Abstract
The presence of the vitamin D receptor in mammary gland and breast cancer has been recognized since the early 1980s, and multiple pre-clinical studies have demonstrated that its ligand 1,25D modulates normal mammary gland development and sensitivity to carcinogenesis. Although studies have characterized many 1,25D responsive targets in normal mammary cells and in breast cancers, validation of relevant targets that regulate cell cycle, apoptosis, autophagy and differentiation, particularly in vivo, has been challenging. Vitamin D deficiency is common in breast cancer patients and some evidence suggests that low vitamin D status enhances the risk for disease development or progression. Model systems of carcinogenesis have provided evidence that both VDR expression and 1,25D actions change with transformation but clinical data regarding vitamin D responsiveness of established tumors is limited and inconclusive. Because breast cancer is heterogeneous, analysis of VDR actions in specific molecular subtypes of the disease is necessary to clarify the conflicting data. Genomic, proteomic and metabolomic analyses of in vitro and in vivo model systems is also warranted to comprehensively understand the network of vitamin D regulated pathways in the context of breast cancer heterogeneity.
Keywords: Vitamin D, breast cancer, mammary gland, VDR
I. PRE-CLINICAL STUDIES ON VITAMIN D AND BREAST CANCER
VDR expression and effects of vitamin D in breast cancer cells
Expression of VDR in breast tissue
In 1980, Colston and Feldman (1) were the first to demonstrate VDR expression, as identified by specific 1,25D binding, in normal breast tissue. Subsequent studies verified that the majority of breast tumors and established breast cancer cell lines retained some VDR expression (2–4). Although some early studies suggested that the presence of tumor VDR independently correlated with better prognosis in breast cancer patients, the cumulative data remains inconclusive (5).
In vitro effects of VDR activation on breast cancer cells
Studies to determine the effect of VDR activation in breast tumor cells have clearly demonstrated inhibition of cell cycle, induction of cell death and (in some model systems) induction of differentiation (6; 7). Detailed analysis of the cell death process revealed that both apoptosis and autophagy are induced by 1,25D and/or synthetic VDR agonists in breast cancer cells in vitro (8–10). In co-treatment paradigms, the VDR analog EB1089 promoted autophagy in combination with irradiation of several breast cancer cell lines, including models of radioresistance such as Hs578T cells (9; 11). More recent follow-up of these data indicated that 1,25D-mediated autophagy is triggered in luminal but not basal-like breast cancer cells (12). These authors used The Cancer Genome Atlas (TCGA) breast dataset to demonstrate that the 1,25D specific autophagy profile was present in the normal mammary gland but was lost upon breast cancer progression. Collectively, these data clearly demonstrate that 1,25D modulates cell cycle, apoptosis and autophagy in VDR positive breast cancer cells.
Effects of VDR agonists on tumor growth in xenograft models
Translation of these in vitro observations to in vivo model systems was initially achieved through the use of synthetic vitamin D analogs that act as VDR agonists. In both chemically induced rat breast carcinomas and human breast cancer cell lines grown as xenografts, treatment with VDR agonists reduced tumor growth in the absence of hypercalcemia (13). Although these synthetic vitamin D analogs were generally more potent in early stage disease models (ie, MCF7 xenografts), effects were also demonstrated in estrogen independent tumor models including MCF-7 cells selected for anti-estrogen resistance (14) and mesenchymal-like SUM159PT cells (15). Quantitative histology of tumor sections demonstrated that these treatments induced cell cycle arrest and apoptosis, confirming that the in vitro results could be recapitulated in vivo. The physiological relevance of these observations was demonstrated in VDR null mice, which exhibited enhanced proliferation in response to hormonal surges during puberty and pregnancy and reduced apoptosis during post-lactation involution (16; 17). More recent studies have utilized dietary vitamin D manipulations to demonstrate that breast tumor growth is sensitive to vitamin D status (18; 19). With respect to VDR mediated autophagy in vivo, Tavera-Mendoza et al (12) demonstrated that dietary vitamin D supplementation enhanced basal rates of autophagy in the mouse mammary gland.
