Abstract
Objectives
Our previous studies suggested that human milk proteases begin to hydrolyze proteins in the mammary gland and continue within the term infant stomach. No research has measured milk protease and pepsin activity in the gastric aspirates of preterm infants after human milk feeding. This study investigated how the concentrations of human milk proteases and protease inhibitors changed in the premature infant stomach.
Methods
Human milk and infant gastric samples were collected from 18 preterm-delivering mother-infant pairs (24–32 wk gestational age). Paired human milk and gastric samples were collected across postnatal age (2–47 days). Protease concentrations were determined by spectrophotometric or fluorometric assays, and the concentrations of protease inhibitors and bioactive proteins were determined by ELISA. Paired t-tests were applied to compare enzymes, antiproteases and bioactive proteins between human milk and gastric samples.
Results
Our study reveals that although human milk proteases, including carboxypeptidase B2, kallikrein, plasmin, cathepsin D, elastase, thrombin and cytosol aminopeptidase are present in the preterm infant stomach, only plasmin and cathepsin D can actively hydrolyze proteins at gastric pH. ELISA and peptidomic evidence suggest that all milk antiproteases as well as lactoferrin and immunoglobulin A are partially digested in the preterm stomach.
Conclusions
Most human milk proteases are active in milk but not at preterm infant gastric pH. Only cathepsin D and plasmin have potential to continue degrading milk proteins within the preterm infant stomach.
Keywords: α1-antitrypsin, preterm milk, fluorometric assays, spectrophotometric assay, peptidomics
Introduction
Premature infants have an underdeveloped digestive system, produce less gastric acid and may have lower gastric and intestinal protease activity compared with term infants (1, 2). Gastric digestion initiates protein digestion, which is continued in the small intestine (3). Knowledge about gastric digestion in the preterm infant is essential to optimize their feeding and improve their growth and development.
Our recent study using synthetic peptide substrate assays demonstrated that an array of proteases are active within human milk, including carboxypeptidase B2, kallikrein, plasmin, elastase, thrombin and cytosol aminopeptidase (4). Our peptidomics-based model predicted that an array of milk proteases was responsible for the cleavages within milk and within the term infant’s stomach (5–7). We hypothesized that the systems of proteases, inhibitors and activators in human milk (recently reviewed (8, 9)) provide controlled and protein-selective digestion in the mammary gland and infant’s stomach. The prediction that milk proteases continue to function within the infant stomach stimulated us to hypothesize that the activity of proteases within human milk is an important component of the infant’s digestive capacity. Milk enzymes may also compensate for the immature digestive system in preterm infants and help the degradation of protein. However, whether these milk proteases are present and active in the premature infant stomach has not been studied. We aimed to determine which milk proteases present and active within the premature infant stomach.
Methods
Study Design
Participants and sample collection
This study was approved by the institutional review boards of the University of California, Davis (UC Davis) and Oregon State University (OSU). Human milk and infant gastric samples were collected from 18 premature-delivering mother-infant pairs ranging in gestational age (GA) at birth from 24–32 weeks) and birth weight from 620–2,210 g (Table 1) during the first seven weeks of postnatal age (2–47 days) at the UC Davis Children’s Hospital Neonatal Intensive Care Unit in Sacramento, California. Preterm infants enrolled in this study each had health conditions related to prematurity but no overt gastrointestinal tract issues. As the infants’ conditions precluded normal breastfeeding, a nasogastric feeding tube was placed into each infant. Human milk samples were collected by pumping on-site or at home with clean electric breast pumps into sterile plastic containers and stored immediately at −20 °C. The breast was cleaned with water on a washcloth before pumping. The infants were fed their mother’s milk fortified with intact bovine milk protein-based fortifier in powder form (Similac Human Milk Fortifier Powder, Abbott, Abbott Park, IL, USA). The fortified milk feedings were delivered via the nasogastric tubes over 30 min. Two hours after the initiation of feeding, 2 mL of each preterm infant’s gastric contents was collected in a 3-mL syringe back through the feeding tube. Gastric samples were stored immediately at −20 °C. Human milk and gastric samples were transported to OSU on dry ice and stored at −80 °C.
TABLE 1.
