Abstract
Cereal grains are the most important food source for humans. As the global population continues to grow exponentially, the need for the enhanced yield and minimal loss of agricultural crops, mainly cereal grains, is increasing. In general, harvested grains are stored for specific time periods to guarantee their continuous supply throughout the year. During storage, economic losses due to reduction in quality and quantity of grains can become very significant. Grain loss is usually the result of its deterioration due to fungal contamination that can occur from preharvest to postharvest stages. The deleterious fungi can be classified based on predominance at different stages of crop growth and harvest that are affected by environmental factors such as water activity (aw) and eco-physiological requirements. These fungi include species such as those belonging to the genera Aspergillus and Penicillium that can produce mycotoxins harmful to animals and humans. The grain type and condition, environment, and biological factors can also influence the occurrence and predominance of mycotoxigenic fungi in stored grains. The main environmental factors influencing grain fungi and mycotoxins are temperature and aw. This review discusses the effects of temperature and aw on fungal growth and mycotoxin production in stored grains. The focus is on the occurrence and optimum and minimum growth requirements for grain fungi and mycotoxin production. The environmental influence on aflatoxin production and hypothesized mechanisms of its molecular suppression in response to environmental changes are also discussed. In addition, the use of controlled or modified atmosphere as an environmentally safe alternative to harmful agricultural chemicals is discussed and recommended future research issues are highlighted.
Keywords: Aflatoxin, Aflatoxin biosynthesis gene cluster, Mycotoxin, Storage fungi, Temperature, Water activity
Cereal grains, including wheat, rice, and corn, are the hard and dry seeds typical of the family of grasses (Gramineae). Grains are one of the most important staple food sources for humans. For example, rice is the major food for Asians, sorghum and millet for Africans and Indians, wheat, rye, and barley for Europeans, and corn for Americans [1]. Historically, the ability to store and distribute food grains has been the foundation of urbanization, as urban societies depend on the effective supply of grains throughout the year. Hence, food grains must be safely stored, after harvesting at specific times of the year, to ensure supply throughout the remainder of the year [2]. The estimated global production of cereal grains in 2017 was 2,593 million metric tons (MMT). Of this amount, the production of coarse grains, wheat, and rice, was 1347, 743, and 502 MMT, respectively [3]. Owing to the growing human population, food shortages have been predicted. For instance, it was estimated that more than one billion people would be malnourished and facing hunger in 2009. Therefore, continuous enhancement of food production and minimization of crop losses are needed to meet the demands of the growing human population [4].
Increased grain production has resulted in extended grain storage period. During storage, grain deterioration by fungal contamination includes discoloration, musty odors, dry matter loss, tissue disintegration, nutritional and processing quality losses, and mycotoxin accumulation [5]. The estimated losses by different causes in cereal grains during storage vary widely. The National Academy of Science [6] estimated the minimum overall losses as around 10% worldwide, with losses of up to 50% in tropical regions. The main cause of quality loss and spoilage is the action of deleterious microorganisms, which interact among themselves, with the grain, and with the environment of the storage facilities [7]. The concept of stored-grain ecosystem is used to describe all these interrelated biotic and abiotic factors affecting the grains, with the goal of preserving the harvested grains with minimum losses in their quantity and quality [7]. A better understanding of all these interacting factors has paved way for the development of efficient grain storage systems in the last decade [8].
Historically, thousands of years ago, the ancient Egyptians were known to store harvested grains. The Bible and Quran describe the famous story of Jacob's son, Joseph, who ordered the storage of harvested grains in ancient Egypt in the years of abundance to overcome seven years of severe grain shortage. Old scriptures have also described the details of several practices used by the ancient Egyptians for protection of stored grains from pests using dusting, fumigation, volatilization, or pyrolysis of incense [9]. More recently, several methods have been developed for efficient mass storage of grains. Efforts to reduce or eliminate the use of harmful chemicals to control deleterious fungi and mycotoxin production in stored grains include the screening and selection of antagonistic bacterial strains with antifungal and anti-mycotoxin activities [10,11,12]. Environmental control by manipulation of the ecological factors at the storage facilities remains a basic and efficient measure for controlling fungal contamination in stored grains. Fungal growth on stored grains is an obvious signs of poor quality grains from the aspects of diminished sensory quality and nutritional value. Mycotoxins produced by the grain fungi also pose a health hazard to animals and humans.
In this review, we discuss (1) the different groups of fungi contaminating grains at different time periods from preharvest to postharvest stages, (2) mycotoxins and their related fungi, (3) the effects of environmental conditions such as temperature and water activity (aw) on deleterious fungi and a detailed consideration of the major mycotoxins with a focus on molecular aflatoxin suppression, and (4) controlled or modified atmosphere conditions for the management of deleterious fungi and mycotoxin production during grain storage.
CLASSIFICATION OF FUNGI CONTAMINATING STORED GRAINS
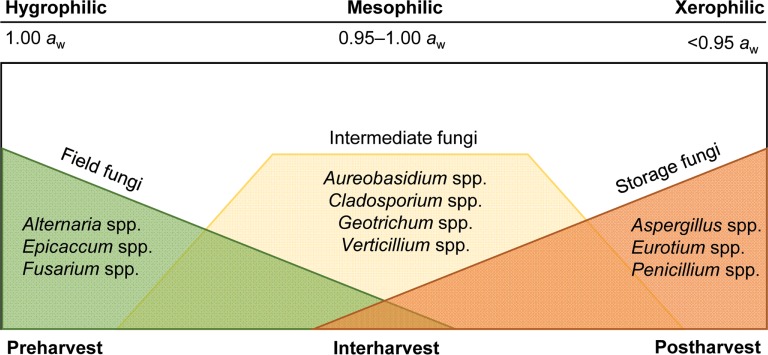
The fungi that contaminate grains are divided into two main groups and a third intermediate group based on their predominance at different stages of crop growth and harvest as affected by environmental conditions (Fig. 1) [5,13,14,15]. The first group is field fungi that can colonize the ripening grains on standing crops in the field prior to harvesting. This group includes species of the genera Alternaria and Fusarium. Most of the field fungal species do not infect the crops after harvesting; however, they can produce mycotoxins before or just after harvesting [5,16]. The second group is the storage fungi that can be present in small numbers prior to harvesting, or can contaminate grains during harvesting and increase in numbers during storage as the environmental conditions favor the growth of these fungi over the growth of other groups. Storage fungi mainly include species of the genera Aspergillus and Penicillium [17,18]. In Korea, Aspergillus candidus, Aspergillus flavus, Aspergillus fumigatus, Penicillium fellutanum, and Penicillium islandicum are the most predominant fungal species observed in stored rice grains [19,20,21,22]. The intermediate fungal group comprises of fungi that continue to develop in storage if aw remains high. This group includes species of the genera Cladosporium, Fusarium, and Trichoderma [13,14,15]. Wilson et al. [23] argued that location must be considered in the classification of field versus storage fungi because the occurrence of storage fungi in the field might increase in lower latitudes. As a consequence, a specific fungal species could be classified as a storage fungus in one location and as a field fungus in another. More information is needed about field and storage fungi from different latitudes to define the effect of location, along with the environmental factors responsible for fungal contamination of grains [23]. Fungal contamination at the preharvest stage is governed mainly by interactions with host plants, genotype, soil types, and biological factors. At the postharvest stage, fungal growth and development are governed by the substrate status (grain damage and nutritional constituents), environmental factors (temperature and moisture), and biotic factors (insect pests and microorganisms) [18,24].
