Abstract
Background:
Recent studies demonstrated that B cells and their chemoattractants are elevated in the nasal mucosa of patients with chronic rhinosinusitis (CRS) with nasal polyposis (CRSwNP). However, the presence of naive B cells and of plasmablasts and memory B-cell subsets in the mucosa and periphery of the same patient with CRS is yet to be characterized.
Objective:
Here we sought to quantify naive, plasmablasts, and memory B cells in mucosal tissue and peripheral blood of patients with CRSwNP, patients with CRS without nasal polyps (CRSsNP), and control patients.
Methods:
Polyps, mucosa, and peripheral blood samples were prospectively collected from the patients with CRS and from the non-CRS controls. We used flow cytometry to distinguish among naive, plasmablast, and memory B cells in sinus tissue and peripheral blood.
Results:
A total of 45 patients were recruited for the study. The patients with CRSwNP had significantly increased mucosal B-cell numbers versus the controls (3.39 ± 4.05% versus 0.39 ± 1.05% of live cells; p < 0.01, Kruskal-Wallis test), which included naive B cells (0.61 ± 0.94 versus 0.11 ± 0.24% of live cells; p < 0.03, Kruskal-Wallis test), plasmablasts (0.06 ± 0.26 versus 0.00 ± 0.00% of live cells; p < 0.055, Kruskal-Wallis test), and memory B cells (0.62 ± 1.26 versus 0.05 ± 0.15% of live cells; p < 0.02, Kruskal-Wallis test).
Conclusion:
Our study identified increased frequencies of different B-cell subtypes in the mucosa of patients with CRSwNP but not in the peripheral blood. We also found that patients with CRSwNP had significantly increased B-cell subtypes compared with the patients with CRSsNP and the controls. These results implied a potential role for mucosal B cells in the ongoing inflammation in patients with CRSwNP.
Keywords: Chronic rhinosinusitis, flow cytometry, nasal polyps, B cells
B cells play a fundamental role in the adaptive immune response at mucosal surfaces. Once activated, naive B cells develop into antibody-secreting plasmablasts, plasma cells, or memory B cells.1,2 The initial and rapid B-cell antibody responses are dominated by plasmablasts located in the peripheral immune organs. These plasmablasts undergo clonal expansion, which leads to the generation of large amounts of terminally differentiated short-lived antibody-producing plasmablast cells.3 These plasmablast cells are able to differentiate further into long-lived plasma cells that continue to produce antibodies long term and are capable of increased survival and circulation throughout the body.4 Memory B cells are long-surviving cells, which, on secondary encounter with the same antigen to which the naive B cells were exposed, respond at a faster rate and with a more robust antibody response.3,5
Chronic rhinosinusitis (CRS) is an inflammatory disease characterized by a unique inflammatory microenvironment. Recent studies demonstrated that B cells and their chemoattractants are elevated in the nasal mucosa of patients with CRS with nasal polyposis (CRSwNP).6–8 Patients with CRSwNP have been documented to have elevated local immunoglobulin E (IgE) antibodies as well as autoantibodies in polyps; however, the study of naive B cells, plasmablasts, and memory B-cell subsets within the same patient with CRS is yet to be elucidated.6,9,10 By using flow cytometry in this study, we identified naive and effector B-cell subsets in the mucosa and the periphery of patients with CRSwNP, patients with CRS without nasal polyposis (CRSsNP), and the non-CRS controls.
METHODS
Patient Sample Collection
This study was approved by the human research ethics committee of the Queen Elizabeth Hospital, Adelaide, Australia. Representative tissue samples (polyps of patients with CRSwNP, and ethmoid mucosa of patients with CRSsNP and the controls) and blood specimens were prospectively collected at the time of endoscopic sinus surgery from controls, patients with CRSsNP, and patients with CRSwNP. The control patients were undergoing endoscopic sinonasal procedures for pituitary tumor resections and were without clinical or radiologic evidence of past or present sinus disease. The patients with CRS fulfilled the diagnostic criteria set out in the recent position papers by the American Academy of Otolaryngology and Head and Neck Surgery11 and the European Position Statement on Chronic Rhinosinusitis.12 The patients with CRS were further subclassified according to the absence or the presence of visible polyps within the middle meatus on nasal endoscopy. Exclusion criteria were minors, <18 years of age; pregnancy; malignancy; and immune disorders; and the use of antibiotics or oral corticosteroids in the month before surgery. All the patients provided informed written consent before enrollment. The patients were classified as atopic if they had positive radioallergosorbent test and/or skin-prick testing results. Demographic and clinical data were collected on all the patients before commencement of the study.