Demographics, pregnancy, and birth data of 18 premature-delivering mother-infant pairs who participated in the study
| Premature-delivering mother-infant pairs (N = 18) |
|
|---|---|
| (a) | |
| GA, weeks | 27 ± 3 (24–32) |
| Postnatal age, days | 24 ± 14 (2–47) |
| BW, g | 964 ± 411 (620–2,210) |
| Birth length, cm | 35 ± 5 (28–46.5) |
| Birth head circumference, cm | 25 ± 3 (22–32) |
| Mother age, years | 30 ± 6 (18–40) |
| (b) | |
| Male, n (%) | 9 (50) |
| Maternal medical conditions | |
| Pregnancy-induced hypertension, n (%) | 5 (27.8) |
| Gestational diabetes, n (%) | 8 (38.9) |
| Caesarean deliveries, n (%) | 14 (77.8) |
| Infant medical conditions at birth | |
| Respiratory distress syndrome, n (%) | 9 (50) |
| Chronic lung disease, n (%) | 4 (22.2) |
| Hypoxic ischemic encephalopathy, n (%) | 2 (11.1) |
| Necrotizing enterocolitis, n (%) | 2 (11.1) |
| Anemia of prematurity, n (%) | 2 (11.1) |
| Sepsis n (%) | 1 (5.6) |
BW = birth weight; GA = gestational age. Data are presented as (a) mean ± SD (range) or (b) number (percentile) of subjects.
General sample preparation
Human milk and gastric samples were thawed at 4 °C, centrifuged at 4,226 × g for 10 min at 4 °C and the infranate was collected by pipette from below the upper fat layer. Multiple aliquots of 50 μL of the supernatant for each sample were stored at −80 °C until use for each assay.
pH
The pH values of the human milk and gastric samples were measured with a pH meter (S220 SevenCompact pH/Ion, Mettler-Toledo, Columbus, OH, USA).
Spectrophotometric and fluorometric assays
The spectrophotometric and fluorometric assays were performed according to the methods described by the manufacturers, with some modifications as described previously (4). Measurements were recorded with a microplate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA, USA) with SoftMax Pro 4.8 Microplate Data Analysis Software (Molecular Devices) and with two replicates of blanks, standards and samples. For each assay (enzyme-linked immunosorbent assay (ELISA), and fluorometric and spectrophotometric assays), values were interpolated from standard calibration curves using the Four Parameter Logistic curve fit for ELISA and linear fit for enzyme concentration assays.
ELISA
All ELISAs were carried out as described by the manufacturer (Innovative Research Inc., Novi, MI, USA) or MyBiosource (San Diego, CA, USA)) (see Table, Supplemental Digital Content 1). Concentrations were expressed as mg/mL for lactoferrin, as μg/mL for α1-antitrypsin, α1-antichymotrypsin and IgA, and as ng/mL for antithrombin III, α2-antiplasmin, plasma serine protease inhibitor and TGF-β1.
Protease concentration assays
Enzyme concentration assays were carried out according to the manufacturer’s instructions and as explained previously (4). The specific assay and manufacturer, amount of milk and gastric samples used, dilution factor and range of the standard curve are shown for each protease (see Table, Supplemental Digital Content 2). The peptide substrate assays were selected based on the cleavage specificity from Merops (10) and preference for specific amino acids (11) for each identified protease (see Table, Supplemental Digital Content 3). There is potential for overlapping cleavage between some enzymes, in particular, plasmin, kallikrein and thrombin. We have discussed these potential overlaps in our previous study (4). In each case, the standard curve was based on concentrations of the standard enzyme, and the result is expressed as enzyme concentration. Except for cathepsin D, all protease concentration assays were carried out at the enzyme’s pH optima for both milk and gastric samples. Concentration of cathepsin D was determined at its pH optima (pH 4.0) for gastric samples; however, as procathepsin D can auto-activate at acidic pH to pseudocathepsin D (which can cleave cathepsin D-specific substrates) (12, 13), milk samples were measured at pH 6.5 to prevent auto-activation. Concentrations are expressed as μg/mL for kallikrein and carboxypeptidase B2, and as ng/mL for plasmin, thrombin, cytosol aminopeptidase, elastase and cathepsin D.
Protease activity assays
As the milk and gastric sample enzyme analyses were carried out at the pH optima for each enzyme and measured enzyme concentration but not activity, we performed additional substrate assays with standard enzymes (10 μg/mL from kits or purchased separately, see Table, Supplemental Digital Content 2) in buffer adjusted to the average pH of milk (pH 6.5) and the stomach (pH 4.5) to determine whether these milk enzymes are potentially active in each sample type. Standard curves were prepared with the fluorophore or chromophore specific to each assay. As activity values were calculated for the standard enzyme, they do not represent the actual activity level of the milk enzyme in the sample and are, therefore, reported only as relative change in activity from milk to gastric sample pH.