Fig. 1. Classification of fungal groups based on their stages of occurrence and dominance from preharvest to postharvest stages of field crops. The top scale represents the alternate classification of fungal species on grains based on their water activity requirements (i.e., optimum water activity [aw]) for fungal growth [5,13,14,15].
The main environmental factors governing the predominance of fungal groups on grains are aw and the eco-physiological requirements of each fungal group. On standing crops before harvest, hygrophilic fungal species dominate and generally disappear after a few months in storage. After harvest, these fungal species are replaced by mesophilic fungal species that persist during storage as long as aw supports their growth. Finally, xerophilic fungal species are the dominant species present under storage conditions, when aw drops beyond the limits of growth for hygrophilic and mesophilic fungal species. The most xerotolerant fungal group is the genus Aspergillus followed by the genus Penicillium [15]. The fungal groups were categorized based on optimum aw for growth of xerophiles, mesophiles, and hygrophiles being < 0.95, 0.95 to < 1.00, and 1.00, respectively [13]. The mesophilic fungal group, in this case, is confusing because this term is usually used to describe temperature requirements of microorganisms rather than aw. Hocking and Pitt [25] suggested a slight modification to the above aw criteria, with xerophiles considered to be fungi capable of growth at aw ≤ 0.85.
Miller [18] identified four different types of pathogenic and mycotoxigenic fungi. The first type comprises fungi that are pathogenic to plants (e.g., Fusarium graminearum); the second type includes fungi that infect stressed and weak plants (e.g., Fusarium moniliforme); the third type comprises fungi that contaminate standing plants and produce mycotoxins after harvest (e.g., A. flavus); and the fourth type includes fungi that are present in soil or decaying plant debris, contaminate developing grains on standing plants, and develop during storage when conditions are suitable for their growth (e.g., Aspergillus ochraceus and Penicillium verrucosum). This classification is somewhat confusing, particularly since types 3 and 4 can be combined due to their similar eco-physiological requirements. Nevertheless, the classification is valuable since it emphasizes the role of environmental factors in determination of the dominant fungi at different stages of grain crop growth and harvest.
MYCOTOXINS IN STORED GRAINS
Mycotoxins are low molecular-weight, secondary metabolites that are produced by certain fungal species mainly belonging to the genera Aspergillus, Fusarium, and Penicillium. Mycotoxins are toxic to humans and animals, and cause a wide range of disorders from gastroenteritis to cancer [26]. Mycotoxin contamination of food and feed was first verified in the 1960s after a large number of turkeys were supplied with A. flavus-contaminated feed [27]. However, in 1881, as described by Pitt [28], extracts from rice infected with P. islandicum were reported to induce animal mortality. The term mycotoxicosis is used to describe the non-infectious, non-contagious, and non-transferable fungus-related toxic effects on humans or animals upon ingestion of mycotoxin-contaminated food or feed [29].
Mycotoxins have been a health threat for millennia. For example, ergotism, originally known as “St. Anthony's fire,” resulted in massive death in Europe during the middle ages. It was shown to be related to toxins (i.e., ergot alkaloids) produced by the ergot fungus (Claviceps purpurea), having various toxic effects on humans and animals [30]. In 1891, toxicity of rice grains contaminated with mold was observed in Japan; this was later referred to as “yellow rice.” This term describes the rice grains contaminated with certain species of Penicillium that are toxic to several animals and cause grain discoloration in rice [31]. Another infamous historic outbreak of a mycotoxin-induced disease or mycotoxicosis is alimentary toxic aleukia (ATA) associated with the Fusarium toxin T-2, in Russia during the World War II. Thousands of ATA cases resulted from the consumption of poor quality and overwintered grains left in the field [18]. Subsequently, the disease was determined to be caused by the T-2 toxin produced by a Fusarium sp. during growth on wet grains overwintered in the fields [32]. More recently, in western India and Kenya, outbreaks of hepatitis with rapidly developing symptoms affected several hundred people and caused many deaths. The culprit was aflatoxicosis, which is developed due to the consumption of grains heavily contaminated with aflatoxins [33,34].