VDR in tumor cells is necessary and sufficient for tumor suppression
Formal demonstration that VDR is necessary and sufficient for the tumor suppressive effects of vitamin D was achieved with mammary tumor cells derived from Vdr null mice (20; 21). Cells derived from wild-type mice expressed Vdr and exhibited cell cycle arrest, apoptosis and inhibition of invasion in response to 1,25D. In contrast, cells derived from VDR null mice were completely unresponsive to 1,25D in vitro. These cell lines were grown as xenografts in immunocompromised mice to determine whether Vdr in the tumor cells was necessary and sufficient for tumor suppression in vivo. In these experiments, tumor bearing mice were administered a VDR agonist (EB1089) or exposed to ultraviolet radiation (UVR) which increased serum 25D two-fold. Both interventions significantly reduced growth of xenografts derived from wild type (Vdr positive) cells but not of xenografts derived from Vdr null cells. These data suggest that VDR agonists and serum 25D suppress tumor growth via direct effects on Vdr in tumor cells rather than systemically or indirectly in accessory cells of the tumor microenvironment (such as immune cells, adipocytes or endothelial cells) which in this model originate from the normal host mice and thus express functional VDR.
Impact of the vitamin D pathway on mammary tumorigenesis in transgenic models
Transgenic mouse models of mammary carcinogenesis typically rely on the mouse mammary tumor virus (MMTV) promoter to drive expression of transgenes that act as oncogenes. The MMTV promoter induces expression in hormone responsive mammary epithelial cells during puberty as estrogen and progestin surge. It is important to note that tumors induced in these models arise from hormone responsive cells but in some cases the resultant tumors become estrogen independent. Specific transgenic mammary tumors that have been demonstrated to be altered by vitamin D (either via dietary manipulation or VDR agonist treatment) are summarized in Table 1 (22–28). Also included are studies which reported that tumor outcomes were affected when tumor prone mice were crossed onto the Vdr null (25; 26) or Cyp27b1 null (27) backgrounds. From these studies, it became evident that vitamin D mediated tumor suppression pathways can be triggered in many distinct mammary tumor models, some of which closely mimic human breast cancers. These studies also demonstrated that tumors accumulated both 25D and 1,25D (27; 28) and that deletion of either Vdr or Cyp27b1 enhanced tumorigenesis (25; 26).
TABLE 1.
Summary of studies that have examined the impact of the vitamin D pathway in transgenic models of breast cancer.
| Model | Study Description | Outcome | Reference |
|---|---|---|---|
| bLHβ-CTP mice: Mammary hyperplasia and spontaneous tumors develop in response to chronic, systemic LH production | Effect of short term treatment of tumor bearing mice with EB1089 on proliferation and tumor burden. | LH-driven tumors had high Vdr expression. EB1089 inhibited tumor cell proliferation and reduced tumor burden in ~50% of treated mice. | Milliken et al, 2002 (23) |
| MMTV-Neu mice: Mammary tumors develop in response to targeted expression of Neu oncogene (models HER2 positive human breast cancer) | MMTV-Neu mice were crossed with Vdr null mice. Ductal morphology, pre-neoplastic lesions and tumor burden were evaluated. | High expression of Vdr in MMTV-Neu tumors and lung metastatic foci. Abnormal ductal morphology in Vdr-null and Vdr-Het mice. . Increased tumor incidence in Vdr-Het vs. Vdr-WT mice on MMTV-Neu background. | Zinser et al, 2004 (26) |
| MMTV-Neu mice were treated with VDR agonist BXL0124 | BXL0124 decreased tumor weight, incidence and multiplicity and inhibited ErbB2, Erk and Akt signaling. | Lee et al, 2010 (24) | |
| MMTV-Neu mice were treated with BXL0124 ± CDDO-Im (synthetic triterpenoid) either before or after tumor onset. | In prevention protocol, both BXL0124 and CDDO-Im delayed tumor development but the combination was most effective. In the therapeutic protocol, administration of the combination did not reduce tumor burden. | So et al, 2013 (22) | |
| MMTV-Ron mice: Metastatic mammary tumors develop in response to Ron oncogene expression. | MMTV-Ron mice were crossed with Vdr null mice. Hyperplasia, tumor burden and β-catenin signaling were evaluated. | Vdr deletion enhanced Ron-mediated mammary hyperplasia, tumor burden and metastasis to lungs and liver. β-catenin signaling was elevated in Vdr ablated tumors. | Johnson et al, 2015 (25) |
| MMTV-PyMT mice: Rapid onset mammary tumors that metastasize to lung. Develop in response to targeted expression of polyoma middle T antigen. | Tumorigenesis was evaluated in MMTV-PyMT mice fed low (25IU/kg) vs standard (1000IU/kg) vitamin D diets and in mice perfused with 25D or 1,25D. Tumor vitamin D metabolites were measured. | Low dietary vitamin D accelerated tumorigenesis relative to standard diet. Systemic perfusion with 25D or 1,25D delayed tumorigenesis and decreased lung metastasis. Both 25D and 1,25D were detected in tumors. | Rossdeutscher et al, 2015 (28) |