Peptidomic analysis
Human milk and gastric samples were analyzed via peptidomics after extraction (7) and mass spectrometric analysis (14), as described previously. Spectra were analyzed by database searching in Thermo Proteome Discoverer (v2.1.0.81) using an in-house human milk protein sequence database. Only peptides identified with high confidence were included (P < 0.01). Peptide counts measured the number of unique peptides identified in a sample.
Statistical analysis
Paired t-tests were applied to compare milk and gastric samples for the concentrations of each protease, antiprotease and bioactive protein. Differences were designated significant at P < 0.05. Sample size, N = 18 paired milk and gastric samples, was selected based on previous literature sample sizes and proved to be adequately powered based on the results.
Results
pH
Gastric samples (pH 4.56 ± 0.06) were more acidic than milk samples (pH 6.35 ± 0.03, P < 0.001).
Protease inhibitors
Expressed as percentage decrease, α1-antitrypsin (75.8%, P < 0.001), α1-antichymotrypsin (35.7%, P = 0.0012), antithrombin III (70.1%, P < 0.001), α2-antiplasmin (43.9%, P < 0.001) and plasma serine protease inhibitor (56.6%, P < 0.001) concentrations in gastric contents were lower than those in human milk (Figure 1A–E). Peptide counts of α1-antitrypsin were 20-fold greater, respectively, in the stomach contents than in the milk (P < 0.001, Figure 1F). No peptides from antithrombin III, α1-antichymotrypsin, α2-antiplasmin and plasma serine protease inhibitor were identified in milk and gastric samples. See Table, Supplemental Digital Content 4).
FIGURE 1.
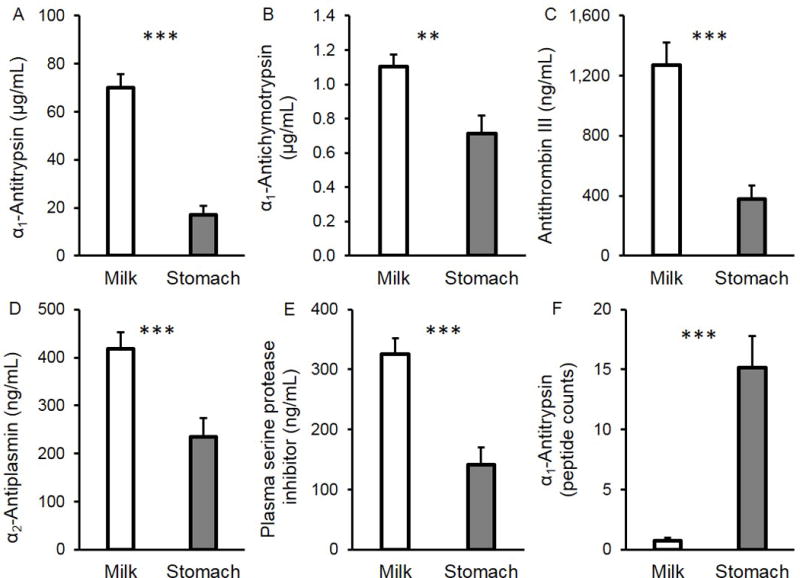
Protease inhibitors in mother’s milk and in the preterm infant stomach (24–32 wk GA, 2–47 days of postnatal age). Concentration of (A) α1-antitrypsin, (B) α1-antichymotrypsin, (C) antithrombin III, (D) α2-antiplasmin and (E) plasma serine protease inhibitor. (F) Peptide counts for α1-antitrypsin. Values are mean ± SEM. Asterisks represent the p-value (*** = P < 0.001; ** = P < 0.01) for N = 18 using the paired t-tests between milk (white bar) and gastric (grey bar) samples.
Bioactive proteins
Lactoferrin and IgA concentrations were 42.9% (P = 0.030) and 46.2% (P = 0.0014) lower, respectively, in gastric contents compared with milk (Figure 2A–B). Lactoferrin-derived peptide counts was 6.6-fold higher in the gastric contents than in milk (P < 0.001, Figure 2C). IgA-derived peptide counts was 16.0-fold higher in the gastric samples than in milk (P < 0.001, Figure 2D). TGF-β1 concentration, on the other hand, was 2.3-fold greater in the gastric contents than in milk (P = 0.0088, Figure 2E). There were no peptides from TGF-β1 (or latency-associated peptide (LAP)) in either milk or gastric samples. See Table, Supplemental Digital Content 5 for concentrations of all bioactive proteins.
FIGURE 2.
