pooled standard deviation of change for the two comparison groups. Credibility and expectancy factor scores were computed as recommended54 and analyzed in relation to change in the primary outcome measure (MFI score) within the SMT and LT active intervention groups. Statistical analyses were carried out using SAS 9.2 software (SAS Institute Inc., 2001). A two-tailed alpha level of 0.05 was used to determine statistical significance. (This analysis plan was specified in the funded grant application.)
Prior to scoring, missing data was imputed for 7 out of 3,780 items (0.18%) within the MFI based on the mean of the relevant individual’s responses to similar items in the same subscale. For example, one record had item 16 (“I tire easily”) filled in based on the subject’s response to item 5 (“I feel tired”) at the same visit. There were no missing data from the PROMIS fatigue scale (out of a total of 1,281 items). Of a total of 3,024 Q-LES-Q items, one subject left item 9 blank at 2 visits; this subject’s Q-LES-Q score was based on the remainder of items 1–14. For most studies, subjects leave the Q-LES-Q item on satisfaction with work (item 3) blank if it is not applicable due to not working; in such cases the Q-LES-Q score is computed as a percentage of the maximum possible score for the number of items answered out of items 1–14 without any imputation.
Within the modified intent-to-treat sample of 57 subjects, only 1 person in the LT group was unable to complete the 6-week study (Figure 1), but this individual’s change from baseline to week 3 contributed to the MMRM analysis. All remaining subjects in the SMT and LT groups had complete outcome data at baseline, week 3, and week 6. MMRM analysis was unaffected by missing data for WLC subjects, who were not assessed at week 3.
Figure 1.
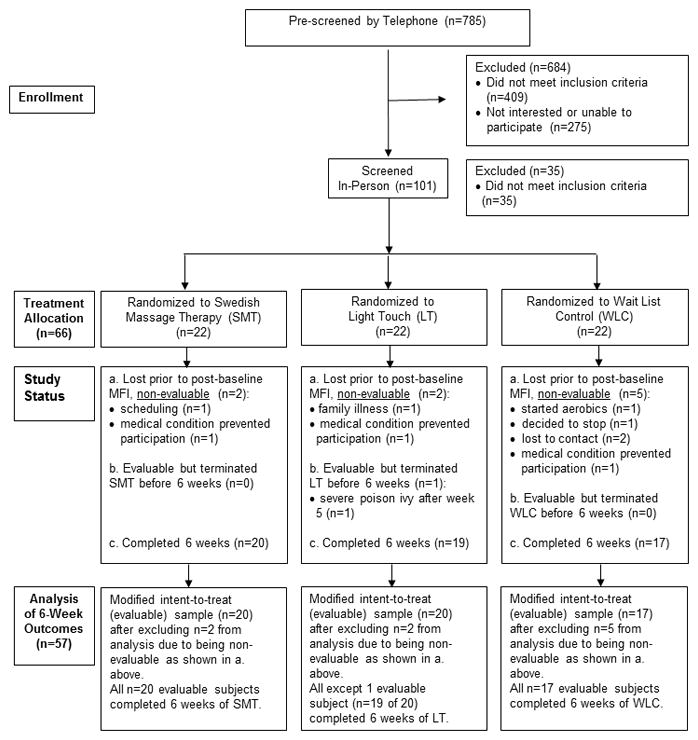
CONSORT Statement Flow Diagram
The sample size of 20 subjects per arm was based on the time and budget limitations for this early phase trial and on our team’s experience recruiting for other breast cancer survivor studies and for other early phase massage trials in normal volunteers and subjects with anxiety disorders.39,49,50 Since there were no published data on which to base power analysis of expected group differences in breast cancer survivors, we focus on standardized treatment effect size differences rather than on statistical power. Based on results from our prior studies, we anticipated that this trial would demonstrate at least a moderate effect size (0.50 or larger) for the superiority of SMT over both LT and WLC on the 3 outcome measures of improvement in fatigue and quality of life.