| Tumor development was evaluated in MMTV-PyMT mice with mammary-specific deletion of Cyp27b1. | Targeted ablation of Cyp27b1 in MMTV-PyMT mice accelerated mammary hyperplasia and tumorigenesis. NfKB and JAK-STAT signaling were increased in Cyp27b1 ablated tumors. Cyp27b1 ablation reduced tumor 1,25D level. | Li et al, 2016 (27) |
Impact of the vitamin D pathway on metastatic spread of mammary tumors
Multiple approaches have been employed to examine the impact of vitamin D signaling on breast cancer metastasis. Early studies indicated that VDR agonists not only reduced primary tumor growth but also suppressed metastatic spread (15). In a transgenic model (MMTV-RON mice), deletion of VDR was associated with more extensive metastasis to liver and lung (25), an effect driven by activation of β-catenin. Using a xenograft model of breast cancer metastasis to bone, Ooi et al (29) demonstrated that osteolytic lesions appeared earlier and were significantly larger in vitamin D-deficient compared to vitamin D-sufficient mice. Inhibition of metastatic growth in bone was attributed to actions of vitamin D on tumor cells as well as effects on the bone microenvironment. Zhang et al (30) utilized clinical tissue from human breast cancers and a syngeneic mouse model of breast cancer with differential metastasis to demonstrate that VDR expression negatively correlated with metastasis. In addition, this group reported that overexpression of VDR in tumor cells reduced metastatic growth, an effect which was mediated through inhibition of TNFα release from tumor-associated macrophages.
Effects of vitamin D on human breast cancer slices ex vivo
Two groups have independently profiled the effects of 1,25D on global gene expression profiles in human breast cancer explants which better mimic the heterogeneity and complexity of the tumor microenvironment. Milani et al (31; 32) identified genes regulated by low (0.5nM) and high (100nM) concentrations of 1,25D and confirmed four of these (CYP24A1, CLMN, EFTUD1, SERPINB1) as up-regulated within 24h of exposure. Subsequently, Sheng et al (33) performed similar studies with both tumor and adjacent normal tissue from breast cancer patients as well as breast tissue from healthy women. Remarkably, the same four genes (CYP24A1, CLMN, EFTUD1 and SERPINB1) were found to be consistently up-regulated by 1,25D in this second study. In a correlative study, higher expression of two of these genes (CLMN and EFTUD1) was associated with significantly improved patient survival, suggesting that induction of these target genes by endogenous vitamin D signaling could be relevant in the context of tumor suppression.
Summary of pre-clinical studies
It is clear from cell, molecular and animal studies that the vitamin D endocrine system is expressed and functional during normal breast development (for more details there are a number of recent reviews (5; 6; 34) on this subject). Furthermore, activation of VDR has consistently been demonstrated to lead to tumor suppression in breast cancer cells and tumors (cell cycle arrest, apoptosis, autophagy, etc). Although systemic effects (ie, on obesity) and paracrine signaling (ie, via cancer cells and immune cells) may contribute to tumor suppression, both VDR and CYP27B1 can independently mediate local effects directly in tumor cells. Evidence also supports the accumulation of vitamin D metabolites (25D and 1,25D) within tumor tissue. The tumor suppressive effects of vitamin D and its receptor have been demonstrated in many different models and multiple pathways and targets have been implicated in vitamin D actions. At least a subset of the identified vitamin D regulated genes have known relevance to progression of human breast cancers. Thus, the pre-clinical data strongly supports the expectation that vitamin D status would correlate with indicators of breast cancer risk, progression and/or survival in observation and intervention studies.
II. UPDATE ON TRANSLATIONAL STUDIES
Are the tumor suppressive effects of vitamin D signaling observed in pre-clinical studies relevant to human breast cancer?
Hundreds of observational and clinical studies have addressed the possibility that vitamin D status alters development or progression of breast cancer. Studies have examined the presence of VDR, CYP27B1 and CYP24A1 in tumors in relation to progression and the impact of vitamin D status (as reflected by serum 25D and 1,25D, UVR exposure, dietary intakes, use of supplemental vitamin D, SNPs in vitamin D pathway genes, etc) on both development and progression of breast cancer. Readers are referred to recent reviews for details (6; 34) as only a few most recent studies will be discussed here.