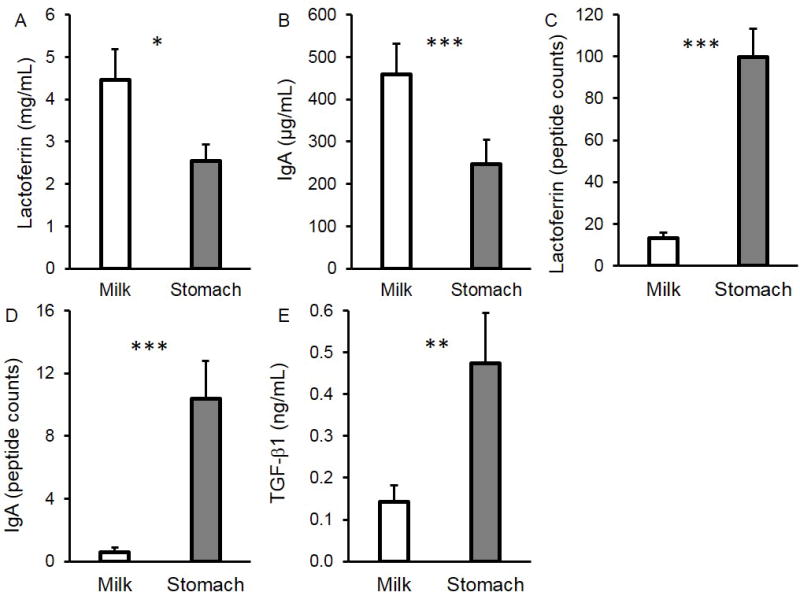
Concentration of (A) lactoferrin, (B) IgA and (E) TGF-β1 in mother’s milk and in the preterm infant stomach (24–32 wk GA, 2–47 days of postnatal age). Peptide counts of (A) lactoferrin and (B) IgA in mother’s milk and in the preterm infant stomach (24–32 wk GA, 2–47 days of postnatal age. Values are mean ± SEM. Asterisks represent the p-value (*** = P < 0.001; ** = P < 0.01; * = P < 0.05) for N = 18 using the paired t-tests between milk (white bar) and gastric (grey bar) samples.
Proteases
Based on the substrate assay, no cathepsin D was detected in human milk; however, cathepsin D was present in the preterm infant stomach (1,390 ± 131 ng/mL) (Figure 3A). Elastase concentration was 118% higher in gastric contents than in milk (P < 0.001, Figure 3B). Cytosol aminopeptidase concentration was 76.7% lower in gastric contents than in milk (P < 0.001, Figure 3C). Kallikrein (13 ± 2 μg/mL), plasmin (123 ± 16 ng/mL), thrombin (70 ± 9 ng/mL) and carboxypeptidase B2 (2.1 ± 0.3 μg/mL) concentrations were similar in milk and gastric samples (P > 0.05) (See Table, Supplemental Digital Content 6). As the enzyme measurements in the milk and gastric samples provided only concentrations, we performed additional testing of each standard enzyme against the same substrate assays at both pH 6.5 (the average milk pH) and 4.5 (the average gastric pH) to determine their potential for having activity in the milk and gastric pH conditions. At pH 6.5, plasmin, carboxypeptidase B2, kallikrein, elastase, thrombin and cytosol aminopeptidase were all active, and cathepsin D was inactive. At pH 4.5, carboxypeptidase B2, kallikrein, elastase, thrombin and cytosol aminopeptidase showed no activity. Plasmin remained active at pH 4.5, but with 71.2% lower activity compared with milk pH (pH 6.5). Cathepsin D was only active at pH 4.5 and not active at pH 6.5.
FIGURE 3.
