Abstract
Background
A growing number of patients survive sepsis but remain chronically critically ill. We sought to define clinical outcomes and incidence of chronic critical illness (CCI) after sepsis and to determine whether selected biomarkers of inflammation, immunosuppression, and catabolism differ between these patients and those that rapidly recover (RAP).
Methods
This three-year prospective observational cohort study (NCT02276417) evaluated 145 surgical intensive care unit patients with sepsis for the development of CCI (≥14 days of ICU resource utilization with persistent organ dysfunction). Patient clinical demographics, outcomes, and serial serum/urine samples were collected for plasma protein and urinary metabolite analyses.
Results
Of 145 sepsis patients enrolled, nineteen (13%) died during their hospitalization and 71 (49%) developed CCI. CCI patients were significantly older (mean 63±15 vs 58 ±13 years, p=0.006), and more likely to be discharged to long-term acute care facilities (32% vs 3%, p<0.0001), whereas those with RAP were more often discharged to home or a rehabilitation facility. 6-month mortality was significantly higher in CCI as compared to RAP cohort (37% vs 2%; p<0.01). Multivariate logistic regression modeling revealed delayed onset sepsis (>48 hours after admission; O.R. 10.93, 95% C.I. [4.15–28.82]), inter-facility transfer (O.R. 3.58, [1.43–8.96]), vasopressor-dependent septic shock (O.R. 3.75, [1.47–9.54]), and SOFA score ≥5 at 72 hours (O.R. 5.03, [2.00–12.62]) as independent risk factors for the development of CCI. CCI patients also demonstrated greater elevations in inflammatory cytokines (IL-6, IL-8, IL-10), and biomarker profiles consistent with persistent immunosuppression (absolute lymphocyte count and sPDL-1) and catabolism (plasma IGFBP3 and urinary 3-methylhistidine excretion).
Conclusions
The development of CCI has become the predominant clinical trajectory in critically-ill surgical patients with sepsis. These patients exhibit biomarker profiles consistent with an immunocatabolic phenotype of persistent inflammation, immunosuppression, and catabolism.
Keywords: sepsis, shock, chronic critical illness, inflammation, immunosuppression
BACKGROUND
Sepsis is a major source of morbidity and mortality (1, 2). Over the past decade, in-hospital mortality after sepsis has progressively declined secondary to advances in the early recognition of sepsis and prompt intervention based upon evidenced-based guidelines (3, 4). Despite these improvements, sepsis remains a morbid disease with poor long-term outcomes including lasting physical, cognitive, and psychiatric deficits, as well as high rates of post-discharge mortality (5, 6). Additionally, at a biologic level sepsis is now evolving into a chronic inflammatory disease. We have observed that while an increasing number of surgical ICU (SICU) patients survive their acute illness and recover from multiple organ failure (MOF), they commonly remain hospitalized for extended periods of time with complicated clinical courses and evidence of persistent inflammation, immunosuppression, and protein catabolism (7, 8).
Several descriptive terms have been used by investigators to convey the chronicity of adverse outcomes which are observed in these patients. These terms include, but are not limited to, ‘chronic critical illness’, ‘post-intensive care syndrome’, ‘myopathy of critical illness’, ‘neuropathy of critical illness’, and ‘ICU-acquired weakness’ (9–12). For the purpose of this study, we will refer to the lasting sequelae after sepsis as chronic critical illness (CCI). At present, there is no well-accepted and widely utilized definition for CCI among the scientific and medical communities, which makes defining its epidemiology difficult. Despite the variance among definitions of CCI, they usually agree in two components: (1) the requirement for prolonged intensive care and (2) presence of persistent organ dysfunction. Therefore, we aimed to determine the incidence of CCI in surgical patients after sepsis using a set of standardized criteria based upon prolonged intensive care resource utilization and persistent, low-grade organ dysfunction.
In addition to benchmarking the epidemiology of CCI, we sought to identify evidence for unifying mechanistic explanation for CCI. We have proposed that adverse outcomes observed in sepsis survivors in the SICU are linked with a syndrome of persistent inflammation, immunosuppression, and catabolism (PICS) (7, 8, 13, 14). In previous work, we showed that severely injured trauma patients who experience complicated clinical outcomes demonstrate persistent alterations in pro- and anti-inflammatory cytokines, leukocyte subsets, and biomarkers of protein catabolism (15). Similarly, we hypothesize that patients who develop CCI after sepsis will exhibit lasting derangements in biomarkers of inflammation, immunosuppression, and protein catabolism as compared to patients who exhibit recovery.