UVR and breast cancer risk
As reviewed by Zamoiski et al (35), twelve published studies (10 population or cohort studies; two case controls) have assessed the relationship between UVR and breast cancer. Of these, two showed inverse relationships, three were null for all analyses and seven reported mixed results (ie, some but not all biomarkers of dermal vitamin D synthesis were correlated with risk). A major new analysis (35) examined the relationship between ambient UVR, time spent outdoors, sun sensitivity factors, and subsequent risk of first primary breast cancer using data from the United States Radiologic Technologists (USRT) study. This was the first study to prospectively examine multiple UVR related factors in a large nationwide cohort, using information on personal sun sensitivity, sun exposure over the lifetime, and detailed lifestyle factors in subjects residing in all 50 states and thus exposed to a wide range of ambient UVR. Over 36,0000 women were included, and satellite based analysis of UVR combined with lifetime residential history and sun susceptibility factors and history (skin pigmentation, eye/hair color, sunburns, use of tanning beds and sunlamps, protective clothing while outdoors, etc) were integrated over five age periods (childhood, puberty, young adult, middle age, post-menopausal). Contrary to the hypothesis that UVR generated vitamin D would reduce breast cancer risk, all analyses in this study were null, meaning there were no significant associations between any of the indices of dermal vitamin D synthesis at any age periods (or overall) with breast cancer development. As pointed out in a comment on this article by Grant (36), limitations of this study included failure to assess sunscreen use and possible confounders such as obesity and diabetes. The authors acknowledge that the focus of their study was UVR and not vitamin D, and that no attempts were made to assess dietary or supplemental vitamin D or serum 25D (37). Another limitation is that the heterogeneity of breast cancer was not taken into account, which could mask a UVR-vitamin D link if the effects of vitamin D are restricted to, or stronger in, specific subtypes of the disease.
Serum 25D and breast cancer risk
Although many studies have addressed the relationship between serum 25D and breast cancer risk, there is no clear consensus on the optimal serum level of 25D (if any) associated with the lowest risk for breast cancer. Although widely considered to be the best indicator of vitamin D status and the most relevant metabolite with respect to tissue actions, most studies measure total 25D (as opposed to free 25D) and fail to assess the known genetic variations that affect 25D. Typically serum 25D is assessed in a single sample which if necessary is corrected for seasonality, a non-trivial process (38). No human cancer studies have assessed tissue levels of vitamin D metabolites. Despite these caveats, several studies (particularly case-control studies) have reported inverse correlations between serum 25D and breast cancer risk, especially for post-menopausal cases. One of these widely cited case-control studies was a 2005 analysis of the Nurses Health Study, a cohort of >120,000 nurses (39). Analysis of 25D in stored blood samples from over 700 cases and 700 controls indicated that women in the highest quintile had a breast cancer relative risk of 0.73 compared to women in the lowest quintile, but this difference did not reach statistical significance. In 2016 this cohort was re-evaluated, now with 1506 cases and 1506 matched controls. Uniquely, this study included two blood samples collected 10 years apart (allowing an assessment of timing of vitamin D status) and a 20 year follow-up. Among all cases, plasma 25D levels [top (32.7 ng/mL) vs. bottom (<17.5 ng/mL) quintile] were not significantly associated with breast cancer risk. Estimates were similar for cases diagnosed less than 10 years and 10–20 years after blood collection. Stratification for the season of blood collection revealed that women with the lowest serum 25D in summer did have a significantly higher risk of breast cancer. The authors speculate that women who were deficient in summer were more likely to be deficient year round, further enhancing their risk of breast cancer relative to those who were deficient only in winter. This data highlights the need for a better understanding of the importance of seasonal variations in vitamin D status. The study also included extensive assessment of tumor prognostic and histologic indices (ER status, cancer subtype, lymph node status, tumor size, etc.), but no significant correlations with serum 25D for any of these biomarkers were found.
Serum 25D and breast cancer survival
The latest study to assess vitamin D status in relation to breast cancer survival was a large prospective analysis of the Pathways cohort (40). This cohort was established within the Kaiser Permanente health care delivery system in northern California. Women with a diagnosis of incident invasive breast cancer were typically enrolled within 2 months of diagnosis. In the 1666 members of this cohort who developed breast cancer, higher serum 25D was strongly associated with superior prognosis. Women with higher levels of 25D had better overall survival, and in premenopausal women, also better breast cancer specific survival (BCSS), relapse free survival (RFS) and invasive disease free survival (IDFS). There were inverse associations of 25D with tumor stage and tumor grade, and among premenopausal women 25D concentrations were the lowest in women with triple negative breast cancer, an aggressive subtype of the disease. Premenopausal women with the highest 25D levels had significantly reduced odds of triple negative tumors compared with those in the lowest third. These data highlight the importance of vitamin D status in breast cancer survivors as well as the heterogeneity of disease associations with serum 25D.