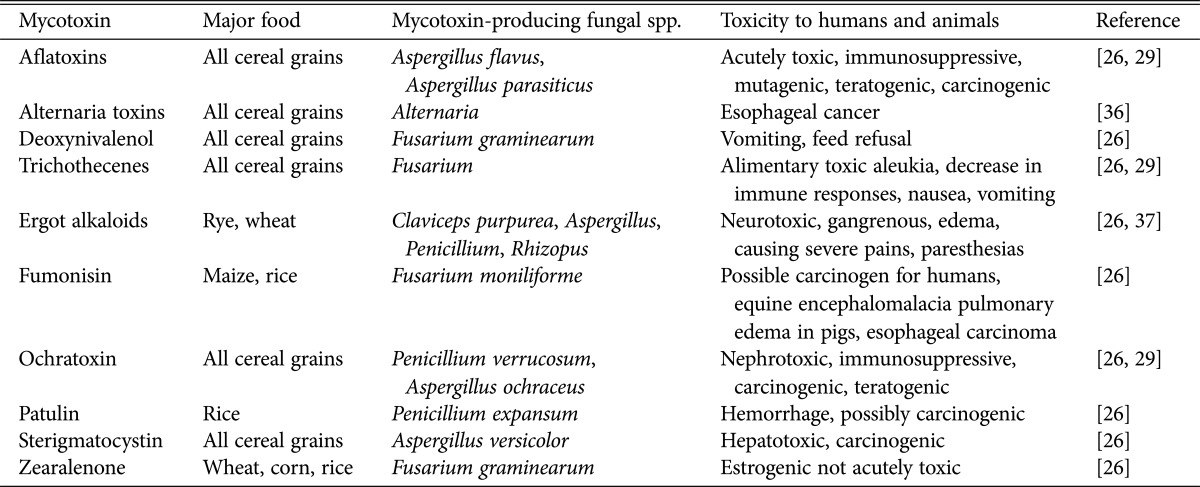
Of the thousands of reported fungal species, only around 100 species can produce various mycotoxins. The mycotoxin-producing fungi primarily belong to the genera Aspergillus, Fusarium, and Penicillium. Several hundred mycotoxins have been isolated and chemically characterized; however, relatively low numbers of mycotoxins can develop on foods and feeds. Among these aflatoxins, ochratoxins, deoxynivalenol, zearalenone, fumonisin, trichothecenes, and patulin significantly contaminate stored grains [35]. According to the Food and Agriculture Organization (FAO), 25% of the world's food crops are contaminated with significant amounts of mycotoxins, mainly in tropical regions [26]. The major mycotoxins, causal fungi, and biological activities are summarized in Table 1 [26,29,36,37]. High humidity and temperature in the tropical and subtropical regions than in the temperate regions increase the susceptibility of crops to mycotoxin contamination [38]. Therefore, global climate changes are expected to cause a more serious problem when some mycotoxigenic fungal species are likely to dominate others. For example, A. flavus is likely to dominate other fungal species that have a lower optimum temperature for growth [39]. Thus, aflatoxin produced by this fungus might become the major mycotoxin of concern. Several studies have indicated that climatic changes could affect important crops and mycotoxigenic fungal contamination [39,40,41].
Table 1. Major mycotoxins and their related fungi and toxicity to humans and animals.
Human and animal health hazards associated with mycotoxin contamination of food and feed have prompted about 100 countries to formulate regulations concerning the allowable levels of mycotoxins [42]. In general, the allowed level of aflatoxins in food destined for human consumption is 4–30 parts per billion (ppb) [43]. The strictest level of total aflatoxin (4 µg/kg body weight [BW]) is in the European Union (EU). In the United States, the acceptable limit is 20 µg/kg BW [42]. On the other hand, for ochratoxin A, the provisional tolerable weekly intake is 100 ng/kg BW [44]. Strict control of food and feed contamination are crucial to reduce the human and animal health risks due to mycotoxins [29]. In the following section, the effect of environmental factors on mycotoxigenic fungi and major mycotoxins found in cereal grains are discussed.
FACTORS AFFECTING FUNGAL GROWTH AND MYCOTOXIN PRODUCTION IN STORED GRAINS
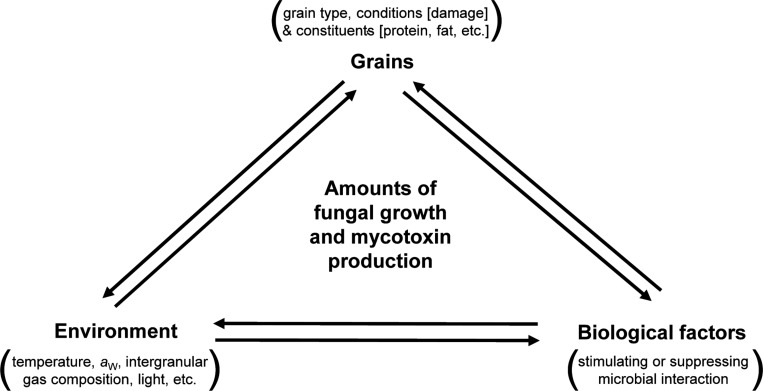
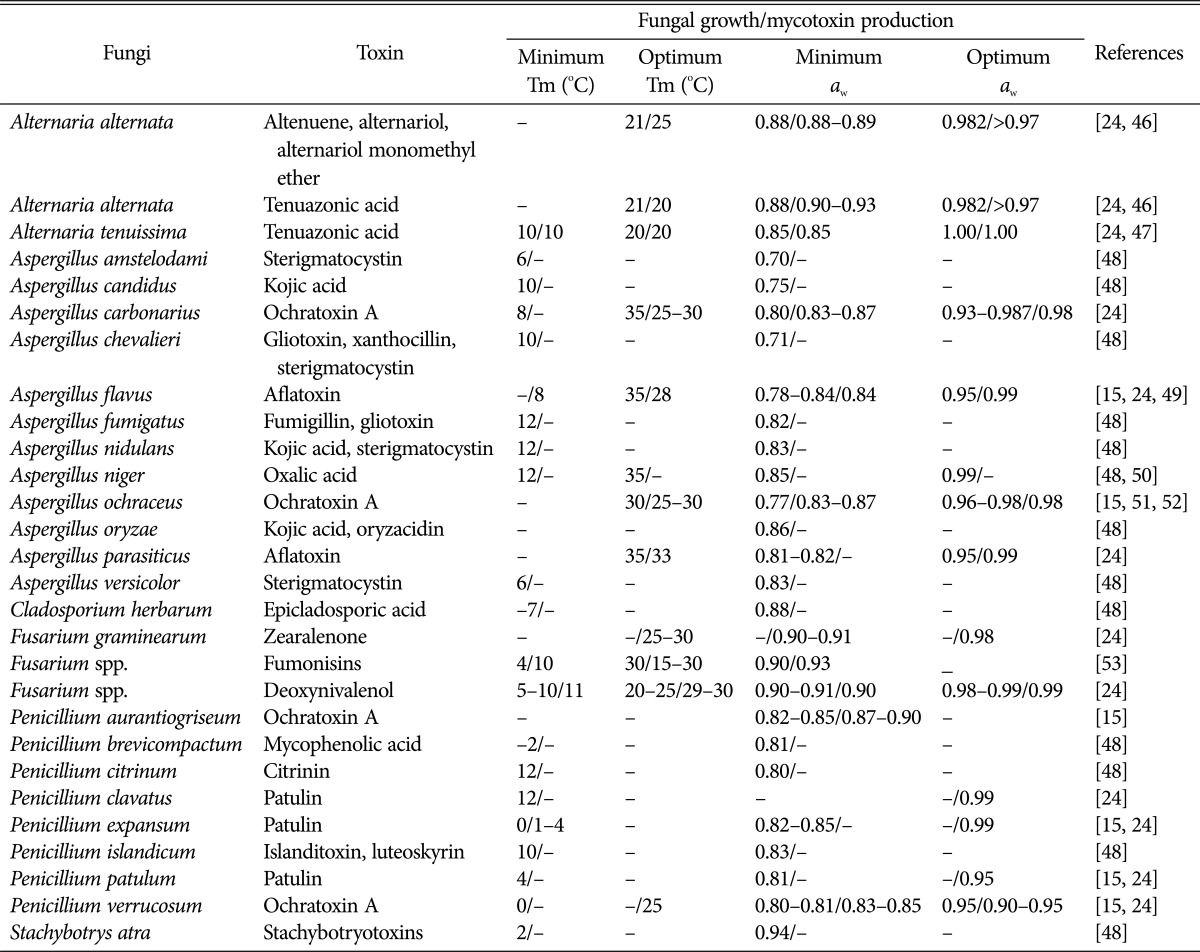
In the stored-grain ecosystem, several intrinsic and extrinsic factors interact and affect the etiology, dominance, and toxin production of mycotoxigenic fungi. It is important to note that the conditions favorable for fungal growth and development in stored grains are not always conducive for mycotoxin production. The general factors influencing fungal growth and mycotoxin production on stored grains are illustrated in Fig. 2. Fungal growth and mycotoxin production are principally controlled by various environmental or ecological factors including temperature, water availability, pH, light, and nature of substrate. These conditions vary immensely among different fungal species, and even within isolates of the same species. Therefore, it is difficult to describe a single set of optimum conditions for fungal growth and mycotoxin production [45]. The minimum and optimum temperatures and aw for growth and mycotoxin production of the major mycotoxigenic fungal species are summarized in Table 2 [15,24,46,47,48,49,50,51,52,53].
Fig. 2. Illustration of the main interrelated factors affecting fungal growth and mycotoxin production in the stored-grain ecosystem. The interactions among the three main factors (grain type, biological factors, and environment) can control the occurrence and dominance of fungal species on grains, and the type and amounts of the mycotoxins produced.
Table 2. Minimum and optimum temperatures (Tm) and water activity (aw) for fungal growth and mycotoxin production.
-, not reported.