METHODS
Study design, site and patients
This prospective observational study was conducted between January 2012 and January 2016 at an academic, 996-bed quaternary medical center. Overall program study design and protocols for the Sepsis and Critical Illness Center research program have been published (16). Additional methodologic details to those described below are available in Supplemental Digital Content 1. The study received Institutional Review Board approval prior to initiation. Signed informed consent from the patient or legal proxy was obtained within 96 hours of study enrollment. Patients included in this study were admitted to the SICU and either admitted with, or subsequently developed sepsis. Sepsis screening was performed using the Modified Early Warning Signs-Sepsis Recognition System (MEWS-SRS) (17). All enrolled patients were managed using a standardized sepsis management protocol based on the Surviving Sepsis guidelines, supplemented by standardized evidenced-based ICU clinical care protocols and utilization of a computerized clinical decision support system to ensure high compliance and timely intervention (17, 18).
Patient Enrollment, classification and outcomes
Inclusion criteria consisted of the following: (1) age ≥18 years; (2) presence in the SICU; and (3) clinical diagnosis of sepsis, severe sepsis, or septic shock as defined by the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (19). Sepsis diagnoses were clinically adjudicated in a prospective fashion at weekly program adjudication meetings.
Exclusion criteria consisted of the following: (1) refractory shock (death <24 hours) or inability to achieve source control (e.g. unresectable dead bowel); (2) pre-sepsis expected lifespan <3 months; (3) patient goals of care not consistent with aggressive management; (4) severe CHF (NYHA Class IV); (5) Child-Pugh Class C liver disease or pre-liver transplant; (6) HIV with CD4+ count <200 cells/mm3; (7) chronic corticosteroids or immunosuppressive agent use, including organ transplant recipients; (8) pregnancy; (9) chemotherapy or radiotherapy within past 30 days; (10) severe traumatic brain injury (evidence of neurologic injury on CT scan and GCS <8); or, (11) spinal cord injury resulting in permanent sensory and/or motor deficits.
CCI was defined as an ICU length of stay (LOS) greater than or equal to 14 days with evidence of persistent organ dysfunction, determined using components of the Sequential Organ Failure Assessment (SOFA) score (cardiovascular SOFA ≥ 1, or score in any other organ system ≥ 2) (20). Therefore, a wide representation of organ dysfunction was incorporated, including patients with respiratory, cardiovascular, neurologic, coagulative, liver, and renal dysfunction. Rapid recovery (RAP) patients were those who did not meet criteria for CCI or early death (death <14 days of sepsis protocol onset). Primary outcomes included in-hospital mortality, hospital and ICU LOS, ventilator days, presence and severity of organ dysfunction, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), development of CCI, and discharge disposition. Six-month mortality was determined by prospective follow-up, with cross-check validation via the United States Social Security Death Index (SSDI).
Laboratory Analyses
Blood samples were collected and analyzed for biomarkers of inflammation (IL-6, IL-8, IL-10), immunosuppression (absolute lymphocyte count and soluble programmed death ligand one [sPDL-1]), and catabolism (insulin-like growth factor binding protein 3 [IGFBP3]) at 12 hours, one, four, seven, and 14 days, and weekly thereafter while hospitalized. Urine was collected for measurement of 3-methylhistidine (3-MH) to creatinine ratios at similar time frames (21).
Statistical Analysis
Data are presented as either frequency and percentage, or mean and standard deviation, or median and 25th/75th percentiles. Student’s t-test, ANOVA and Kruskal–Wallis tests were used for comparison of continuous variables as appropriate. Measured biomarkers were compared using non-parametric rank tests to determine significant differences between groups at each time point. Mixed model analysis was also performed to determine differences between groups over time.
Two multivariate logistic regression models at 24 and 72 hours after sepsis onset were constructed by a stepwise model selection at a significance level of 0.10. Adjusted odds ratios (OR) with 95% confidence intervals (95% CI) and the area under the receiver operating characteristics curve values (AUC) and Hosmer–Lemeshow goodness-of-fit test were used. All significance tests were two-sided, with p-value ≤0.05 considered statistically significant. Statistical analyses were performed with SAS (v.9.4, Cary, NC).
RESULTS
Of the 145 study patients, 29 (20%) were diagnosed with sepsis, 56 (39%) with severe sepsis, and 60 (41%) with septic shock. Mean age was 61 ± 14 (SD) years with a slight predominance of male patients (n=82, 56%). Seventy-one percent of patients (n=103) had at least one medical comorbidity, while almost half (n=57, 45%) had two or more comorbidities. Sixty-one patients (42%) were transfers from another acute care facility at the time of index admission. There was a broad range of sepsis severity, with increasing APACHE II scores proportionate to sepsis severity (sepsis, mean 14 ± 6; severe sepsis, mean 21 ± 7; septic shock, mean 25 ± 8). Detailed cohort characteristics are listed in Supplemental Digital Content 2.