III. UNDERSTANDING THE FREQUENT DISCONNECTS BETWEEN PRE-CLINICAL AND TRANSLATIONAL STUDIES
The importance of breast cancer heterogeneity
It is well appreciated that human breast cancer can be classified into several subtypes with different incidences, treatment strategies and prognoses. As noted above in the Pathways study, vitamin D status may be particularly important for survival of women with triple negative breast cancer, an aggressive form of the disease with few effective treatment options. Although the genesis of the multiple subtypes of breast cancer remains to be elucidated, it is likely that they arise from distinct epithelial cell types normally present in the breast. The two major types of epithelial cells are the basal (myo-epithelial) and luminal (ductal and lobular) cells, both of which arise from a common mammary stem cell. Although little is known about vitamin D actions in specific breast cell lineages, recent studies have provided some insight into the expression of the vitamin D pathway during the transition of mammary stem cells into basal and luminal cells. Lim et al (41) conducted unbiased transcriptomic analysis of sorted populations of mammary stem cells, luminal progenitor cells and mature luminal cells from both mouse and human breast tissue to identify unique gene signatures associated with each cell type. Surprisingly, CYP24A1 was one of only three genes demonstrated to be uniquely expressed in the luminal progenitor cell type isolated from both human and mouse breast tissue. This was the first data to suggest that a gene that encodes a vitamin D related protein was present (and presumably functional) during mammary cell lineage development.
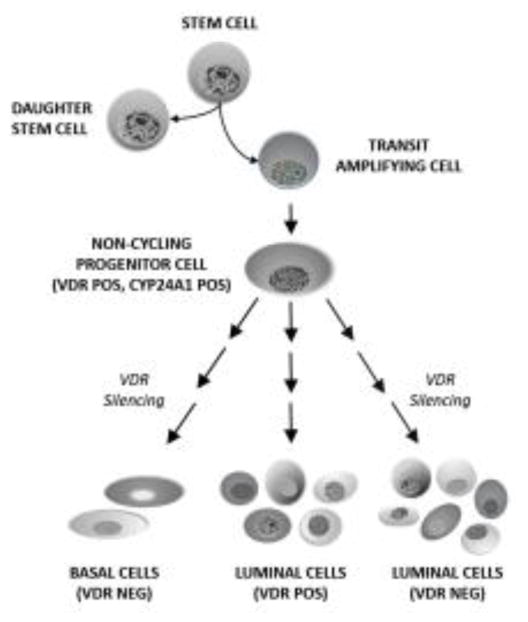
A related study by an independent group focused on identification of proteins that are differentially expressed in basal and luminal cell types in the normal human breast (42). Of fifteen hormone receptors examined, only three (ER, VDR, AR) were found to have bimodal expression in basal versus luminal cells. Multiplex immunofluorescence was therefore used to characterize ER, VDR and AR expression in >15,000 normal breast cells, leading to the identification of eleven differentiation states for luminal cells and two for basal cells. Co-expression of cytokeratins and proliferation markers was used to develop a hierarchy of these cellular differentiation states in relation to hormone receptor expression (43). Regarding expression of the VDR protein, several important observations were made. First, VDR positive cells were always negative for the proliferation marker Ki67, consistent with the concept that VDR signaling is anti-proliferative. Second, VDR was not expressed in the earliest progenitor (transit amplifying) cells but was acquired in post-mitotic progenitor cells, consistent with the cell type shown to express CYP24A1 in the Lim et al study (41). Third, of the eleven distinct mature luminal cell types, five expressed VDR (either as the only hormone receptor or in association with AR, ER or both). This finding suggests that VDR becomes repressed in a percentage of luminal progenitors as they transition into distinct subsets of differentiated luminal epithelial cells. The mechanism of VDR repression is unknown, but the implication is that breast cancers derived from these lineages would be expected to be VDR negative and insensitive to vitamin D status. Fourth, mature myoepithelial cells did not express VDR and it was suggested that loss of VDR expression in the post-mitotic progenitor cells might be permissive for myoepithelial cell differentiation. These concepts are incorporated into the schematic shown in Figure 1. It is important to note that the cell types identified in normal breast were found to be recapitulated in breast cancers, and that patient survival data demonstrated that women whose tumors expressed all three hormone receptors (ER, AR and VDR) had the best prognosis.
Figure 1. Model depicting VDR expression during mammary stem cell lineage determination.
Mammary stem cells are capable of asymmetric division which generates daughter stem cells and transit amplifying cells. The transit amplifying cells proliferate slowly and give rise to non-cycling progenitor cells, the earliest cell type in this hierarchy that has been demonstrated to express VDR. This cell population (or a slightly later VDR positive progenitor cell) also expresses CYP24A1. Both basal and luminal cell populations originate from the VDR positive progenitor cell. In the course of basal cell differentiation VDR expression is silenced. During luminal cell differentiation both VDR positive and VDR negative cells arise. Eleven luminal cell populations have been identified based on expression of cytokeratins and hormone receptors (ER, AR, VDR). Five of the eleven luminal cell populations express VDR. Any of the cell populations shown here could theoretically give rise to breast cancer, leading to extraordinary heterogeneity in VDR expression. Schematic is based on data from refs 41–43; see text for additional details.