Environmental factors also affect several physiological processes that are important for fungal survival and competition. The expressions of hydrolytic enzymes including β-D-galactosidase, α-D-galactosidase, N-acetyl-β-D-glucosaminidase, β-D-fucosidase, and β-D-xylosidase are affected by aw, particularly during the early stages of development. These enzymes play an important role in the establishment of fungal populations on substrates [54]. In addition, carbon source utilization is affected by environmental conditions. The use of carbon sources and niche overlap index (NOI) of individual fungal species depend on aw and temperature [50,55]. The NOI is based on the difference between the number of carbon sources utilized by an individual fungal species and those commonly utilized by the coexisting fungal species. This index is a useful tool to illustrate the competition ability of a fungal species in a given ecosystem [54].
The main environmental determinants affecting grain fungi and mycotoxin production during storage are water availability and temperature [7]. Estimation of the water content of grains is not sufficient to indicate the water available for microbial growth. However, equilibrium relative humidity (ERH), aw, or water potential (Ψ) can be used to measure water availability. ERH and aw are the main parameters used in the studies for evaluating the effect of water availability on grain fungi. ERH represents the percent relative humidity of the air between grains in equilibrium with water in the grains. On the other hand, aw is the ratio of the water vapor pressure above the grains to that above pure water at the same temperature and pressure [56]. The aw as a measurement of water availability has replaced moisture content measurement as a more useful and precise expression of water availability for microbial growth. As the water available for microbial growth is reduced, aw becomes lower [48]. The significance of the major mycotoxins contaminating stored grains and the influence of temperature and aw on fungal growth and mycotoxin production are discussed next.
Aflatoxins
Aflatoxins are the most significant mycotoxins that contaminate various agricultural and food products. They are the most potent carcinogens with immunosuppressive, mutagenic, and teratogenic activities in humans and animals [29,57]. There are several types of aflatoxins. For example, aflatoxins B1, B2, G1, and G2 are most important; aflatoxins M1 and M2 are the metabolic products of oxidation of aflatoxin B1 in humans and animals following ingestion [29]. Aflatoxin B1 is the most toxic substance and is classified as a Class 1 carcinogen by the International Agency for Research on Cancer (IARC), based on the evidence of its carcinogenicity in humans after the evaluation of epidemiological and laboratory results [58]. Continuous exposure to low doses of aflatoxins might be carcinogenic and high doses can result in acute toxicity leading to death [59]. Malnutrition, along with the chronic intake of aflatoxin, may also result in immunosuppression, impaired growth, and other diseases [60]. An association between the dietary intake of aflatoxins in contaminated food and high incidence of liver cancer was concluded from epidemiological studies conducted on human subjects exposed to aflatoxin-contaminated food [35].
Aflatoxins are produced by species of Aspergillus section Flavi, primarily A. flavus and A. parasiticus. These two species are ubiquitous present in some foods and stored agricultural products. Other species reported to produce aflatoxins include Aspergillus arachidicola, Aspergillus minisclerotigenes, and Aspergillus nomius [61,62]. The major source of contamination in cereal grains is the conidia of toxin-producing fungi in the field, which can continuously grow and contaminate the grains [63]. The aflatoxin-producing fungi may form sclerotia in damaged grains before harvest, which can be dispersed in the soil during harvesting. The sclerotia can survive in soil and remain viable to regenerate conidiophores and conidia in subsequent growing seasons, leading to repeated contamination of grains [64].