Results
Study Participants
As shown in Figure 1, 785 subjects were pre-screened for this study, 101 subjects were screened in person, and 66 were randomized, of whom 9 dropped out before attending the third intervention session, leaving 57 subjects evaluable for the outcome analyses. Fifty-six of these 57 subjects completed the entire 6-week study period.
Demographic characteristics and baseline fatigue and quality of life scores for the analysis sample are shown in Table 1. Groups randomized to SMT vs. LT vs WLC did not differ at P<0.10, on any variable except the percentage receiving chemoprevention treatment during the study, which was highest in the WLC group.
Table 1.
Baseline Demographic and Clinical Characteristics of Evaluable Subjects (All Female), Overall and by Randomized Treatment Group.
| Characteristics | All Evaluable Subjects (N=57) | Swedish Massage Therapy (SMT) (N=20) | Light Touch (LT) (N=20) | Wait List Control (WLC) (N=17) | |
|---|---|---|---|---|---|
|
| |||||
| DEMOGRPAHICS | |||||
| Age (Years) | Mean (sd) [Range] | 54.1 (10.4) [32–72] | 54.5 (12.4) [32–71] | 55.6 (9.0) [40–72] | 51.8 (9.6) [34–67] |
| Race | |||||
| White/Caucasian | N (%) | 33 (57.9) | 12 (60.0) | 10 (50.0) | 11 (64.7) |
| Black/African American | N (%) | 22 (38.6) | 8 (40.0) | 8 (40.0) | 6 (35.3) |
| Asian | N (%) | 2 (3.5) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Ethnicity | |||||
| Hispanic/Latino | N (%) | 3 (5.3) | 2 (10.0) | 0 (0.0) | 1 (5.9) |
| Not Hispanic/Latino | N (%) | 54 (94.7) | 18 (90.0) | 20 (100.0) | 16 (94.1) |
| Marital Status | |||||
| Married/Living Together | N (%) | 33 (57.9) | 11 (55.0) | 12 (60.0) | 10 (58.8) |
| Separated/Widowed/Divorced | N (%) | 19 (33.3) | 9 (45.0) | 6 (30.0) | 4 (23.5) |
| Never Married | N (%) | 5 (8.8) | 0 (0.0) | 2 (10.0) | 3 (17.7) |
| Educationa | |||||
| High School or Less | N (%) | 6 (10.7) | 2 (10.5) | 2 (10.0) | 2 (11.8) |
| Some College | N (%) | 16 (28.6) | 6 (31.6) | 6 (30.0) | 4 (23.5) |
| Bachelor’s Degree | N (%) | 16 (28.6) | 4 (21.0) | 6 (30.0) | 6 (35.3) |
| Master’s Degree or PhD | N (%) | 18 (32.1) | 7 (36.8) | 6 (30.0) | 5 (29.4) |
| Employment Status | |||||
| Employed | N (%) | 32 (56.1) | 12 (60.0) | 12 (60.0) | 8 (47.1) |
| Unemployed | N (%) | 6 (10.5) | 1 (5.0) | 2 (10.0) | 3 (17.6) |
| Housewife | N (%) | 2 (3.5) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Student | N (%) | 2 (3.5) | 1 (5.0) | 0 (0.0) | 1 (5.9) |
| Retired | N (%) | 6 (10.5) | 3 (15.0) | 2 (10.0) | 1 (5.9) |
| Other, Unspecified | N (%) | 9 (15.8) | 3 (15.0) | 2 (10.0) | 4 (23.5) |
|
| |||||
| BASELINE MEASURES | |||||
| Multidimensional Fatigue Inventory (MFI) | Mean (sd) [Range] | 59.40 (12.42) [32–86] | 62.95 (9.54) [44–82] | 55.00 (12.48) [32–86] | 60.41 (14.34) [39–84] |
| PROMIS Fatigue Short Form 7a | Mean (sd) [Range] | 22.47 (4.04) [13–33] | 22.25 (2.77) [17–28] | 22.05 (4.42) [16–33] | 23.24 (4.91) [13–33] |
| Quality of Life Enjoyment & Satisfaction (16 item Q-LES-Q): % of maximum possible for items 1–14 | Mean (sd) [Range] | 66.35 (13.80) [30.4–92.9] | 65.26 (11.99) [42.9–85.7] | 68.74 (9.52) [50.0–89.3] | 64.82 (19.43) [30.4–92.9] |
|
| |||||
| BASELINE CLINICAL CHARACTERISTICS | |||||
| Ongoing chemoprevention during study periodb | (N) | (52) | (17) | (19) | (16) |
| Yes | N (%) | 38 (73.1) | 12 (70.6) | 11 (57.9) | 15 (93.8) |
| No | N (%) | 14 (26.9) | 5 (29.4) | 8 (42.1) | 1 (6.2) |
| Number of months between end of chemotherapy/radiation and baseline visitc | (N) Mean (sd) [Range] | (53) 20.13 (13.32) [6–49] | (18) 19.78 (14.00) [6–49]d | (20) 21.65 (14.62) [6–48] | (15) 18.53 (10.67) [6–44] |
Level of education is missing for one subject in the SMT group.