The primary source of sepsis was intra-abdominal infection, followed by pneumonia, necrotizing soft tissue infection, urinary tract infection, surgical site infection, and central line-associated bloodstream infection (Supplemental Digital Content 3). More than half (55%) of patients required a surgical or interventional procedure for source control. Although most patients (62%) had sepsis ‘present on admission’ (diagnosed within 48 hours), 38% of patients were diagnosed with ‘delayed onset’ sepsis (sepsis occurring >48 hours after hospital admission secondary to a post-surgical or post-traumatic injury infection), with a median onset of eight days. The most common causes of delayed onset sepsis included post-operative bowel perforation/anastomotic leaks, ventilator-associated pneumonia, and urinary tract infections. Subgroup analysis of sepsis, severe sepsis, and septic shock revealed that septic shock patients were older (sepsis, 58 ± 13 years; severe sepsis, 58 ± 15 years; septic shock, 65 ± 14 years; p=0.01), but there were no significant differences found in race, gender, BMI or number of comorbidities. However, the incidence of AKI increased with sepsis severity (sepsis, 38%; severe sepsis, 54%; septic shock, 77%; p=0.0002).
Clinical Outcomes and the Incidence of Chronic Critical Illness
Overall in-hospital mortality for this sepsis cohort was thirteen percent. As expected, those with septic shock exhibited the highest mortality rates (sepsis, 3%; severe sepsis, 5%; septic shock, 25%; p=0.003), longest ICU LOS (median days: sepsis, 4; severe sepsis, 12; septic shock, 12; p<0.0001), and the greatest number of ventilator days (median days: sepsis, 4; severe sepsis, 7; septic shock, 10; p=0.01).
Clinical trajectories after sepsis and their patient characteristics are shown in Table 1. The eight patients experiencing ‘early death’ (median 6 days) were older, had more severe organ dysfunction within the first 24 hours, and presented with an early overwhelming response to infection as indicated by a higher frequency of septic shock (Table 1). Roughly half (n=71, 49%) of all septic patients developed CCI (Table 1). In comparison to patients with rapid recovery (RAP), CCI patients were older, exhibited greater sepsis severity and early physiologic derangement, and were more likely to have been admitted as an inter-facility hospital transfer (Table 1). There were no significant differences between the groups in number of comorbidities, need for sepsis source control procedure, or infection type.
Table 1.
Baseline Characteristics by Trajectory
| Characteristics | RAP (n=66) | CCI (n=71) | Early death (n=8) | p-value† | p-value‡ |
|---|---|---|---|---|---|
| Male, n (%) | 31 (47) | 47 (66.2) | 4 (50) | 0.07 | 0.03 |
| Age in years, mean (SD) | 57.6 (13.3) | 62.6 (14.7) | 69.3 (10.5) | 0.004 | 0.006 |
| Age ≥65 years, n (%) | 17 (25.8) | 36 (50.7) | 6 (75) | 0.001 | 0.003 |
| BMI, median (25th, 75th) | 27.9 (24.2, 34.3) | 28.8 (24.2, 34.8) | 25.1 (20.9, 42.2) | 0.96 | 0.97 |
| Number of comorbidities*, n (%) | 0.4 | 0.22 | |||
| 0 | 19 (28.8) | 22 (31) | 1 (12.5) | ||
| 1 | 20 (30.3) | 16 (22.5) | 2 (25) | ||
| 2 | 10 (15.2) | 20 (28.2) | 3 (37.5) | ||
| ≥3 | 17 (25.8) | 13 (18.3) | 2 (25) | ||
| Charlson comorbidity index score, mean (SD) | 3.6 (2.6) | 4.5 (3) | 5 (1.9) | 0.06 | 0.04 |
| APACHE II score, mean (SD) | 18.4 (7.8) | 23.6 (7.9) | 24.9 (7.5) | 0.001 | <0.001 |
| Delayed sepsis onset**, n (%) | 10 (15.15) | 44 (62) | 1 (12.5) | <0.001 | <0.001 |
| Inter-facility hospital transfer, n (%) | 21 (31.8) | 34 (47.9) | 6 (75) | 0.03 | 0.08 |
| Sepsis severity, n (%) | <0.001 | 0.002 | |||
| Sepsis | 21 (31.8) | 8 (11.3) | 0 (0) | ||
| Severe sepsis | 28 (42.4) | 27 (50.7) | 1 (12.5) | ||
| Septic shock | 17 (25.8) | 36 (38) | 7 (87.5) | ||
| Sepsis diagnosis, n (%) | 0.13 | 0.15 | |||
| Intra-abdominal sepsis | 24 (36.4) | 33 (46.5) | 7 (87.5) | ||
| Pneumonia | 9 (13.6) | 17 (23.9) | 0 | ||
| NSTI | 15 (22.7) | 8 (11.3) | 0 | ||
| UTI | 8 (12.1) | 3 (4.2) | 0 | ||
| Surgical site infection | 4 (6.1) | 4 (5.6) | 0 | ||
| Other | 6 (9.1) | 6 (8.5) | 1 (12.5) | ||
| Sepsis source control procedure, n (%) | 33 (50) | 40 (56.3) | 6 (75) | 0.38 | 0.5 |
Definition of Abbreviations: BMI, body mass index; APACHE II, acute physiology and chronic health evaluation II; NSTI, necrotizing soft tissue infection; UTI, urinary tract infection.