As mentioned above, the optimum conditions for the germination and growth of mycotoxigenic fungi are not always conducive for toxin production. This has been observed in the growth and aflatoxin production by A. flavus and A. parasiticus. The fungal growth occurs over a narrower range of conditions than germination. Moreover, aflatoxin production occurs over a narrower range of conditions than fungal growth. Hill et al. [65] reported that the optimum temperature and aw for fungal growth were 35℃ and 0.95, respectively; however, the optimum temperature and aw for aflatoxin production by these two species were 33℃ and 0.99, respectively. The effects of environmental conditions on aflatoxin production by A. flavus and A. parasiticus have been studied. Klich [66] reported that the optimum temperature for aflatoxin production can vary between 24–30℃ depending on the strain and substrate type. Earlier, Sorenson et al. [49] reported that the optimum temperature for aflatoxin production by A. flavus on rice grains was 28℃, with considerable toxin production still evident at 32℃. Temperatures above 32℃ markedly hinder aflatoxin production even though fungal growth was enhanced. The toxins are not produced at 8℃. Abdel-Hadi et al. [67] reported that optimal growth of A. flavus is at 30–35℃ and 0.99 aw, whereas the optimum conditions for aflatoxin production are 25–30℃ at 0.99 aw and 30–35℃ at 0.95 aw. The suppression of aflatoxin production at temperatures higher than 32–35℃ has been described in several other studies [68,69,70]. Kheiralla et al. [71] demonstrated that aflatoxin production was not correlated to fungal growth at temperatures higher than 30℃. In that study, variations in the optimum temperature for aflatoxin production were observed between different isolates of A. flavus. They also reported that maximum aflatoxin production occurred at 25–30℃ 14 days after incubation. However, the fungal growth expressed as mycelial dry weight in that study continuously increased with increasing temperature, with maximum growth at 35℃. A recent study on corn grains described the significant increase in aflatoxin production by increasing the temperature from 30 to 37℃ [72]. In contrast, Yu et al. [73] reported almost complete cessation of aflatoxin production at 37℃ on laboratory media. Medina et al. [72] suggested that the short incubation period of broth cultures could be responsible for the lack of aflatoxin production at 37℃ in the latter study. However, other possible explanations for the two contrary findings are the use of different strains and, in the study by Medina et al. [72], use of corn as the substrate. This substrate could possibly create a micro-environment within individual kernels that might offer slightly different conditions than expected. Thus, the comparison of fungal strains cultured in broth might yield different results.
While evaluating the effect of aw on aflatoxin production, Faraj et al. [74] reported that aw has a higher influence on aflatoxin production than temperature. On the contrary, Mousa et al. [68] found that temperature had a higher influence on aflatoxin production than aw. This dichotomy may be due to the different values of aw used in both studies, which could be below the optimum range for toxin production in the first study. More precisely, Medina et al. [72] reported that variations in aw exert a more profound effect on aflatoxin production, which was confirmed by higher changes in aflatoxin-related gene expression. Mousa et al. [68] found that aflatoxins were produced at temperatures ranging from 20 to 40℃, with continuous increase noted with increasing aw in the range of 0.82 to 0.92. At 40℃, only a minute quantity of aflatoxins was observed at aw of 0.92. Cuero et al. [75] reported that the optimum growth for A. flavus on maize extract agar occurred at 0.95 aw, while maximum aflatoxins were produced at 0.98 aw. Similarly, Zhang et al. [76] observed more aflatoxin production at 0.99 than at 0.93 aw, which correlated with the corresponding regulation of the aflatoxin biosynthesis gene cluster.
The characterization of aflatoxin biosynthesis pathway has facilitated the understanding of the roles of different structural and regulatory genes in the aflatoxin production process [77,78]. Recently, sequencing and annotation of the A. flavus genome has paved the way for different molecular approaches, such as reverse transcriptase real-time polymerase chain reaction, microarrays, and RNA-sequencing (RNA-Seq), which are being used to study the behavior of aflatoxigenic fungal species under various environmental conditions [79]. The effect of temperature on aflatoxin production by A. flavus and A. parasiticus could be explained by understanding the changes in the expression of aflatoxin-related genes in the fungi with temperature [80]. In the latter study, at 37℃, the level of expression of aflatoxin production-related genes was reduced, with the consequent suppression of aflatoxin production. Abdel-Hadi et al. [81] confirmed the positive correlation between the expression of an early structural gene (aflD) and aflatoxin B1 production. In a similar study, Schmidt-Heydt et al. [82] reported that two transcriptional pathway regulatory genes, aflR and aflS, are expressed at lower levels at temperatures above 37℃, which results in suppression of aflatoxin production. Additionally, the interactions between temperature and aw were correlated with the ratio of these transcriptional regulatory genes. Increasing aflR and aflS ratios were associated with increasing aflatoxin B1 production [82,83]. Likewise, Yu et al. [73] studied the gene expression and aflatoxin production at 30 and 37℃ using RNA-Seq technology. Aflatoxin production was confirmed to be one of the most tightly regulated processes in fungal cells. They also reported that higher aflatoxin production was correlated with a 50% increase in the expression of aflatoxin biosynthesis genes at 30℃ in comparison to that at 37℃. Moreover, higher expression levels were observed in the two transcriptional regulatory genes, aflR and aflS, at 30℃ than at 37℃. Therefore, it can be concluded that at a temperature higher than 37℃, aflatoxin production is negatively affected by the down-regulation of the transcription of aflR and aflS [73]. Previously, Chang [87,88] demonstrated that AFLR proteins encoded by aflR in association with AFLS proteins encoded by aflS together regulate aflatoxin biosynthesis. They also showed a role of AFLS as a coactivator to the AFLR pathway regulator for transcription of the aflatoxin gene cluster. However, O'Brian et al. [80] observed no difference in the expression levels of aflR and aflS in A. flavus between 28 and 37℃. Consequently, they suggested that the aflatoxin biosynthesis regulatory protein does not function at elevated temperatures regardless of its expression level. Further, Gallo et al. [84] reported high expression of the aflR and aflS regulatory genes under conditions non-conducive for aflatoxin production. Likewise, in a more recent study, Bernáldez et al. [85] found a weak correlation between the expression level of aflR and aflatoxin production. The authors demonstrated that aflR expression is not a good indicator for aflatoxin production in A. flavus. Further molecular research will be needed to identify other genes as indicators for aflatoxin biosynthesis. A possible explanation for the inconsistency could be that the mycotoxin production pathway-related gene expression levels in response to changing environmental factors may vary between strains. Variable observations and comments on the environment-induced aflatoxin suppression and roles of the regulatory genes in several previous studies are summarized in Table 3 [67,72,73,80,83,84,85,86]. Based on the findings of the previous studies, a hypothetical mechanism of aflatoxin suppression under conducive or non-conducive temperature and aw conditions highlighting the possible roles of aflR and aflS in the regulation of transcription of the aflatoxin gene cluster is illustrated in Fig. 3 [78,87,88,89,90]. Further studies are required to confirm the hypothesized mechanism of aflatoxin transcription regulation and to identify novel target sites for controlling aflatoxin production, which may be helpful for the management of aflatoxin levels in food and feed.