Deregulation of VDR during carcinogenesis
In addition to the heterogeneity of VDR expression in normal breast epithelial cells, changes in the function and expression of this receptor likely occurs during the initiation and progression of breast cancer. Through analysis of VDR expression in normal and breast cancer cells using ENCODE data, Saccone et al (44) reported considerable VDR heterogeneity at the transcript level. Fourteen distinct VDR transcripts were identified in ENCODE datasets and these have been sequenced verified from various cell types. These transcripts arise from four different promoters, some of which are tissue specific. In breast cells at least four different transcripts were validated, two which are predicted to produce full length VDR and two of which are predicted to produce truncated VDRs of 395 or 36 amino acids. The functionality of such truncated VDRs is unknown. Expression of specific VDR variants was examined by Marik et al (45) who found that the levels and patterns of splice variants of VDR were markedly different in breast cancer compared to normal breast epithelial tissue. Breast cancer tissues showed extensive heterogeneity and variability, particularly in the shorter variants which are barely detectable in normal tissue. The authors also reported that the levels of full-length VDR transcripts were markedly lower in breast cancer tissue compared to normal breast tissue. This data is consistent with reports of VDR deregulation in response to oncogene expression in breast epithelial cells (46) and demonstration that explants from breast cancers are less sensitive to 1,25D than explants from adjacent normal tissue or healthy breast epithelium (33).
In addition to the transcript variants, emerging data has highlighted novel mechanisms for VDR regulation at the epi-genetic level. Marik et al (45) utilized bisulfite sequencing of the VDR promoter region to identify two CpG hypermethylated regions in breast cancer cell lines and in primary breast cancers obtained from patients. To validate the notion that VDR is methylated in breast cancer, quantitative methylation-specific PCR was performed with DNA from eight human breast cancers and seven adjacent normal breast samples. This technique confirmed highly methylated CpG islands (40–65%) in primary breast tumors but low levels in adjacent normal breast tissue (5–15%). In vitro, treatment of breast cancer cell lines with demethylating agents coordinately enhanced the expression of VDR and sensitivity to 1,25D mediated growth inhibition.
Collectively, these recent studies suggest that VDR functions during mammary cell lineage development and that its regulation in healthy human breast tissue is complex. In human breast cancers, distinct VDR transcript variants have been identified. Breast cancers also exhibit extensive VDR methylation patterns leading to receptor silencing. Thus, failure of translational studies to take the heterogeneity of VDR in breast cancer tissue into account could underestimate the effects of vitamin D status on disease occurrence. Additional studies are warranted to explore the heterogeneity of other genes in the vitamin D pathway (CYP24A1, CYP27B1) during breast cancer progression.
IV. SUMMARY
Vitamin D signaling in normal breast and in breast cancer is highly heterogeneous and incompletely understood. Data from pre-clinical studies needs to be re-interpreted in the context of this VDR heterogeneity. Depending on the specific cell type of origin, an individual’s breast tumor may be inherently vitamin D responsive or vitamin D resistant. In addition, vitamin D resistance could emerge during tumor progression through mechanisms such as VDR methylation or CYP24A1 amplification. Transgenic breast cancer models which employ the MMTV-promoter drive transgene expression in estrogen responsive luminal cells, but VDR is not expressed in all ER positive cell populations and is expressed in some ER negative luminal cell populations. Transgenic models utilizing promoters other than MMTV and/or other approaches that model the heterogeneous breast cell populations that express VDR need to be explored in the context of vitamin D signaling. In addition the relevance of cell lineage data derived from genetically inbred mouse strains to human breast tissue needs to be addressed.
Other issues which remain to be clarified regarding vitamin D and breast cancer include tissue uptake, storage and metabolism in specific breast compartments. The adipose-rich stroma surrounding the epithelial tissue expresses CYP27B1 (47; 48) and may serve as a storage pool of vitamin D3 or 25D3. Better understanding of how chronic vitamin D deficiency or seasonal variations in vitamin D status alter vitamin D metabolite pools in breast tissue is needed. Studies to determine whether there are critical windows during breast development (puberty, pregnancy or menopause for example) when vitamin D status is especially important are also warranted.