Comorbidities included those defined within the Charlson Comorbidity Index
Delayed sepsis onset, defined as sepsis occurring >2 days after hospital admission due to post-surgical or post-trauma infection
p-value comparing three groups overall
p-value comparing two groups (RAP vs CCI)
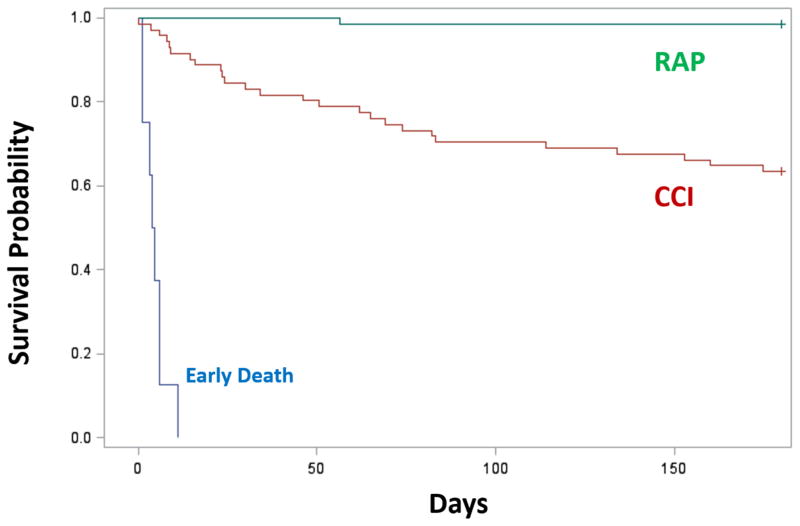
Clinical outcomes clearly differed among the CCI and RAP groups (Table 2). CCI patients had significantly higher maximum SOFA scores and a greater rate of MOF than RAP patients. Additionally, CCI patients had both higher incidence and severity of AKI. Hospital length of stay for CCI patients was almost three times as long as that for RAP patients. Ultimately, 80% of patients who developed CCI had a ‘poor’ hospital discharge disposition. Of CCI patients who survived to discharge, nearly two thirds required placement in either a long-term acute care facility, skilled nursing facility, another inpatient hospital, or hospice. In contrast, over 70% of RAP patients were discharged to home or to a rehabilitation facility (Table 2). In addition to requiring higher levels of care upon discharge, CCI patients demonstrated significantly increased post-discharge mortality, with over one-third (37%) of CCI patients dying within the first 6 months of sepsis diagnosis, as compared to 2% in the RAP group (p<0.01) (Figure 1).
Table 2.
Outcomes by Clinical Trajectory
| Outcomes | RAP (n=66) | CCI (n=71) | p-value |
|---|---|---|---|
| Hospital mortality, n (%) | 0 (0) | 11 (15.5) | 0.0007 |
| ICU LOS, median (25th, 75th) | 6 (3, 9) | 22 (16, 34) | <0.001 |
| Hospital LOS, median (25th, 75th) | 10 (7, 17) | 29 (19, 44) | <0.001 |
| Ventilator days, median (25th, 75th) | 3 (2, 5) | 15.5 (7.5, 22.5) | <0.001 |
| Maximum SOFA score*, median (25th, 75th) | 5 (3, 8) | 10 (7, 12) | <0.001 |
| Multiple organ failure**, n (%) | 26 (30.6) | 36 (70) | <0.001 |
| ARDS | 0 (0) | 6 (8.5) | 0.03 |
| AKI, n (%) | 31 (47) | 49 (69) | 0.01 |
| AKI KDIGO Stage, n (%) | 0.07 | ||
| 1 | 17 (25.8) | 23 (32.4) | |
| 2 | 9 (13.6) | 14 (19.7) | |
| 3 | 5 (7.6) | 12 (16.9) | |
| ESRD | 2 (3) | 0 (0) | |
| Discharge disposition, n (%) | |||
| “Good” Disposition | 47 (71.2) | 14 (19.7) | <0.001 |
| Home | 29 (43.9) | 0 (0) | |
| Rehab | 14 (21.2) | 6 (8.5) | |
| Home healthcare services | 4 (6.1) | 8 (11.3) | |
| “Poor” Disposition | 19 (28.8) | 57 (80.3) | <0.001 |
| Long Term Acute Care facility | 2 (3) | 23 (32.4) | |
| Skilled Nursing facility | 17 (25.8) | 7 (9.9) | |
| Another Hospital | 0 (0) | 12 (16.9) | |
| Hospice | 0 (0) | 4 (5.6) | |
| Death | 0 (0) | 11 (15.5) |
Definition of Abbreviations: RAP, rapid recovery; CCI, chronic critical illness; BMI, body mass index; LOS, length of stay; SOFA, sequential organ failure assessment; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury; KDIGO, Kidney disease improving global outcomes staging; ESRD, end-stage renal disease; UTI, urinary tract infection.
SOFA score, defined as a composite score of six different organ systems (respiratory, cardiovascular, hepatic, coagulation, renal, and neurological systems) used to determine the extent of a patient’s organ dysfunction.