Table 3. Observations and comments regarding the influence of temperature and water activity (aw) on the regulatory genes governing the transcription of the aflatoxin biosynthesis gene cluster.
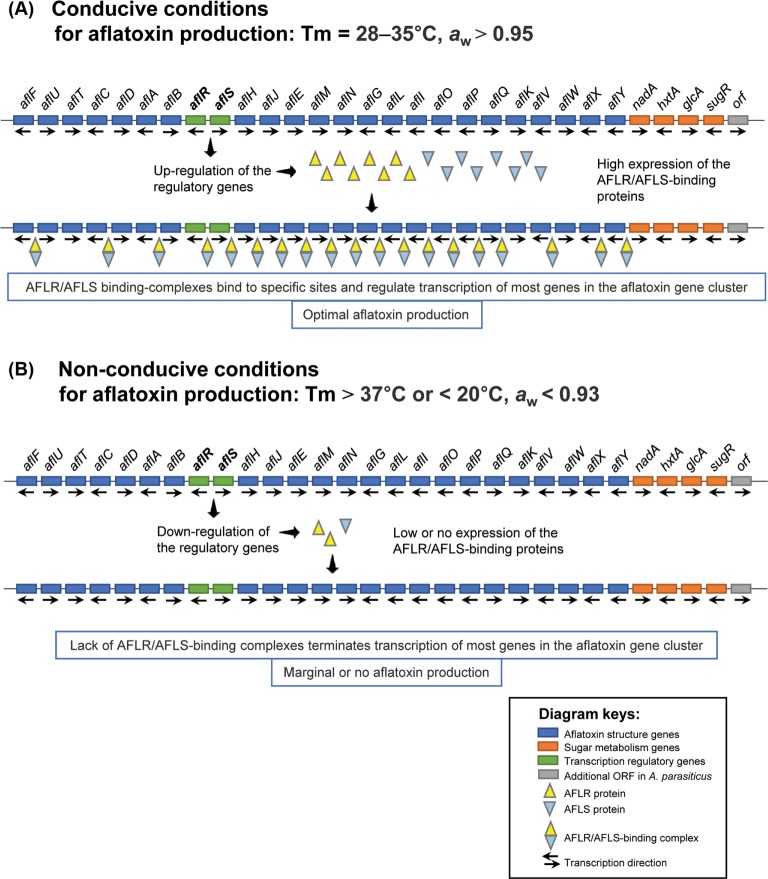
Fig. 3. Influence of temperature (Tm) and water activity (aw) on the aflatoxin biosynthesis gene cluster (~80 kb DNA region) under conducive production conditions at optimum Tm (28–37℃) and aw (> 0.95) (A), and non-conducive conditions at high (> 37℃) or low (< 20℃) Tm and low aw (< 0.93) (B). The diagram shows the aflatoxin gene cluster in the toxin-producing fungi (e.g., Aspergillus flavus and Aspergillus parasiticus), emphasizing the roles of two transcription regulatory genes, aflR and aflS, in the regulation of aflatoxin production [78,89]. The two genes are located in divergently adjacent positions within the gene cluster. It is hypothesized that the AFLR proteins (encoded by aflR) in association with AFLS proteins (encoded by aflS) bind to specific sites on the aflatoxin gene cluster to activate the transcription of most structural genes [84,85,90]. Aflatoxin production is a tightly regulated process that is affected dramatically by changing environmental conditions. The unfavorable conditions cause reduction in expression of the two regulatory genes, aflR and aflS, which results in the suppression of aflatoxin production.
Alternaria toxins
Fungal species of the genus Alternaria can colonize a wide variety of plants as saprophytes or in some cases as fungal pathogens of specific crops. The most common species include Alternaria alternata, Alternaria arborescens, Alternaria radicina, and Alternaria tenuissima, which occur in several crops including cereals [54]. The significance of contamination of cereal grains with Alternaria spp. is the production of mycotoxins, particularly on grains that are not appropriately dried or which are damaged after harvesting [91]. Unlike Aspergillus and Penicillium spp., Alternaria spp. might not survive on grains with low moisture content [92]. A. tenuissima is the major species that predominates on cereals compared with other Alternaria spp. [93]. Several secondary metabolites with toxic activities have been isolated and chemically characterized from Alternaria spp. Among them, altenuene, alternariol, and alternariol monomethyl ether occur most frequently on cereal grains [94,95]. Previously, Liu et al. [36] demonstrated that A. alternata is the causal agent of esophageal cancer in humans. The fungal isolate was obtained from grains in Linxian County, China, where the incidence of esophageal cancer was high. The extracts of this isolate were shown to be tumorigenic in mice.
For cereal grains (e.g., wheat), A. alternata and Alternaria triticina are the causal agents of the black point disease, which results in discoloration of the kernels and reduces grain quality. These Alternaria spp. can tolerate low temperatures and cause spoilage in vegetables during cooled transport [24]. Regarding the effects of aw and temperature, it was reported that the limits for germination are lower than those for A. alternata growth, being 0.86 aw for germination and 0.88–0.89 aw for fungal growth and toxin production. Moreover, a narrower range of temperature is required for the production of toxic altenuene and alternariol monomethyl ether than for alternariol. Maximum production of the three toxins was observed at 25℃ and aw > 0.97 [24]. Studies on the influence of aw and temperature on tenuazonic acid production by Alternaria spp. from sorghum and cottonseeds also revealed that aw and time affected the optimum production [92]. On sorghum-based media, the minimum aw for production was about 0.93–0.90. In another study conducted on A. tenuissima from cottonseed, the maximum tenuazonic acid production was obtained as 20℃ and 37% water content (= 1.00 aw), and the minimum toxin production was observed at 14.9% water content (= 0.85 aw) [47]. In the same study, tenuazonic acid production was halved at 0.95 aw. The results from these studies indicate that conditions for toxin production by different Alternaria species may vary immensely with respect to growth.