Cell Preparation
Tissue samples, which weighed >100 mg, were included in this study. The tissue was washed and dissected into pieces of ≤2 mm before being prepared in a single-cell suspension by enzymic digestion with 2 mg/mL collagenase type II (Sigma-Aldrich, St. Louis, MO) and 0.04 mg/mL DNAse I (Roche Applied Sciences, Vilvoorde, Belgium) for 45 minutes at 37°C. Cell suspensions were filtered through a 100-μm nylon mesh and washed in phosphate-buffered saline solution. Heparinized peripheral blood was lysed for 15 minutes by using Pharm Lyse (Becton Dickinson Biosciences, San Jose, CA).
Flow Cytometric Immunophenotyping
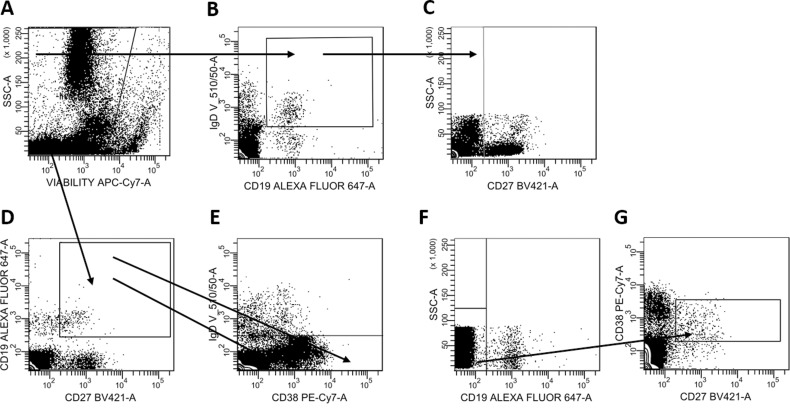
The cells were stained with Fixable Viability Dye eFluor 780 (eBioscience, San Diego, CA) at 4°C for 30 minutes to exclude dead cells before staining with the following antibodies listed in Online Supplemental Table 1. Eight-color flow cytometry was performed by using a gating strategy based on fluorescence minus one controls as specified in Figure 1. Naive B cells were defined as CD19+ CD27− IgD+; plasmablasts were defined as CD19+ CD27+ IgD− CD38high; memory B cells were defined as CD19+ CD27+ IgD− CD38−.
Figure 1.
(A) Live cells were identified and (B) CD19+ immunoglobulin D+ (IgD), (C) CD27− cells were gated to identify CD19+ CD27− IgD+ naive B cells. (D) CD19+ CD27+ cells were identified and gated on (E) IgD− CD38high, and CD38− to identify CD19+ CD27+ IgD− CD38high plasmablasts. Plasma cells were gated on (F) CD19− and (G) CD27+ CD38+.
Statistical Analysis
The data were summarized by using means ± standard deviations, and medians (ranges). The Kruskal-Wallis test was used for the comparison of data from the three independent disease groups of patients. The Mann-Whitney test was used for comparison of two independent disease groups. All tests were two-tailed, and significance was assessed at the 5% alpha.
RESULTS
Patients
Samples from a total 45 patients (16 patients with CRSwNP, 16 patients with CRSsNP, and 13 controls) were used for this study. Patient demographic information is summarized in Online Supplemental Table 2.
B Cells Were Increased in CRSwNP Mucosa
The patients with CRSwNP had significantly more CD19+ B cells in their sinus mucosal polyps versus the controls (11.9-fold increase, 3.39 ± 4.05 versus 0.39 ± 1.05% of live cells; p < 0.01, Kruskal-Wallis test) (Fig. 2). Further subclassification of total CD19+ B-cell numbers showed that all B-cell subtypes were significantly increased in tissue of patients with CRSwNP compared with the controls, which included CD19+ CD27− IgD+ naive B cells (13.3-fold increase, 0.61 ± 0.94 versus 0.11 ± 0.24% of live cells, respectively; p < 0.03, Kruskal-Wallis test) (Fig. 3 A), CD19+ CD27+ CD38high IgD− plasmablasts (0.06 ± 0.26 versus 0.00 ± 0.00, respectively; p < 0.055, Kruskal-Wallis test) (Fig. 3 B), CD19+ CD27+ CD38− IgD− memory B cells (0.62 ± 1.26 vs 0.05 ± 0.15, respectively; p < 0.02, Kruskal-Wallis test) (Fig. 3 C) in the patients with CRSwNP compared with the controls. No differences were seen in CD19+ cell numbers or B-cell subtypes in the peripheral blood of the different patient groups (Online Supplemental Fig. 1).