Multiple organ failure, defined as SOFA component score ≥3 in at least two organs systems.
Figure 1. 6-month mortality after sepsis based on clinical trajectory.
Six-month mortality was determined by prospective follow-up, with cross-check validation via the United States Social Security Death Index (SSDI). Trajectories were classified as Early Death (blue), Rapid Recovery (RAP; green) and chronic critical illness (CCI; red) as defined within the methods section. Kaplan-Meier survival curve shows significant difference between 3 groups (p<0.01) and also between CCI vs RAP (p<0.01). 6-month mortality rate for CCI cohort is 26/71 (37%), and 1/66 (2%) in RAP.
We developed two multivariate risk factor models in order to facilitate prediction of CCI based on readily available clinical data at 24 and 72 hours after sepsis diagnosis (Table 3). At 24 hours, delayed onset of sepsis, inter-facility transfer at admission, and vasopressor-dependent septic shock were identified as independent predictors of development of CCI. Persistent multiple organ dysfunction at 72 hours from sepsis onset was also an independent predictor of CCI, but did not significantly improve risk factor model prediction (Table 3).
Table 3.
Multivariate Clinical Prediction Models for CCI
| Model | O.R. | 95% C.I. |
|---|---|---|
| Baseline (24-hour) model* | ||
| Delayed sepsis (onset ≥2 days after admission) | 12.06 | (4.64, 31.36) |
| Inter-facility transfer | 3.41 | (1.39, 8.37) |
| Septic shock | 3.49 | (1.30, 9.36) |
| SOFA score ≥6 in 1st 24 hours | 2.12 | (0.83, 5.43) |
| Post resuscitation (72-hour) model** | ||
| Delayed sepsis (onset ≥2 days after admission) | 11.37 | (4.27, 30.28) |
| Inter-facility transfer | 2.75 | (1.11, 6.83) |
| Septic shock | 2.80 | (1.04, 7.52) |
| SOFA score ≥5 at 72 hours | 5.03 | (2.00, 12.62) |
Definition of abbreviations: O.R., odds ratio; C.I., confidence interval; SOFA, sequential organ failure assessment score.
Area under receiver operator curve = 0.84 (0.77, 0.91); Hosmer-Lemeshow goodness of fit = 0.076
Area under receiver operator curve = 0.86 (0.80, 0.92); Hosmer-Lemeshow goodness of fit = 0.734
Adjusted odds ratios were derived using the multivariable logistic regression that included all listed variables in the model simultaneously. Baseline (24 hour) model included age, gender, Charlson comorbidity score, delayed sepsis, inter-facility transfer, septic shock status, and dichotomized SOFA scores where cut-off was calculated by maximizing sensitivity and specificity along the receiver operating characteristic curves. Final reduced set of variables were selected by a stepwise model selection at a significance level of 0.10.
CCI and Persistent Inflammation, Immunosuppression and Catabolism
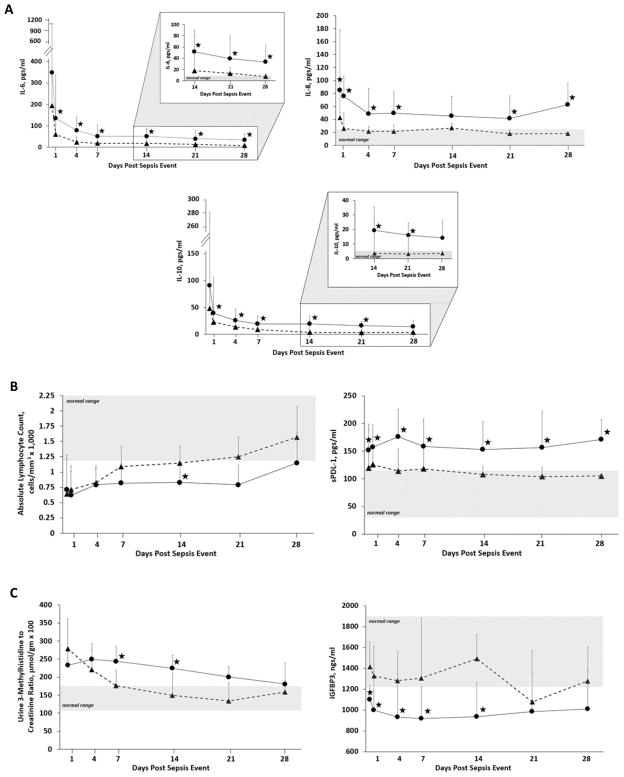
There were significant differences in biomarkers of inflammation, immunosuppression and catabolism between CCI and RAP patients. Patients who developed CCI demonstrated a more robust early inflammatory response, with pronounced and sustained elevations in plasma IL-6, IL-8 and IL-10 out to 28 days (Figure 2). These patients also demonstrated a significantly greater initial degree, and persistence of immune suppression as reflected by measured sPDL-1 levels (Figure 2). Furthermore, absolute lymphocyte counts in CCI patients remained suppressed and significantly decreased (Figure 2). Finally, there were significant differences between groups in markers of catabolism, as measured by urine 3-methylhistidine to creatinine ratio and plasma IGFBP3 levels (Figure 2).