Despite the heterogeneous nature of breast tumors with respect to vitamin D signaling, exploring the concept of VDR as a therapeutic or preventive target for human breast cancer is warranted. The predicted number of new cases of breast cancer in the US exceeds 250,000 for 2017. Even if only half of these women develop tumors with functional VDR (VDR expression estimates vary from 90% in ER positive tumors to 27% in basal/triple negative tumors), approximately 125,000 women would be potentially responsive to vitamin D based therapy. Even if such therapies were only effective in patients with pre-existing deficiency, over 50,000 newly diagnosed women could conceivably benefit from vitamin D supplementation based on the 42% prevalence of deficiency (serum 25D < 50nM) reported in US women (49). Although these calculations represent rough estimates, they suggest that attention to vitamin D status could significantly impact a large percentage of women who are living with a breast cancer diagnosis.
Highlights.
Vitamin D triggers actions consistent with cancer prevention in breast cells
VDR is differentially expressed during murine mammary cell lineage determination
Genetic and epi-genetic changes in VDR may alter vitamin D responses in breast cancer
Correction of vitamin D deficiency in women with breast cancer is recommended
Acknowledgments
Work in the Welsh laboratory has been driven by multiple graduate students and fellows and funded by operating grants and fellowships from the Department of Defense Breast Cancer Research Program (BC962168, BC961128, BC980037, BC996634, BC021532, BC021506, BC050193, BC100980), the National Institutes of Health (RO1CA69700, RC1CA144963, R21CA166434, F31AT007276, RO1CA194500) and the American Institute for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colston K, Hirt M, Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980;107:1916–22. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- 2.Colston K, Wilkinson JR, Coombes RC. 1,25-Dihydroxyvitamin D3 binding in estrogen-responsive rat breast tumor. Endocrinology. 1986;119:397–403. doi: 10.1210/endo-119-1-397. [DOI] [PubMed] [Google Scholar]
- 3.Berger U, McClelland RA, Wilson P, Greene GL, Haussler MR, et al. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer research. 1991;51:239–44. [PubMed] [Google Scholar]
- 4.Buras RR, Schumaker LM, Davoodi F, Brenner RV, Shabahang M, et al. Vitamin D receptors in breast cancer cells. Breast cancer research and treatment. 1994;31:191–202. doi: 10.1007/BF00666153. [DOI] [PubMed] [Google Scholar]
- 5.Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Molecular and cellular endocrinology. 2017 doi: 10.1016/j.mce.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 7.Colston KW, Chander SK, Mackay AG, Coombes RC. Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochemical pharmacology. 1992;44:693–702. doi: 10.1016/0006-2952(92)90405-8. [DOI] [PubMed] [Google Scholar]
- 8.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. The Journal of steroid biochemistry and molecular biology. 1996;58:367–76. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, et al. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy. 2012;8:739–53. doi: 10.4161/auto.19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell death and differentiation. 2005;12:1297–309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 11.Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Hormones & cancer. 2011;2:272–85. doi: 10.1007/s12672-011-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavera-Mendoza LE, Westerling T, Libby E, Marusyk A, Cato L, et al. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E2186–e94. doi: 10.1073/pnas.1615015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139:2102–10. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 14.Nolan E, Donepudi M, VanWeelden K, Flanagan L, Welsh J. Dissociation of vitamin D3 and anti-estrogen mediated growth regulation in MCF-7 breast cancer cells. Molecular and cellular biochemistry. 1998;188:13–20. [PubMed] [Google Scholar]
- 15.Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. The Journal of steroid biochemistry and molecular biology. 2003;84:181–92. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Molecular endocrinology (Baltimore, Md) 2004;18:2208–23. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 17.Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development (Cambridge, England) 2002;129:3067–76. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 18.Swami S, Krishnan AV, Williams J, Aggarwal A, Albertelli MA, et al. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocrine-related cancer. 2016;23:251–64. doi: 10.1530/ERC-15-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong Y, Swami S, Krishnan AV, Williams JD, Martin S, et al. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Molecular cancer therapeutics. 2015;14:1951–61. doi: 10.1158/1535-7163.MCT-15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Molecular and cellular endocrinology. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 21.Valrance ME, Brunet AH, Welsh J. Vitamin D receptor-dependent inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology. 2007;148:4887–94. doi: 10.1210/en.2007-0267. [DOI] [PubMed] [Google Scholar]