In an attempt to understand the roles of mycotoxins in a competing environment for fungal species in a given ecosystem, Müller et al. [96] incubated wheat kernels with two isolates of Fusarium spp. and two isolates of A. tenuissima. Before fungal inoculation, the wheat kernels were amended with the mycotoxins, alternariol and tetramic acid (Alternaria toxins), and deoxynivalenol and zearalenone (Fusarium toxins). Growth of Alternaria strains and mycotoxin production in wheat kernels supplemented with Fusarium mycotoxins were enhanced. Additionally, enhanced growth of Alternaria spp. resulted in degradation of the Fusarium mycotoxins on the wheat kernels substrate [96]. These results are meaningful for understanding the complex interactions between fungal populations of different genera and the possible roles of mycotoxins.
Fumonisins
Fumonisins are mycotoxins produced primarily by Fusarium verticillioides (formerly F. moniliforme = Gibberella fujikuroi) and other related fungal species. These mycotoxins were discovered and characterized about 20 years ago [97]. Since then, at least 15 fumonisin compounds were identified. Of these, fumonisins B1 and B2 are the most toxic and abundant, whereas fumonisins B3, B4, A1, and A2 are less toxic and occur in lower amounts [29]. Fumonisins have been associated with increased incidence of human esophageal cancer and a number of animal diseases [98]. It is classified by IARC as a class 2B possible human carcinogen [58].
Corn is the most susceptible host to contamination with fumonisin-producing fungi. A wide range of corn-based foods have been reported to be contaminated with these mycotoxins worldwide [99]. Contamination might initially occur in the field. During harvest, if the grains have a high moisture content, fungal growth can continue after harvest and fumonisins can accumulate to significant levels before drying and storage [24]. Fumonisin B1 production is also subject to a narrow range of temperature and aw than that conducive for growth of the fungus. Although values might vary depending on the isolates, fungal growth was reported to occur between 4–37℃ with an optimum temperature of 30℃, whereas fumonisin production can occurs between 10–37℃, with 15–30℃ as the optimum temperature. Regarding the aw required for growth and toxin production, 0.90 and 0.93 were recognized as the minimum values for growth and fumonisin B1 production, respectively. Increased aw was correlated with enhanced fungal growth and toxin production [24,53].
Ochratoxins
Ochratoxin A is a potent nephrotoxin that is teratogenic, immunosuppressive, and carcinogenic. It is classified as a group 2B, possible human carcinogen based on sufficient evidences of its carcinogenicity in animals [58]. Ochratoxin A could increase the mutagenic ability of aflatoxin B1 in cases of simultaneous occurrence in certain crops [100]. However, other studies showed that detection of ochratoxin A at high level was associated with the absence or reduction of aflatoxin B1, suggesting a possible competition between these toxin-producing fungi on the substrate [35]. The major ochratoxin A-producing fungi are Aspergillus carbonarius, Aspergillus melleus, A. ochraceus, Aspergillus sclerotiorum, Aspergillus sulphureus, and P. verrucosum. Other species including Aspergillus niger and Penicillium purpurascens are also considered minor ochratoxin A producers [44].
Most studies related to the influence of temperature and aw on the production of ochratoxin A have focused on A. ochraceus, which belongs to Aspergillus section Circumdati and is the major ochratoxin A-producing fungus contaminating cereal grains, particularly in temperate and tropical regions [101]. The niche overlap and dominance by A. ochraceus and consequent ochratoxin A production are influenced by temperature, aw, and the interaction and competition with other fungi [102]. In a study on maize grains, at 30℃, the production of ochratoxin A was significantly higher at 0.95 than at 0.995 aw. Regardless of the aw level, ochratoxin production was significantly reduced by interaction with other fungal spp., such as A. candidus, on maize grains [102]. In another study, the influence of temperature and aw on A. ochraceus growth and ochratoxin A production on barley grains was investigated, in which the toxin production occurred within a narrower range of aw and temperature than the fungal growth [103]. In this study, the minimum aw for fungal growth and toxin production was 0.85 and 0.90, respectively; the optimal growth and toxin production conditions were 30℃ and 0.99 aw. The differences in the reported minimum and optimum conditions for growth and ochratoxin production in the different studies could be related to the use of different substrates. The nutrient source can affect the minimum aw for fungal growth and toxin production [104]. Madhyastha et al. [105] suggested that ochratoxin A production is not associated with rapid growth of A. ochraceus. Moreover, Häggblom [106] reported that the ochratoxin A production could be reduced at higher growth rates of the fungus.
The other major ochratoxin A-producing species is P. verrucosum, which is particularly problematic in cool climatic regions, contaminating inappropriately dried wheat and barley. This fungus can grow over a wide temperature range (0–35℃), with 25℃ being the optimum temperature for toxin production on grains. It can produce the toxin at lower temperature (5–10℃) at its optimum aw. The optimum aw for ochratoxin A production is between 0.90 and 0.95, whereas the minimum aw is 0.83–0.85. However, the optimum aw for fungal colonization of stored grains is 0.95 [24]. Studies on the effects of temperature and aw on the causal fungi have consistently shown that the range of temperature and aw for toxin production is narrower than that for growth. However, ochratoxin A production by P. verrucosum could be an exception, as the fungus grows and produces ochratoxin A under similar temperature and aw conditions [107].
Trichothecenes and zearalenone
Trichothecenes are a group of metabolites produced by a number of fungi, including the genera Fusarium, Myrothecium, Phomopsis, and Trichoderma, [27]. More than 148 trichothecenes have been identified; however, only a few toxins, such as deoxynivalenol (also known as vomitoxin), nivalenol, and T-2, are common contaminants of food and feed. Ingestion of contaminated food or feed in high doses can result in immune response reductions, nausea, and vomiting [29]. On the other hand, zearalenone is another mycotoxin produced by several Fusarium spp., primarily F. graminearum and other related species such as Fusarium crookwellense, Fusarium culmorum, and Fusarium equiseti, which are common contaminants of grains [108]. Zearalenone toxicosis causes estrogenic effects in many animals, which can lead to infertility, vulval edema, and feminization of males [29].
Magan and Lacey [109] studied the effect of temperature and aw on the growth of several Fusarium spp. such as Fusarium avenaceum, F. culmorum, Fusarium poae, and Fusarium tricinctum. The optimum temperature for fungal growth was 20–25℃, with reduced growth at 5–10 and 35℃. At the optimum temperature, the optimum aw was 0.98–0.995 and minimum aw was 0.90–0.91 and 0.88 for germination. In another study, the optimum temperatures for deoxynivalenol production on corn grains having 30% moisture content was 29–30℃ for F. graminearum and 25–26℃ for Fusarium roseum, with about 11℃ as the minimum temperature for toxin production by both fungi [110].