Figure 2. Biomarker concentrations for inflammation, immunosuppression and protein catabolism in patients with chronic critical illness (CCI) and rapid recovery (RAP).
Blood and urine samples were collected at 0.5, 1, 4, 7, 14, 21, and 28 days after sepsis management protocol onset. Plasma concentrations of IL-6, IL-8 and IL-10 were used to assess the inflammatory status of the subject (panel A), plasma sPDL-1 and absolute lymphocyte count to assess immune suppression (panel B), and urinary 3-methylhistidine excretion normalized to creatinine excretion and plasma IGFBP3 concentrations were used as markers of protein catabolism (panel C). Complete cases for laboratory results were binned to the closest time point. Differences in concentrations between CCI (●) and RAP (▲) cohorts at individual time points are identified with a (★) at a p-value <0.05 using non-parametric tests. The results of mixed model analyses reveal that the levels of all analytes over time were significantly different between groups (p<0.05). Patients with CCI had significantly increased early and late inflammation, as well as prolonged immune suppression and evidence of increased muscle protein catabolism (3-MH) and reduced anabolic signals (IGFBP3). (★) indicates p<0.05 at that time point; shaded areas are normal ranges.
DISCUSSION
Although inpatient mortality to sepsis has recently declined due to implementation of evidence based care, this success is paralleled by an emerging epidemic of CCI. Although variably defined, CCI is consistently linked to poor long-term outcomes and high health care expenditures (22, 23). In this prospective observational study, we document the incidence of CCI in 145 critically ill patients who developed sepsis, using a definition based on our previous work (24, 25). We found that CCI is not only prevalent within the SICU, but represents the predominant clinical trajectory in patients who survive sepsis. Of the entire cohort, 87% survived their initial septic insult; however, 52% of these surviving patients progressed to a state of CCI. These patients, by definition, had persistent, low-grade organ dysfunction and were more likely to be discharged to long term acute care facilities, skilled nursing facilities, other hospitals, and hospice in comparison to those with rapid recovery. These findings have significant long-term implications, since one third of patients discharged to skilled nursing facilities never return home, and nearly another third (29%) are dead at one-year (26).
To develop therapeutic interventions aimed at preventing progression to CCI after sepsis, early identification of those likely to progress to CCI will be essential. Here we show that independent risk factors for the development of CCI can be determined within 24 hours of sepsis onset, and include delayed onset of sepsis, vasopressor-dependent septic shock, transfer from another hospital, and persistent organ dysfunction. Delayed onset sepsis (i.e. hospital-acquired sepsis) was by far the strongest independent predictor of CCI and was frequently observed in patients who were initially admitted for a planned surgical procedure or following severe traumatic injury. This suggests that recurrent inflammatory insults may redirect a patient’s trajectory to that of CCI and PICS. Aside from this “second-hit” hypothesis, another possibility is that patients with delayed onset sepsis acquire more resistant and difficult to treat pathogens in the hospital, as opposed to patients who were admitted from the community with sepsis. Vasopressor-dependent septic shock and hospital transfer status were anticipated risk factors for CCI, suggesting greater physiologic derangement or possible delays in sepsis treatment. With further refinement and subsequent analyses, including addition of the biomarkers and future validation studies, it may be possible to identify the cohort of CCI patients who may potentially benefit from interventions such as targeted immunomodulation with emerging, novel agents.
In previous studies, we showed that evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) is found in trauma patients who experience complicated clinical outcomes (7, 15). In this study, we now demonstrate that this biologic phenotype is also present in CCI patients following sepsis. Compared to patients who rapidly recover, CCI biomarker profiles indicate a more robust and persistent inflammatory response that lasts at least four weeks after sepsis onset. Simultaneously, these patients exhibit polarization of innate inflammatory responses over adaptive immunity with indirect evidence of adaptive immune suppression, including reduced absolute lymphocyte counts and elevated sPDL-1 concentrations. This has clinical implications since previous studies in sepsis patients demonstrate that lower absolute lymphocyte counts are linked to reactivation of latent viral infections (27) and place patients at increased risk for recurrent bacterial infections (28). Persistently elevated levels of the anti-inflammatory cytokine, IL-10, in the CCI group, may also reflect the hosts attempt to down-regulate the inflammatory response in order to limit ongoing immune-mediated damage. Therefore, increased levels of IL-10 may also serve as a surrogate of immune suppression.
In addition to immunosuppression, CCI patients demonstrate evidence of increased protein catabolism given their elevated mean urinary 3-MH to creatinine excretion ratios and lower levels of IGFBP3, which is a carrier protein of insulin-like growth factor. We chose this as a marker of catabolism since suppression of the somatotropic axis is linked to critical illness (29–31). Taken together, this constellation of findings is supportive of the presence of PICS in patients with CCI, affords an underlying mechanistic explanation, and suggests potential areas of future targeted therapies.