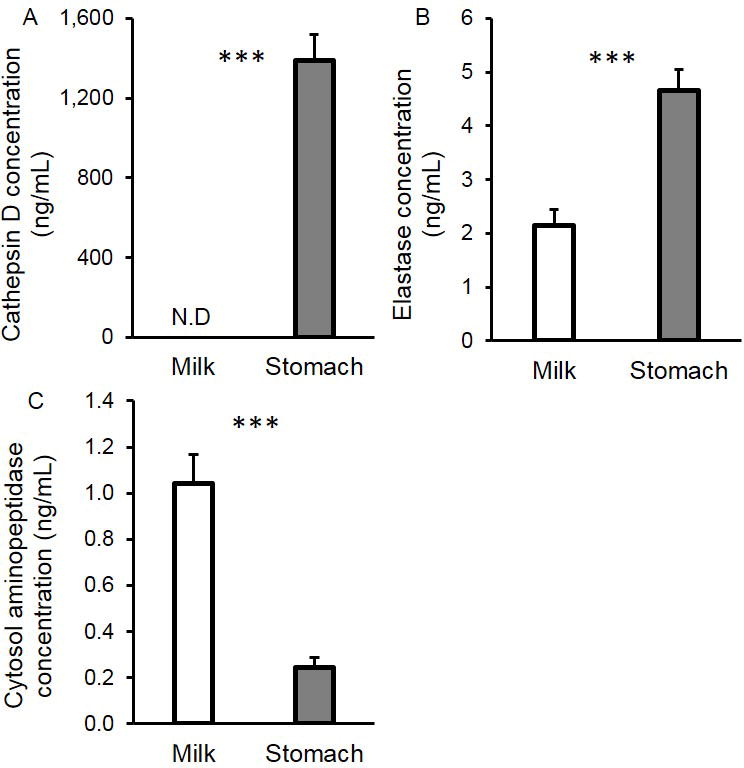
Concentrations of active (A) cathepsin D, (B) elastase and (C) cytosol aminopeptidase in mother’s milk and in the preterm infant stomach (24–32 wk GA, 2–47 days of postnatal age). Values are mean ± SEM. Asterisks represent the p-value (*** = P < 0.001) for N = 18 using the paired t-tests between milk (white bar) and gastric (grey bar) samples.
Discussion
This study aimed to determine how concentrations of proteases, antiproteases and bioactive proteins changed from premature-delivering mothers’ milks to their infants’ stomachs. The extensive system of human milk proteases and antiproteases was recently reviewed (8) and examined in preterm milk samples (4). Gastric samples were aspirated 2 h after initiation of feeding to obtain the maximum feasible length of gastric digestion time (based on gastric emptying times (15)).
The lower concentration of all measured protease inhibitors in the stomach contents than in milk suggests that these specific proteins are partially digested in the preterm infant stomach. The greater number of α1-antitrypsin-derived peptides in the gastric contents than in milk confirms that α1-antitrypsin is further digested in the stomach. The absence of peptides from other protease inhibitors in the peptidomics data is most likely due to the parent protein’s much lower abundance in milk.
The lower IgA and lactoferrin concentrations and their greater peptide counts in the gastric contents than in milk confirmed that these bioactive proteins were further digested in the stomach. These findings are congruent with our previous findings for term infants that lactoferrin and IgA-derived peptide counts were higher in the stomach than in milk (16). The increase of TGF-β1 concentration in the gastric contents likely resulted from acid-induced gastric release of TGF-β1 (AA 279–390) from the LAP latent complex (AA 30–390) (17–19). The decrease in concentration of the protease inhibitors, IgA and lactoferrin could also be partially due to denaturation at the lowered gastric pH and, for protease inhibitors, increased binding to the active proteases, both of which could decrease binding with the ELISA antibodies.
The lower pH of the gastric contents than of milk was expected as premature infants produce some gastric acid in response to milk ingestion (20). The average gastric pH identified corresponds well to that reported in the literature (21, 22).
Concentrations of proteases (measured at each protease’s optimal pH) changed from human milk to the infant stomach for some proteases but not for others. The lower concentration of cytosol aminopeptidase in the gastric contents could be due to partial digestion or increased binding to an inhibitor. Kallikrein, plasmin, thrombin and carboxypeptidase B2 concentrations remained stable in the stomach. Elastase and cathepsin D concentrations, on the other hand, increased. The increase in elastase concentration could be due to increased proteolytic conversion from the zymogen to enzyme form or due to lowered binding to protease inhibitors. Indeed, α1-antitrypsin, the most abundant milk antiprotease, can inhibit elastase (23) and was reduced in the stomach.
The increased cathepsin D concentration in the stomach measured at pH 4.0 was expected, as acidic conditions lead to the auto-activation of procathepsin D to pseudocathepsin D, which has cathepsin D cleavage activity (12, 13). However, we also tested standard pepsin against the cathepsin D substrate at pH 4.0, as they have overlapping cleavage specificity (both cleave after Leu/Phe (P1) (24, 25). We found that pepsin can cleave the cathepsin D substrate at pH 4.0 (data not shown). Thus, some of the measured cathepsin D concentration is likely due to pepsin. In attempt to determine cathepsin D’s contribution to this measurement, we measured cathepsin D concentration in human milk (which does not contain pepsin) using a pH 4.0 reaction buffer (promoting its auto-activation) and found 199 ± 57 ng/mL of cathepsin D. Therefore, the measured cathepsin D concentration in the gastric contents (1,390 ± 131 ng/mL) likely represents a mixture of cathepsin D and pepsin. As aspartic proteases such as pepsin can activate cathepsin D (26), true cathepsin D could contribute more than that predicted by its measurement in milk at acid conditions. Pepsin is known to be produced in the infant stomach as early as 16 wk of GA (27). Hemoglobin substrate-based pepsin activity assays have shown pepsin activity both pre-and post-prandially in preterm infants (21, 28–30). Though measured preterm gastric pepsin activity is much lower than that of adults (21), whether it is significantly lower than term infants remains unknown (2).