Chemoprevention information was not asked of 4 early subjects, and 1 subject did not provide this data. In a test of the difference across randomized groups, chi-square=5.76; df=2; P=0.056.
The study protocol allowed subjects to enter with no chemotherapy or radiation if they were currently on chemoprevention; this was the case for 2 SMT subjects and 2 WLC subjects, who are omitted from the summary above..
The protocol required subjects to be 3 months to 4 years past the end of chemotherapy and/or radiation; one subject was allowed to enter the study one month beyond this limit, at 49 months.
Treatment Outcomes
At baseline mean (sd) MFI scores, PROMIS fatigue scores and Q-LES-Q scores were not significantly different between groups (Table 1). The mean Q-LES-Q score of 66.35 for the overall study sample is more than one standard deviation below the community norm of mean (sd) 78.4 (11.4) for a large sample that includes subjects with and without current or past psychiatric disorder.48
MMRM analysis revealed a significant treatment-by-time interaction for all 3 study outcomes, with large standardized treatment effect sizes indicating superiority of SMT over LT and WLC, as well as for superiority of LT over WLC (Table 2). There was a statistically significant 6-week reduction in MFI total scores within the SMT and LT groups, but a significant increase in MFI fatigue scores within the WLC group. The mean (sd) decrease in MFI fatigue for the SMT group exceeded the minimum clinically meaningful difference of 10 points.47 PROMIS Fatigue scores also decreased significantly for SMT and LT groups over 6 weeks, while remaining the same for WLC. Q-LES-Q scores increased substantially (mean (sd) of +8.11 (8.20) points) for the SMT group, while there was a slight increase of +1.85 (8.37) points for LT and a decrease in life quality of −5.78 (8.24) points for WLC over the 6-week study period.
Table 2.
Change in Fatigue and Quality of Life from Baseline to Week 6.
| Change from Baseline to Week 6 | Standardized Effect Size (ES) at Week 6 (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Assessment Scale | Swedish Massage Therapy (SMT) (N=20) LS-Mean (sd) |
Light Touch (LT) (N=20) LS-Mean (sd) |
Wait List Control (WLC) (N=17) LS-Mean (sd) |
Significance of Treatment-by-Time Interaction F df P |
SMT vs WLC | LT vs WLC | SMT vs LT |
|
| |||||||
| Fatigue: | |||||||
| Multidimensional Fatigue Inventory (MFI) | −16.50 (6.37) | −8.06 (6.50) | +5.88 (6.48) | 24.31 2, 144 <0.0001 |
−3.39 (−4.42 to −2.37) | −2.09 (−2.90 to −1.28) | −1.28 (−1.96 to −0.60) |
| PROMIS Fatigue Short Form 7a | −5.49 (2.53) | −3.24 (2.57) | −0.06 (1.88) | 7.55 2, 144 0.0008 |
−2.06 (−2.87 to −1.25) | −1.20 (−1.90 to −0.49) | −0.86 (−1.51 to −0.21) |
| Quality of Life Enjoyment & Satisfaction: | |||||||
| (Q-LES-Q) % of Max. Possible, Items 1–14 | +8.11 (8.20) | +1.85 (8.37) | −5.78 (8.24) | 6.54 2, 137 0.0019 |
1.64 (0.89 to 2.40) | 0.89 (0.21 to 1.57) | 0.74 (0.10 to 1.38) |
Although differences in percentages of subjects currently receiving chemoprevention treatment approached the level of statistical significance (P=0.056, Table 1), t-tests showed non-significant comparisons of subjects with vs. without current chemoprevention on both baseline MFI scores and change in that primary outcome measure during the 6-week study (P=0.957 and P=0.352, respectively). Randomized groups did not differ significantly on the number of months since the end of chemotherapy and/or radiation (P=0.787, Table 2) and ANOVA based on categories of time since treatment showed a non-significant relationship to baseline MFI scores and change in those scores over the 6-week study period (P=0.189 and P=0.877, respectively).