The underlying mechanisms of dysfunctional inflammation and immunosuppression in these patients remains unclear. However, we recently reported that a population of immature myeloid cells, termed myeloid-derived suppressor cells (MDSCs), are persistently elevated in the blood of septic patients. Importantly, MDCSs suppress T lymphocyte proliferation and decrease the release of Th1 and Th2 cytokines. Exaggerated MDSC expansion correlated with early mortality, and persistent MDSC expansion was a strong independent predictor of nosocomial infections and poor discharge dispositions (13). Interestingly, the increases in MDSCs seen in post-sepsis patients are remarkably similar to those seen in advanced cancer (32, 33). These similarities provide a rationale for using therapies successful in reversing T-cell immunosuppression in patients with sepsis. As an example, Bristol-Myers-Squibb has recently initiated a clinical trial with nivolumab (Opdiva™, a monoclonal antibody against PD-1) in septic patients (NCT:CA209-923).
This study is unique in that it is the first to link poor clinical outcomes associated with CCI to the patient’s immunophenotypic response to sepsis. However, it is important to note that the criteria we used to define CCI differs from other utilized definitions. Whereas previous definitions heavily relied on the presence of respiratory failure requiring prolonged mechanical ventilation (34–37), our definition was more comprehensive in terms of organ dysfunction. We felt the definition for CCI needed incorporate both ICU resource utilization, and a more broad representation of organ dysfunction which includes neurologic, renal, and cardiovascular dysfunction, among others, since non-pulmonary organ dysfunction, such as acute and chronic kidney disease, have been linked to inflammation, immunosuppression, protein catabolism and poor long-term outcomes (38). Our definition also incorporates the chronicity of illness associated with previous definitions which is supported by past investigations that found that patients with ICU LOS >14 days demonstrated higher in-patient mortality, greater complication rates, worse discharge dispositions, and early genomic differences of blood leukocytes when compared to those who experienced a more rapid recovery (24, 25, 39).
There are a number of limitations of this study that require acknowledgement and discussion. We enrolled septic patients exclusively from surgical ICUs composed primarily of trauma, emergency general surgery, and non-cardiac elective surgery patients. While we suspect the underlying mechanisms of CCI to be similar across critically ill populations, surgical patients are somewhat unique in that their complicated clinical course is characterized by recurrent inflammatory insults which likely the nidus of the persistent dysregulated immune response seen in these patients. Additionally, non-surgical patients who develop sepsis in the community are generally older with advanced co-morbidities, so the incidence of CCI and its underlying phenotype in other septic patient populations will require additional investigation. Second, these studies primarily focused on the patient’s in-hospital experience, examining factors that can predict and ultimately define CCI and PICS. While we report inpatient hospital disposition, the long-term outcomes of CCI and PICS remain unclear. We currently are conducting a prospective longitudinal cohort study to assess long-term outcomes (disposition, functional status, physical performance, cognitive ability and 1-year mortality) of these patients (NCT02276417). We conducted this study at a quaternary, academic medical and Level 1 trauma center that has well-established implementation of standardized screening and evidenced-based sepsis management protocols. Whether these results are applicable across the world-wide spectrum of intensive care centers, where sepsis management is more variable, is not known. Finally, in the future, additional analyses such as functional assays and indirect calorimetry will be required to supplement the biomarker data and validate PICS in CCI patients.
In conclusion, while inpatient mortality from surgical sepsis continues to decrease, there is a growing proportion of patients that survive to develop the morbid trajectory of CCI. Over half of patients with sepsis in surgical ICUs will develop CCI, and those that do, have discharge dispositions known to be associated with poor long-term outcomes. These patients exhibit biomarker profiles consistent with an immunocatabolic phenotype of persistent inflammation, immune suppression and protein catabolism. Therefore, it is likely that a multimodal therapeutic approach including interventions such as physical therapy, nutritional intervention, and targeted immunotherapy is required to change the trajectory of CCI to one of recovery.
Supplementary Material
Acknowledgments
Supported, in part, by National Institute of General Medical Sciences grants: R01 GM-040586 and R01 GM-104481 (LM), R01 GM-113945 (PE), and P50 GM-111152 (FM, SB, LM, PE, BB, MS, AB) awarded by the National Institute of General Medical Sciences (NIGMS). In addition, this work was supported, in part, by a postgraduate training grant T32 GM-008721 (JS, JM, TL) in burns, trauma, and perioperative injury by NIGMS. All authors report no conflict of interest.
The authors would like to acknowledge the staff of the University of Florida Sepsis and Critical Illness Research Center, and Laboratory for Inflammation Biology and Surgical Science for their invaluable contributions, including Jennifer Lanz, Ruth Davis, Jillianne Brakenridge, Ashley McCray, Bridget Baisden, Ricky Ungaro, Dina Nacionales, Marvin Dirain, Angela Avery, Tabitha Johns and Ada Malcolm.