Though we detected each of these milk proteases in the gastric samples, this finding does not confirm that these enzymes were actively hydrolyzing proteins at the gastric sample pH. To determine whether these enzymes could be active in the stomach, we compared the activities of the standard enzyme at pH 4.5 (the average gastric pH) and pH 6.5 (the average milk pH). We found that enzyme standards for kallikrein, thrombin, cytosol aminopeptidase, carboxypeptidase B2 and elastase were active at pH 6.5 but not at pH 4.5, and thus unlikely to contribute to gastric protein digestion. Standard plasmin was active at pH 4.5, but 71.2% lower than at pH 6.5. Standard cathepsin D was active at pH 4.5 but not at pH 6.5. Previous studies also found that carboxypeptidase B (31), kallikrein (32), aminopeptidase (33), elastase (34) and thrombin (35) were not active below pH below 4.5. Thrombin was denatured below pH 4.5 (35) and kallikrein was unstable at pH 4.0–5.0 (32). Previous studies also corroborate our findings that plasmin activity is reduced but still active at pH 4.5 compared to pH 6.5 (36). Previous studies also demonstrate that cathepsin D is most active at pH 4.0 and not active at more neutral pH (12, 13). Therefore, the only milk proteases that can hydrolyze milk proteins in the infant stomach are plasmin and cathepsin D.
Though kallikrein, thrombin, cytosol aminopeptidase, carboxypeptidase B2 and elastase are inactive at preterm post-prandial gastric pH, these milk proteases could potentially become active again in the intestine after pancreatic secretions neutralize the pH. Plasmin would also likely increase in activity within the intestine as it is most active at neutral pH. However, cathepsin D will most likely become inactive in the intestine due to the pH change.
Gastric samples included a small amount of powdered bovine-protein based human milk fortifier. The addition of fortifier to mother’s milk did not likely contribute to the measured concentrations of milk proteases (based on substrate assays), as the vast majority of native milk proteases are inactivated by the heat treatments involved in powdered milk fortifier manufacturing (37). Moreover, the fortifier did not likely contribute to ELISA-based protein concentrations, as 1) the antibodies used were species-specific for human proteins rather than bovine proteins; and 2) the vast majority of heat-treated spray-dried proteins are denatured, which would inhibit their binding to the ELISA’s monoclonal antibodies which are specific to the native protein conformation (38, 39). We did not verify this assumption for every protein tested. However, we tested the bovine milk-based fortifier (at 0.1 g/mL in water) for human IgA and human α1-antitrypsin ELISAs and found no detectable cross-reactivity. If our assumption is incorrect and some bovine proteins in the fortifier cross-reacted with the human ELISA antibodies, this addition would only increase the significance of the difference between the human milk and gastric sample values for α1-antichymotrypsin, antithrombin III, α2-antiplasmin, plasma serine protease inhibitor and lactoferrin, as concentrations in the stomach would decrease. As the ELISA-detected concentration for TGF-β1 increases in the stomach, there is potential that this additional protein could arise from cross-reactivity with bovine TGF-β1 from the fortifier. We examined the fortifier for cathepsin D and plasmin activity at pH 4.5 and found no activity. We did not examine the other milk proteases’ activities in the fortifier, as we already demonstrated that they are inactive at preterm infant gastric pH.
A limitation of our study is that gastric dilution by gastric secretions was not measured, which could cause variance in the protein measurements. However, as the contribution of gastric secretions is relatively small (less than 10% of total gastric volume) (15), the gastric volume in the preterm stomach represents mostly the remaining feeding volume.
Our study reveals for the first time that though carboxypeptidase B2, kallikrein, plasmin, cathepsin D, elastase, thrombin and cytosol aminopeptidase are present in the milk of preterm-delivering mothers and the preterm infant stomach, only plasmin and cathepsin D retain activity at preterm infant gastric pH. Milk protease inhibitors and several bioactive proteins were partially digested in the infant stomach, likely by a combination of milk proteases and pepsin. This research contributes to a better understanding of gastric digestion in preterm infants, which is an essential step, prior to examining small intestinal digestion.
Supplementary Material
What is known
Milk contains an array of proteases that actively hydrolyzed milk proteins.
Peptidomic evidence indicates that human milk proteases remain active in the term infant stomach.