Role of Credibility, Expectancy and Preference
Prior to randomization, 52 (78.8%) of the 66 randomized subjects and 44 (77.2%) of the 57 evaluable subjects indicated they preferred to receive SMT rather than LT. Subjects endorsed SMT as a more logical therapy for reducing CRF (Credibility Factor Score: SMT vs. LT: 1.17 (2.14 vs. −1.24 (2.96); t=2.91, df 37, p= 0.006). However, expectancy of the assigned intervention to improve CRF symptoms was not significantly higher for those assigned to SMT than those assigned to LT (Expectancy Factor Score: SMT vs. LT: 0.26 (2.50) vs. −0.38 (3.24); t=0.79, df 37, p= 0.740).
There were low, non-significant correlations between preference, credibility, or expectancy responses and the amount of change in MFI scores within the SMT and LT groups (data available on request), suggesting that the change we found in these groups’ MFI fatigue scores is not an artifact of preference, credibility, or expectancy bias.
Safety
Adverse events were assessed at every visit: the most common ones being bruising at the venipuncture site (12/39 subjects) and discomfort due to hyperextension from lying on the massage table (2/39 subjects). There were no differences between treatment groups and no serious adverse events were reported.
Discussion
CRF is a frustrating and disabling long-term sequela of cancer treatment, impacting both the cancer survivor and his/her family’s quality of life and functioning. At this time, mild exercise is the non-pharmacological intervention with the best body of evidence supporting some level of symptom improvement. In this early phase clinical trial we demonstrate that 6 weekly sessions of a manualized form of SMT was superior to both a WLC and an active control, LT, in reducing symptoms of fatigue as assessed by 2 different well validated self-report measures. These findings indicate that patients suffering from chronic CRF may receive at least short-term relief of their symptoms from weekly Swedish massage. SMT also caused a statistically significant improvement in the quality of life of study participants when compared to both the WLC and LT condition.
One of the potential confounds researchers face when attempting to investigate the subjective effect of a manual massage therapy is the impact that subjects’ preference or credibility or expectancy bias may have on the results of the study. This is particularly worrisome since it is unavoidable for the subject to know which intervention he/she is receiving. CPES responses from the screening visit demonstrate that SMT was preferred to LT by the great majority of the subjects. The post-randomization CEQ responses reveal that subjects randomized to SMT thought that their assigned intervention was more credible or logical as a method to reduce their CRF symptoms than did subjects randomized to LT. Interestingly, while credibility was significantly higher for SMT than for LT, expectancy as measured by the CEQ was not higher for SMT. This suggests that belief in the credibility of a therapy can be distinguished from the expectation that the intervention will actually improve one’s symptoms. Neither CPES preference nor the CEQ scores correlated with change from baseline in MFI scores over six weeks of SMT or LT. This suggests that improvement in the primary outcome measure, the MFI, is not an artifact of subject preference, credibility, or expectancy bias.