Footnotes
Level of Evidence: Level II, prognostic
Authorship: JS, JM, SR, TL, MS, AB, AM, PE, LM, FM, and SB contributed to the conception and design of this project. JS, TO, ZW, GG, BB, LM, FM, and SB performed data analysis and interpretation. All authors (JS, JM, SR, TL, TO, ZW, GG, CL, MS, AB, BB, AM, PE, LM, FM, SB) contributed in drafting the manuscript and/or revising it critically for important intellectual content.
References
- 1.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 2.Moore LJ, McKinley BA, Turner KL, Todd SR, Sucher JF, Valdivia A, Sailors RM, Kao LS, Moore FA. The epidemiology of sepsis in general surgery patients. J Trauma. 2011;70(3):672–80. doi: 10.1097/TA.0b013e31820e7803. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–31. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43(1):3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T, Bozza FA, LaRosa SP, Ranieri VM, Angus DC. Long-Term Quality of Life Among Survivors of Severe Sepsis: Analyses of Two International Trials. Crit Care Med. 2016;44(8):1461–7. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10 Suppl):S309–15. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 10.Elliott D, Davidson JE, Harvey MA, Bemis-Dougherty A, Hopkins RO, Iwashyna TJ, Wagner J, Weinert C, Wunsch H, Bienvenu OJ, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42(12):2518–26. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 11.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;371(3):287–8. doi: 10.1056/NEJMc1406274. [DOI] [PubMed] [Google Scholar]
- 12.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–54. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal MD, Moore FA. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surg Infect (Larchmt) 2016;17(2):167–72. doi: 10.1089/sur.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, Gentile LF, Nacionales DC, Cuenca AL, Bihorac A, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–9. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, Brumback BA, Bihorac A, Segal MS, Anton SD, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. doi: 10.1136/bmjopen-2016-015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft CA, Moore FA, Efron PA, Marker PS, Gabrielli A, Westhoff LS, Lottenberg L, Jordan J, Klink V, Sailors RM, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76(2):311–7. doi: 10.1097/TA.0000000000000121. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 18.Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. J Subst Abuse Treat. 2011;40(3):215–23. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 21.Long CL, Birkhahn RH, Geiger JW, Betts JE, Schiller WR, Blakemore WS. Urinary excretion of 3-methylhistidine: an assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism. 1981;30(8):765–76. doi: 10.1016/0026-0495(81)90022-6. [DOI] [PubMed] [Google Scholar]
- 22.Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, Bellomo R. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–73. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 23.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, Yende S, Carson SS ProVent Study Group I. The epidemiology of chronic critical illness in the United States*. Crit Care Med. 2015;43(2):282–7. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–9. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakkarainen TW, Arbabi S, Willis MM, Davidson GH, Flum DR. Outcomes of Patients Discharged to Skilled Nursing Facilities After Acute Care Hospitalizations. Ann Surg. 2016;263(2):280–5. doi: 10.1097/SLA.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–7. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesotten D, Van den Berghe G. Changes within the growth hormone/insulin-like growth factor I/IGF binding protein axis during critical illness. Endocrinol Metab Clin North Am. 2006;35(4):793–805. ix–x. doi: 10.1016/j.ecl.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Ross R, Miell J, Freeman E, Jones J, Matthews D, Preece M, Buchanan C. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf) 1991;35(1):47–54. doi: 10.1111/j.1365-2265.1991.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 31.Van den Berghe G, de Zegher F, Lauwers P, Veldhuis JD. Growth hormone secretion in critical illness: effect of dopamine. J Clin Endocrinol Metab. 1994;79(4):1141–6. doi: 10.1210/jcem.79.4.7962286. [DOI] [PubMed] [Google Scholar]
- 32.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18(3):461–76. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 35.Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, Chai E, Nelson JE. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA. 2016;316(1):51–62. doi: 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahn JM, Werner RM, David G, Ten Have TR, Benson NM, Asch DA. Effectiveness of long-term acute care hospitalization in elderly patients with chronic critical illness. Med Care. 2013;51(1):4–10. doi: 10.1097/MLR.0b013e31826528a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacIntyre NR. Ventilator-associated pneumonia: the role of ventilator management strategies. Respir Care. 2005;50(6):766–72. discussion 72–3. [PubMed] [Google Scholar]
- 38.Feng L, Yap KB, Yeoh LY, Ng TP. Kidney function and cognitive and functional decline in elderly adults: findings from the Singapore longitudinal aging study. J Am Geriatr Soc. 2012;60(7):1208–14. doi: 10.1111/j.1532-5415.2012.04043.x. [DOI] [PubMed] [Google Scholar]
- 39.Cuenca AG, Gentile LF, Lopez MC, Ungaro R, Liu H, Xiao W, Seok J, Mindrinos MN, Ang D, Baslanti TO, et al. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit Care Med. 2013;41(5):1175–85. doi: 10.1097/CCM.0b013e318277131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.