Milk protease inhibitors are present in human milk and limit overall milk protease activity.
What is new
Among the human milk proteases (carboxypeptidase B2, kallikrein, plasmin, cathepsin D, elastase, thrombin and cytosol aminopeptidase) only plasmin and cathepsin D were able to hydrolyze proteins at gastric pH.
Milk protease inhibitors, lactoferrin and immunoglobulin A were partially degraded in the preterm stomach.
Acknowledgments
Statement of authors’ contributions to manuscript. The authors thank C. J. Dillard for editing the manuscript. V. D. M. conducted the research and analyzed data. S. D. N. conducted peptidomic analysis. M. A. U. and R. B. provided milk samples. V. D. M. and D. C. D. designed the study and wrote the paper. V. D. M. and D. C. D. have primary responsibility for the final content. All authors read and approved the final manuscript.
Supported by the K99/R00 Pathway to Independence Career Award, Eunice Kennedy Shriver Institute of Child Health & Development of the National Institutes of Health (R00HD079561) (DCD).
Footnotes
Author disclosures: V. Demers-Mathieu, S. D. Nielsen, M. A. Underwood, R. Borghese and D. C. Dallas have no conflicts of interest.
References
- 1.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85:629S–34S. doi: 10.1093/ajcn/85.2.629S. [DOI] [PubMed] [Google Scholar]
- 2.Dallas DC, Underwood MA, Zivkovic AM, German JB. Digestion of protein in premature and term infants. J Nutr Disord Ther. 2012;2:112–21. doi: 10.4172/2161-0509.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson RH, Kim YS. Digestion and absorption of dietary protein. Annu Rev Med. 1990;41:133–9. doi: 10.1146/annurev.me.41.020190.001025. [DOI] [PubMed] [Google Scholar]
- 4.Demers-Mathieu V, Nielsen SD, Underwood MA, Borghese R, Dallas DC. Analysis of milk from mothers who delivered prematurely reveals few changes in proteases and protease Inhibitors across gestational age at birth and infant postnatal age. J Nutr. 2017:jn244798. doi: 10.3945/jn.116.244798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaldi N, Vijayakumar V, Dallas DC, Guerrero A, Wickramasinghe S, Smilowitz JT, Medrano JF, Lebrilla CB, Shields DC, German JB. Predicting the important enzymes in human breast milk digestion. J Agric Food Chem. 2014;62:7225–32. doi: 10.1021/jf405601e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holton TA, Vijayakumar V, Dallas DC, Guerrero A, Borghese RA, Lebrilla CB, German JB, Barile D, Underwood MA, Shields DC, et al. Following the digestion of milk proteins from mother to baby. J Proteome Res. 2014;13:5777–83. doi: 10.1021/pr5006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas DC, Smink CJ, Robinson RC, Tian T, Guerrero A, Parker EA, Smilowitz JT, Hettinga KA, Underwood MA, Lebrilla CB, et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J Nutr. 2015;145:425–33. doi: 10.3945/jn.114.203646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallas DC, Murray NM, Gan J. Proteolytic Systems in Milk: Perspectives on the Evolutionary Function within the Mammary Gland and the Infant. J Mammary Gland Biol Neoplasia. 2015;20:133–47. doi: 10.1007/s10911-015-9334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallas DC, German JB. Intestinal Microbiome: Functional Aspects in Health and Disease. Karger Publishers; 2017. Enzymes in Human Milk; pp. 129–36. [Google Scholar]
- 10.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42:D503–9. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JL, Backes BJ, Leonetti F, Mahrus S, Ellman JA, Craik CS. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc Natl Acad Sci U S A. 2000;97:7754–9. doi: 10.1073/pnas.140132697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briozzo P, Morisset M, Capony F, Rougeot C, Rochefort H. In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res. 1988;48:3688–92. [PubMed] [Google Scholar]
- 13.Larsen LB, Boisen A, Petersen TE. Procathepsin D cannot autoactivate to cathepsin D at acid pH. FEBS Lett. 1993;319:54–8. doi: 10.1016/0014-5793(93)80036-t. [DOI] [PubMed] [Google Scholar]