Figure 2.
The percentage of CD19+ cells of total live cell numbers in the mucosa of the controls, the patients with chronic rhinosinusitis without nasal polyposis (CRSsNP), and the patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). Medians (interquartile ranges). p ≤ 0.05, Kruskal-Wallis test.
Figure 3.
(A) The percentage of naive B cells, (B) plasmablasts, (C) memory B cells of total live cell numbers in the mucosa of the controls, the patients with chronic rhinosinusitis without nasal polyposis (CRSsNP), and the patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). Medians (interquartile ranges). p ≤ 0.05, Kruskal-Wallis test.
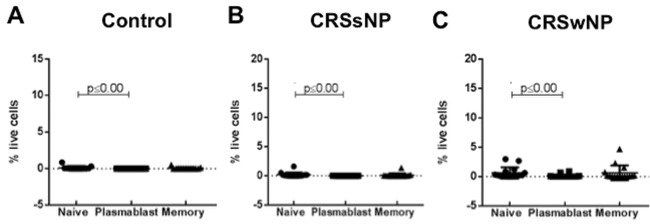
Naive B Cells Were the Most Abundant B-cell Type in Mucosal Tissue
In mucosal tissue samples from controls and patients with CRSsNP, naive B cells were the most abundant cell type. Naive B cells were significantly more abundant compared to plasmablasts in controls, CRSsNP and CRSwNP patients (Fig. 4). In the peripheral blood of the control patients and the patients with CRSsNP, there were more naive cells than plasmablasts and memory B cells (25.4- and 3.4-fold more naive cells in control and 21.8- and 8.01-fold more in CRSsNP, respectively) (Fig. 5 A and B). In the peripheral blood of the patients with CRSwNP, there were no differences seen in the different B-cell types (Fig. 5 C).
Figure 4.
(A) The percentage of naive B cells, plasmablasts and memory B cells of the total live cell numbers within mucosa of the controls, (B) the patients with chronic rhinosinusitis without nasal polyposis (CRSsNP), and (C) the patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). Medians (interquartile ranges). p ≤ 0.001, Kruskal-Wallis test.
Figure 5.
The percentage of naive B cells, plasmablasts and memory B cells of total live cell numbers within the peripheral blood of (A) the controls, (B) the patients with chronic rhinosinusitis without nasal polyposis (CRSsNP), and (C) the patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). Medians (interquartile range). p ≤ 0.001, Kruskal-Wallis test.
DISCUSSION
CRS is an inflammatory condition characterized, in part, by a dysregulated adaptive immune response. Earlier studies that used flow cytometry and immunohistochemistry found increased mucosal B-cell numbers in patients with CRSwNP.7,13,14 When using flow cytometry and a combination of lineage markers, we further defined B-cell subsets in patients with CRS and showed increased numbers of naive B cells, plasmablasts, and memory B cells in CRSwNP tissue but not in peripheral blood, which was in support of studies by Psaltis et al.14 that showed an increase in total B-cell numbers as well as different B-cell subpopulations in CRSwNP tissue compared with that of tissue in the controls.14
In addition, the B-cell cytokine B-cell activating factor, important in B-cell IgG class switching, and its receptor transmembrane activator and calcium-modulating cyclophilin ligand interactor, required for the survival of activated B cells and plasmablasts in vitro, are found elevated in patients with CRSwNP compared with healthy subjects.6,15 Most recently, a study that investigated B cells in patients with nonatopic CRSsNP found an influx of IgE-expressing plasmablasts present in the mucosa that were virtually absent in control tissue or peripheral blood.16 Our study also demonstrated that plasmablasts were increased in numbers within tissue of the patients with CRSsNP and of the patients with CRSwNP, which further indicated the presence of an ongoing active immune response in these patients.