An important ancillary finding of this study is that SMT resulted in a statistically significant within-group improvement in the quality of life of subjects suffering from CRF, which was also significantly better than for the LT group, as well as for the WLC group which experienced significantly worse quality of life at the end of 6 weeks of non-intervention. The mean baseline Q-LES-Q score for our overall sample was 66.35 (sd=13.80); this is more than one standard deviation lower than Q-LES-Q scores (mean=78.4; sd=11.4) for a large (N=527) community normative sample.48 A mean improvement of more than 8 points on the Q-LES-Q brought the mean score for the SMT group to within 5 percentage points (0.44 sd) of the community sample mean. This preliminary finding, albeit one that requires replication in a larger sample, suggests that the benefits of SMT treatment of CRF may extend beyond specific symptom reduction.
There are limitations to this NIH-funded early phase clinical trial. Although we found clinical and statistical evidence supporting the efficacy of carefully developed LT and SMT protocols in decreasing CRF, this work needs to be replicated in a larger study. A second limitation is that fatigue is a highly subjective and complex entity to define and study. Future studies would benefit from including both clinician-rated and objective measures that may correlate with fatigue, such as activity levels measured from wearable devices. A third concern is that we do not know if this finding is generalizable to a more heterogeneous population of cancer survivors with CRF and so further studies with cancer survivors, including men, with other types of cancer, who are suffering from CRF are warranted. While results of this early phase feasibility study did not indicate that time since the end of chemotherapy and/or radiation or the presence vs. absence of ongoing chemoprevention treatment during the study period had an impact on change in the primary measure of fatigue, the potential influence of these factors on the effects of massage should be examined in a larger study. It is also important to note that this study’s focus is on the treatment of sustained fatigue that persists for survivors in the post-treatment period and not the effect of SMT on fatigue caused by ongoing chemotherapy or radiation therapy. Future studies need to prospectively investigate the durability of the acute improvements in the fatigue and QOL observed in this study. Is a short-term intervention sufficient or is there a need for continuation or even maintenance therapy? If these studies are informative, the next challenge is how to translate this work, performed in a research setting, into every day practice in a feasible and meaningful manner.
In conclusion, we found that SMT was more effective than two control conditions in decreasing CRF on two self-report measures of fatigue. Further, we observed that SMT enhanced quality of life in these subjects. Future studies confirming and extending these results, as well as exploring the mechanisms responsible for the beneficial actions of SMT on CRF, are warranted.
Acknowledgments
Supported by: National Center for Complementary & Alternative Medicine, National Cancer Institute, Grant # R21AT007090 to MR. Clinicaltrials.gov registration number: NCT01926678.
The authors would like to acknowledge and thank the Staff of the Atlanta School of Massage, Leticia Allen, as well as the research massage therapists: Shanese Armstrong-Marks, Melissa Comer, Sarah Istambouli, Suzanne Ledoux, Grace Prior, and Brittney Turner. This study could not have been performed without their partnership.
Footnotes
Conflict of interest disclosures:
MT Paid consultant for Blue Earth Diagnostics where she has served on the Advisory Board
BD Research support from Acadia, Assurex, Axsome, Bristol-Myers Squibb, Janssen, GlaxoSmithKline, NIMH, Otsuka, Pfizer, and Takeda. He has served as a consultant to Pfizer and Medavante.
JJR r Research support from from Assurex and Takeda
ERL, DC, MS, JN All employed by the Atlanta School of Massage.
BK, PJS, SAE, AHM, MR no disclosures
Author Contributions:
Conceptualization: B Kinkead, P Schettler, A Miller, M Torres, M Rapaport
Methodology: B Kinkead, P Schettler, E Larson, D Carroll, A Miller, M Torres, M Rapaport
Validation: S Edwards, E Larson, D Carroll, M Sharenko
Formal analysis: B Kinkead, P Schettler, J Nettles, M Rapaport
Investigation: E Larson, D Carroll, M Sharenko, S Edwards, J Rakofsky, B Dunlop
Resources: M Torres
Writing – original draft: B Kinkead, P Schettler, M Rapaport
Writing – review and editing: All authors
Supervision: B Kinkead, M Rapaport
Project Administration: B Kinkead
Funding acquisition: M Rapaport
References
- 1.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 2.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence DP, Kupelnick B, Miller K, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 4.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–39. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nail LM. My get up and go got up and went: fatigue in people with cancer. J Natl Cancer Inst Monogr. 2004:72–5. doi: 10.1093/jncimonographs/lgh021. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27:6033–40. doi: 10.1200/JCO.2008.20.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower JE. Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol. 2005;23:8280–2. doi: 10.1200/JCO.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Institute. NC. Fatigue(PDQ) 2010 Available from: www.cancer.gov/cancertopics/pdq/supportivecare/fatigue/HealthProfessional.