- 14.Dallas DC, Citerne F, Tian T, Silva VL, Kalanetra KM, Frese SA, Robinson RC, Mills DA, Barile D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016;197:273–84. doi: 10.1016/j.foodchem.2015.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel M, Lebenthal E, Topper W, Krantz B, Li P. Gastric emptying in prematures of isocaloric feedings with differing osmolalities. Pediatr Res. 1982;16:141–7. doi: 10.1203/00006450-198202000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Dallas DC, Guerrero A, Khaldi N, Borghese R, Bhandari A, Underwood MA, Lebrilla CB, German JB, Barile D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J Nutr. 2014;144:815–20. doi: 10.3945/jn.113.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhwa M, Dilger P, Tubbs J, Mire-Sluis A, Barrowcliffe T, Thorpe R. Identification of transforming growth factor-beta as a contaminant in factor VIII concentrates: a possible link with immunosuppressive effects in hemophiliacs. Blood. 1994;84:2021–30. [PubMed] [Google Scholar]
- 18.Srivastava MD, Srivastava A, Brouhard B, Saneto R, Groh-Wargo S, Kubit J. Cytokines in human milk. Res Commun Mol Pathol Pharmacol. 1996;93:263–87. [PubMed] [Google Scholar]
- 19.Kalliomäki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-β in breast milk: A potential regulator of atopic disease at an early age. J Allergy Clin Immunol. 1999;104:1251–7. doi: 10.1016/s0091-6749(99)70021-7. [DOI] [PubMed] [Google Scholar]
- 20.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35:215–20. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 21.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–37. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Babbott FL, Johnston JA, Haskins CH. Gastric acidity in infantile tetany. Am J Dis Child. 1923;26:486–501. [Google Scholar]
- 23.Cohen AB. Interrelationships between the human alveolar macrophage and alpha-1-antitrypsin. J Clin Invest. 1973;52:2793–9. doi: 10.1172/JCI107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Lou X, Shan Q, Zhang J, Zhu X, Zhang J, Wang Y, Xie Y, Xu N, Liu S. Proteolytic characteristics of cathepsin D related to the recognition and cleavage of its target proteins. PloS one. 2013;8:e65733. doi: 10.1371/journal.pone.0065733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn J, Cao M-J, Yu YQ, Engen JR. Accessing the reproducibility and specificity of pepsin and other aspartic proteases. Biochim Biophys Acta. 2013;1834:1222–9. doi: 10.1016/j.bbapap.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent-Matha V, Derocq D, Prébois C, Katunuma N, Liaudet-Coopman E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B. J Biochem. 2006;139:363–71. doi: 10.1093/jb/mvj037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keene MFL, Hewer EE. Digestive enzymes of the human foetus. Lancet. 1929;213:767–9. [Google Scholar]
- 28.Weisselberg B, Yahav J, Reichman B, Jonas A. Basal and meal-stimulated pepsinogen secretion in preterm infants: a longitudinal study. J Pediatr Gastroenterol Nutr. 1992;15:58–62. doi: 10.1097/00005176-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Yahav J, Carrion V, Lee PC, Lebenthal E. Meal-stimulated pepsinogen secretion in premature infants. J Pediatr. 1987;110:949–51. doi: 10.1016/s0022-3476(87)80421-3. [DOI] [PubMed] [Google Scholar]
- 30.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GBJ. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Dig Dis Sci. 1969;14:400–14. doi: 10.1007/BF02239360. [DOI] [PubMed] [Google Scholar]
- 31.Wolff EC, Schirmer E, Folk J. The kinetics of carboxypeptidase B activity. J Biol Chem. 1962;237:3094–9. [PubMed] [Google Scholar]
- 32.Takahashi H, Nagasawa S, Suzuki T. Studies on Prekallikrein of Bovine Plasma. J Biochem. 1972;71:471–83. [PubMed] [Google Scholar]
- 33.Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from β-lipotropin60–65. FEBS letters. 1984;175:135–9. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- 34.Ohlsson K, Odsson I. The neutral proteases of human granulocytes. Eur J Biochem. 1974;42:519–27. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- 35.Seegers WH, Smith H. Factors which influence the activity of purified thrombin. The American journal of physiology. 1942;137:348–54. [Google Scholar]
- 36.Kaminogawa S, Mizobuchi H, Yamauchi K. Comparison of bovine milk protease with plasmin. Agric Biol Chem. 1972;36:2163–7. [Google Scholar]
- 37.Alkanhal H. The fate of milk and bacterial lipases and proteases in raw milk during the production of milk powder. Egyption J Dairy Sci. 2006;34:159–66. [Google Scholar]
- 38.Jocson MA, Mason EO, Schanler RJ. The effects of nutrient fortification and varying storage conditions on host defense properties of human milk. Pediatrics. 1997;100:240–3. doi: 10.1542/peds.100.2.240. [DOI] [PubMed] [Google Scholar]
- 39.Singh H. Interactions of milk proteins during the manufacture of milk powders. Le Lait. 2007;87:413–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