The accumulation of B-cell activating factor and the elevation of plasma and plasmablast cells in the patients with CRSwNP supported the theory of a secondary lymphoid microenvironment, which favors the activation of naive B cells in patients with polyposis.17 Although the effector B-cell presence and antibody production have historically been thought to be protective, studies indicated that the accumulation of antibodies, such as IgA and IgG, results in the accumulation and degranulation of eosinophils, one of the main factors associated with polyp formation.18–20 Together, our finding of increased B-cell subtypes in the tissue but not in the blood of the patients with CRSwNP supported the hypothesis that there is a local immune microenvironment within the chronically inflamed sinonasal mucosa that contributes to the ongoing inflammation in CRS and, potentially, to polyp formation.
CONCLUSION
Our study identified increased frequencies of different B-cell subtypes in mucosa in the patients with CRSwNP but not in peripheral blood. These results implied a potential role for B cells in the chronic inflammation in the patients with CRSwNP.
Footnotes
This study was supported by a Conjoint grant from The Garnett Passe and Rodney Williams Memorial Foundation to P.J. Wormald and S. Vreugde
The authors have no conflicts of interest to declare pertaining to this article
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Tarlinton D. B-cell memory: Are subsets necessary? Nat Rev Immunol. 2006; 6:785–790. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005; 175:7867–7879. [DOI] [PubMed] [Google Scholar]
- 3. Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996; 381:751–758. [DOI] [PubMed] [Google Scholar]
- 4. Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006; 6:741–750. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996; 272:54–60. [DOI] [PubMed] [Google Scholar]
- 6. Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008; 121:1385–1392, 1392.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013; 131:1075–1083, 1083.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arjomandi H, Gilde J, Zhu S, et al. Relationship of eosinophils and plasma cells to biofilm in chronic rhinosinusitis. Am J Rhinol Allergy. 2013; 27:e85–e90. [DOI] [PubMed] [Google Scholar]
- 9. Gevaert P, Holtappels G, Johansson SG, et al. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005; 60:71–79. [DOI] [PubMed] [Google Scholar]
- 10. Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968, 968.e1–e6. [DOI] [PubMed] [Google Scholar]
- 11. Lin SY, Nnacheta LC. American Academy of Otolaryngology—Head and Neck Surgery Foundation (AAO-HNSF) will publish its latest “Clinical practice guideline: Allergic rhinitis (AR) in February, 2015”. Am J Rhinol Allergy. 2015; 29:82. [DOI] [PubMed] [Google Scholar]
- 12. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 23:3 p preceding table of contents. 2012; 1–298. [PubMed] [Google Scholar]
- 13. Drolet JP, Frangie H, Guay J, et al. B lymphocytes in inflammatory airway diseases. Clin Exp Allergy. 2010; 40:841–849. [DOI] [PubMed] [Google Scholar]
- 14. Psaltis AJ, Schlosser RJ, Yawn JR, et al. Characterization of B-cell subpopulations in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:621–629. [DOI] [PubMed] [Google Scholar]
- 15. Bossen C, Cachero TG, Tardivel A, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008; 111:1004–1012. [DOI] [PubMed] [Google Scholar]
- 16. Rashan AR, Goshn E, Peterson A, et al. Characterization of immunoglobulin E plasma cells that are elevated in the upper airway mucosa of nonatopic patients with chronic rhinosinusitis without nasal polyps. Int Forum Allergy Rhinol. 2016; 6:378–384. [DOI] [PubMed] [Google Scholar]
- 17. Yoon YH, Jin J, Kwon KR, et al. The role of B cell Activating Factor (BAFF) expression on pathogenesis of nasal polyp in chronic rhinosinusitis with nasal polyposis. Rhinology. 2014; 52:390–396. [DOI] [PubMed] [Google Scholar]
- 18. Abu-Ghazaleh RI, Fujisawa T, Mestecky J, et al. IgA-induced eosinophil degranulation. J Immunol. 1989; 142:2393–2400. [PubMed] [Google Scholar]
- 19. Kita H, Abu-Ghazaleh RI, Gleich GJ, Abraham RT. Regulation of Ig-induced eosinophil degranulation by adenosine 3′,5′-cyclic monophosphate. J Immunol. 1991; 146:2712–2718. [PubMed] [Google Scholar]
- 20. Shah SA, Ishinaga H, Takeuchi K. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm (Lond). 2016; 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.