- 9.Stone P, Richards M, A’Hern R, et al. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11:561–7. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 11.Curran SL, Beacham AO, Andrykowski MA. Ecological momentary assessment of fatigue following breast cancer treatment. J Behav Med. 2004;27:425–44. doi: 10.1023/b:jobm.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- 12.Bruera E, Yennurajalingam S. Challenge of managing cancer-related fatigue. J Clin Oncol. 2010;28:3671–2. doi: 10.1200/JCO.2010.29.8984. [DOI] [PubMed] [Google Scholar]
- 13.Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010:CD006704. doi: 10.1002/14651858.CD006704.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger AM, Mitchell SA, Jacobsen PB, et al. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J Clin. 2015;65:190–211. doi: 10.3322/caac.21268. [DOI] [PubMed] [Google Scholar]
- 16.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008:CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Pachman DR, Price KA, Carey EC. Nonpharmacologic approach to fatigue in patients with cancer. Cancer J. 2014;20:313–8. doi: 10.1097/PPO.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 18.McQuade JL, Prinsloo S, Chang DZ, et al. Qigong/tai chi for sleep and fatigue in prostate cancer patients undergoing radiotherapy: a randomized controlled trial. Psychooncology. 2016 doi: 10.1002/pon.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PL, Tam KW, Yeh ML, et al. Acupoint stimulation, massage therapy and expressive writing for breast cancer: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2016;27:87–101. doi: 10.1016/j.ctim.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Egan MY, McEwen S, Sikora L, et al. Rehabilitation following cancer treatment. Disabil Rehabil. 2013;35:2245–58. doi: 10.3109/09638288.2013.774441. [DOI] [PubMed] [Google Scholar]
- 21.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013:CD007730. doi: 10.1002/14651858.CD007730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Mills PJ. Biofield Therapies: Helpful or Full of Hype? A Best Evidence Synthesis. Int J Behav Med. 2010;17:1–16. doi: 10.1007/s12529-009-9062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer. 2008;44:1097–104. doi: 10.1016/j.ejca.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 24.Vapiwala N, Mick R, Hampshire MK, et al. Patient initiation of complementary and alternative medical therapies (CAM) following cancer diagnosis. Cancer J. 2006;12:467–74. doi: 10.1097/00130404-200611000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Reif M, Field T, Ironson G, et al. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int J Neurosci. 2005;115:495–510. doi: 10.1080/00207450590523080. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Reif M, Ironson G, Field T, et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J Psychosom Res. 2004;57:45–52. doi: 10.1016/S0022-3999(03)00500-2. [DOI] [PubMed] [Google Scholar]
- 27.Jane SW, Wilkie DJ, Gallucci BB, et al. Systematic review of massage intervention for adult patients with cancer: a methodological perspective. Cancer Nurs. 2008;31:E24–35. doi: 10.1097/01.NCC.0000339242.51291.85. [DOI] [PubMed] [Google Scholar]
- 28.Krohn M, Listing M, Tjahjono G, et al. Depression, mood, stress, and Th1/Th2 immune balance in primary breast cancer patients undergoing classical massage therapy. Support Care Cancer. 2011;19:1303–11. doi: 10.1007/s00520-010-0946-2. [DOI] [PubMed] [Google Scholar]
- 29.Kutner JS, Smith MC, Corbin L, et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med. 2008;149:369–79. doi: 10.7326/0003-4819-149-6-200809160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Listing M, Krohn M, Liezmann C, et al. The efficacy of classical massage on stress perception and cortisol following primary treatment of breast cancer. Arch Womens Ment Health. 2010;13:165–73. doi: 10.1007/s00737-009-0143-9. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson S, Barnes K, Storey L. Massage for symptom relief in patients with cancer: systematic review. J Adv Nurs. 2008;63:430–9. doi: 10.1111/j.1365-2648.2008.04712.x. [DOI] [PubMed] [Google Scholar]