- 22.So JY, Wahler JE, Yoon T, Smolarek AK, Lin Y, et al. Oral administration of a gemini vitamin D analog, a synthetic triterpenoid and the combination prevents mammary tumorigenesis driven by ErbB2 overexpression. Cancer prevention research (Philadelphia, Pa) 2013;6:959–70. doi: 10.1158/1940-6207.CAPR-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milliken EL, Zhang X, Flask C, Duerk JL, MacDonald PN, Keri RA. EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer letters. 2005;229:205–15. doi: 10.1016/j.canlet.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, So JY, DeCastro A, Smolarek A, Paul S, et al. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. The Journal of steroid biochemistry and molecular biology. 2010;121:408–12. doi: 10.1016/j.jsbmb.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson AL, Zinser GM, Waltz SE. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of beta-catenin activity. Oncotarget. 2015;6:16304–20. doi: 10.18632/oncotarget.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25:2361–72. doi: 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Luco AL, Ochietti B, Fadhil I, Camirand A, et al. Tumoral Vitamin D Synthesis by CYP27B1 1-alpha-Hydroxylase Delays Mammary Tumor Progression in the PyMT-MMTV Mouse Model and Its Action Involves NF-kappaB Modulation. Endocrinology. 2016;157:2204–16. doi: 10.1210/en.2015-1824. [DOI] [PubMed] [Google Scholar]
- 28.Rossdeutscher L, Li J, Luco AL, Fadhil I, Ochietti B, et al. Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast cancer. Cancer prevention research (Philadelphia, Pa) 2015;8:120–8. doi: 10.1158/1940-6207.CAPR-14-0110. [DOI] [PubMed] [Google Scholar]
- 29.Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer research. 2010;70:1835–44. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Guo Q, Zhang Z, Bai N, Liu Z, et al. VDR status arbitrates the prometastatic effects of tumor-associated macrophages. Molecular cancer research : MCR. 2014;12:1181–91. doi: 10.1158/1541-7786.MCR-14-0036. [DOI] [PubMed] [Google Scholar]
- 31.Milani C, Katayama ML, de Lyra EC, Welsh J, Campos LT, et al. Transcriptional effects of 1, 25 dihydroxyvitamin D(3) physiological and supra-physiological concentrations in breast cancer organotypic culture. BMC cancer. 2013;13:119. doi: 10.1186/1471-2407-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milani C, Welsh J, Katayama ML, Lyra EC, Maciel MS, et al. Human breast tumor slices: a model for identification of vitamin D regulated genes in the tumor microenvironment. The Journal of steroid biochemistry and molecular biology. 2010;121:151–5. doi: 10.1016/j.jsbmb.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 33.Sheng L, Anderson PH, Turner AG, Pishas KI, Dhatrak DJ, et al. Identification of vitamin D3 target genes in human breast cancer tissue. The Journal of steroid biochemistry and molecular biology. 2016;164:90–7. doi: 10.1016/j.jsbmb.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Frontiers in physiology. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamoiski RD, Freedman DM, Linet MS, Kitahara CM, Liu W, Cahoon EK. Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environmental research. 2016;151:419–27. doi: 10.1016/j.envres.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant WB. Re: Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environmental research. 2017;152:517–8. doi: 10.1016/j.envres.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Zamoiski RD, Linet MS, Cahoon EK. Response. Environmental research. 2017;152:519. doi: 10.1016/j.envres.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Ahn J, Yu K. Comparing statistical methods for removing seasonal variation from vitamin D measurements in case-control studies. Statistics and its interface. 2011;4:85–93. doi: 10.4310/SII.2011.v4.n1.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 40.Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, et al. Association of Serum Level of Vitamin D at Diagnosis With Breast Cancer Survival: A Case-Cohort Analysis in the Pathways Study. JAMA oncology. 2017;3:351–7. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast cancer research : BCR. 2010;12:R21. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santagata S, Thakkar A, Ergonul A, Wang B, Woo T, et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. The Journal of clinical investigation. 2014;124:859–70. doi: 10.1172/JCI70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santagata S, Ince TA. Normal cell phenotypes of breast epithelial cells provide the foundation of a breast cancer taxonomy. Expert review of anticancer therapy. 2014;14:1385–9. doi: 10.1586/14737140.2014.956096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561:171–80. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Marik R, Fackler M, Gabrielson E, Zeiger MA, Sukumar S, et al. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer biology & therapy. 2010;10:44–53. doi: 10.4161/cbt.10.1.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemmis CM, Welsh J. Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. Journal of cellular biochemistry. 2008;105:980–8. doi: 10.1002/jcb.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson AL, Zinser GM, Waltz SE. Loss of vitamin D receptor signaling from the mammary epithelium or adipose tissue alters pubertal glandular development. American journal of physiology. Endocrinology and metabolism. 2014;307:E674–85. doi: 10.1152/ajpendo.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D(3) and signal via vitamin D(3) receptor, modulating mammary epithelial cell growth. Journal of cellular biochemistry. 2011;112:3393–405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutrition research (New York, NY) 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]