- 32.Boyd C, Crawford C, Paat CF, et al. The Impact of Massage Therapy on Function in Pain Populations-A Systematic Review and Meta-Analysis of Randomized Controlled Trials: Part II, Cancer Pain Populations. Pain Med. 2016;17:1553–68. doi: 10.1093/pm/pnw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014:346–58. doi: 10.1093/jncimonographs/lgu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao JJ, Wagner KE, Seluzicki CM, et al. Integrating Oncology Massage Into Chemoinfusion Suites: A Program Evaluation. JOP2016015081. J Oncol Pract. 2017 doi: 10.1200/JOP.2016.015081. [DOI] [PMC free article] [PubMed]
- 35.Listing M, Reisshauer A, Krohn M, et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psychooncology. 2009;18:1290–9. doi: 10.1002/pon.1508. [DOI] [PubMed] [Google Scholar]
- 36.Shin ES, Seo KH, Lee SH, et al. Massage with or without aromatherapy for symptom relief in people with cancer. Cochrane Database Syst Rev. 2016:CD009873. doi: 10.1002/14651858.CD009873.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 38.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Rapaport MH, Schettler P, Larson ER, et al. Acute Swedish massage monotherapy successfully remediates symptoms of generalized anxiety disorder: A proof-of-concept, randomized controlled study. J Clin Psychiatry. 2016;77:e883–91. doi: 10.4088/JCP.15m10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- 41.Choi SW, Reise SP, Pilkonis PA, et al. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Qual Life Res. 2010;19:125–36. doi: 10.1007/s11136-009-9560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershon RC, Rothrock N, Hanrahan R, et al. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11:304–14. [PMC free article] [PubMed] [Google Scholar]
- 43.Rothrock NE, Hays RD, Spritzer K, et al. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63:1195–204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 45.Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wichers MC, Koek GH, Robaeys G, et al. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med. 2005;35:433–41. doi: 10.1017/s0033291704003526. [DOI] [PubMed] [Google Scholar]
- 47.Purcell A, Fleming J, Bennett S, et al. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer. 2010;18:307–15. doi: 10.1007/s00520-009-0653-z. [DOI] [PubMed] [Google Scholar]
- 48.Schechter D, Endicott J, Nee J. Quality of life of ‘normal’ controls: association with lifetime history of mental illness. Psychiatry Res. 2007;152:45–54. doi: 10.1016/j.psychres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapaport MH, Schettler P, Bresee C. A Preliminary Study of the Effects of a Single Session of Swedish Massage on Hypothalamic-Pituitary-Adrenal and Immune Function in Normal Individuals. J Altern Complement Med. 2010 doi: 10.1089/acm.2009.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapaport MH, Schettler P, Bresee C. A preliminary study of the effects of repeated massage on hypothalamic-pituitary-adrenal and immune function in healthy individuals: a study of mechanisms of action and dosage. J Altern Complement Med. 2012;18:789–97. doi: 10.1089/acm.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalauokalani D, Cherkin DC, Sherman KJ, et al. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine (Phila Pa 1976) 2001;26:1418–24. doi: 10.1097/00007632-200107010-00005. [DOI] [PubMed] [Google Scholar]
- 52.Licciardone JC, Russo DP. Blinding protocols, treatment credibility, and expectancy: methodologic issues in clinical trials of osteopathic manipulative treatment. J Am Osteopath Assoc. 2006;106:457–63. [PubMed] [Google Scholar]
- 53.Myers SS, Phillips RS, Davis RB, et al. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med. 2008;23:148–53. doi: 10.1007/s11606-007-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]