Most fungal species produce higher levels of mycotoxins at their optimum growth temperature. However, outbreaks of mycotoxicoses related to Fusarium species have occurred after exposure of the substrates to lower temperatures [111]. After the initial growth of Fusarium spp. at room temperature, subsequent incubation at a low temperature of 12–14℃ resulted in higher toxin productions [112]. In agreement with this finding, Ryu and Bullerman [113] observed maximum deoxynivalenol and zearalenone production by F. graminearum after incubation on rice cultures at 25℃ for 2 wk followed by 4 wk at 15℃. These results indicate that low temperature stress on certain Fusarium spp. increases toxin production. On the contrary, the same study showed that increased incubation temperature resulted in enhanced fungal growth expressed as the amount of produced free ergosterol, which was not correlated with the production of deoxynivalenol and zearalenone. In another study conducted on a wheat-based agar medium, the optimum conditions for growth, and maximum production of deoxynivalenol and nivalenol by F. culmorum were observed to be 25℃ and aw of 0.995 and 0.981, respectively [114]. In this study, the observed difference in optimum aw for the production of deoxynivalenol and nivalenol was, as claimed by the authors, related to the fungal response to stress conditions by producing the more toxic deoxynivalenol to improve competitiveness. Moreover, the production of both toxins in this study occurred at a narrower aw range than that for fungal growth [114].
MODIFIED ATMOSPHERE FOR MANAGEMENT OF MYCOTOXIGENIC FUNGI DURING GRAIN STORAGE
Modified atmosphere storage is a method of food preservation that aims to extend the storage life and maintain the quality of food products. This is achieved mainly by creating an atmosphere around the food products that is enriched in CO2 and low in O2. These conditions reduce the respiration rate of food products and inhibit the activity of microorganisms [115]. The primarily advantage of this method is the reduction or even elimination of the use of harmful chemicals. However, there are practical, technical, and biological factors that might restrict the use of modified-atmosphere approach [45]. The modified atmosphere is different from controlled-atmosphere storage. The gas composition in the modified atmosphere is initially modified and then changes with the respiration rate or biological activity of the food products. In the controlled atmosphere, the gas composition is continuously controlled throughout the storage period [115].
Several studies have reported the inhibitory effect of modified atmosphere with raised CO2 and reduced O2 levels on the growth and mycotoxin production of deleterious fungal species. Earlier, reduction in aflatoxin production by A. flavus on peanuts using increased CO2 concentration was reported [116]. Similarly, Shih and Marth [117] reported that aflatoxin production by A. parasiticus in a liquid medium was inhibited by increasing CO2 or N2 levels, with complete inhibition of the toxin production at 100% of both gases. Moreover, penicillic acid production was inhibited on corn kernels inoculated with P. martensii by increasing the CO2 concentrations. The level of toxin reduction varied depending on the incubation temperature [118]. Paster et al. [119] observed the complete inhibition of ochratoxin A production by A. ochraceus at ≥ 30% CO2, regardless of the O2 level, with partial inhibition of colony growth at 60% CO2 and no observed growth at 80% CO2. A significant reduction was also observed in the T-2 production by F. tricinctum at CO2 levels up to 50% and the O2 level close to normal atmospheric level. Additionally, at CO2/O2 levels of 60%/20% and 80%/20%, the reduction of T-2 production was accompanied with reduction of the fungal growth expressed as colony diameter with 2–4 days delay in the appearance of colonies [120]. In another study, Paster et al. [121] observed almost complete inhibition of zearalenone production by F. equiseti in high moisture corn grains by increasing the CO2 levels (20, 40, and 60%). More recently, Samapundo et al. [122] reported that increased initial headspace CO2 concentration resulted in inhibition of fumonisin B1 production by F. verticillioides and also affected Fusarium proliferatum growth.
Although most stored-grain fungi seem to be sensitive to elevated CO2 levels, resistance to high CO2 has been reported in several fungi, such as Penicillium roqueforti, which was found in sealed silos containing barley grain at 0.80–0.87 aw and high CO2 levels. Notably, fungal response to a modified atmosphere can vary immensely between different fungal species, and even between strains of the same species [45]. Furthermore, interactions with grain types (substrate), storage temperature, and aw can also affect the fungal tolerance to low O2 and high CO2 [8].
CONCLUSIONS
Cereal grains are a very important source of food for the growing human population worldwide. Improved preservation and quantity and quality control of cereal grains are urgently needed to meet the increasing food demands. Grain contamination with mycotoxigenic fungi often deteriorates the quality of grains and leads to mycotoxin production. Mycotoxins are dangerous metabolic substances produced by deleterious storage fungi and can cause severe outbreaks of diseases in humans and animals. Therefore, they should be eliminated or minimized in food and feed by using various effective control measures. Environmental factors, such as temperature and aw, can significantly influence fungal growth and mycotoxin production in stored grains. Understanding the effects of such factors on the stored-grain fungi will help in the development of efficient strategies for controlling or managing fungal contamination. In addition, there is a need for conducting studies on the influence of temperature and aw on the molecular regulatory processes of mycotoxigenic fungi, which will facilitate the development of control measures to minimize mycotoxin production by the identification of targets for molecular control approach such as RNA silencing. The use of controlled or modified atmosphere is a potential alternative for the use of harmful agricultural chemicals during grain storage. Besides, further understanding of fungal interactions with different grain types at different storage temperatures and aw will help to improve the storage systems for grains. In this review, results of the considerable research efforts involving different grain types, fungal species, environmental conditions, and locations have been described. Thus, we hope that this review will not only help in the use of controlled or modified environmental conditions during grain storage, but also contribute towards understanding the diverse responses of different mycotoxigenic fungi to the environmental factors. Future studies should consider the integration of various fungal control methods, including environmental and biological measures, which would assure environmentally safe and sustainable storage of grains.
ACKNOWLEDGEMENTS
Mohamed Mannaa was supported by the Korean Government Scholarship Program (KGSP) during his Ph.D. study at Korea University, Seoul, Korea.
References
- 1.Serna-Saldivar SO. Cereal grains: properties, processing, and nutritional attributes. Boca Raton (FL): CRC Press; 2016. [Google Scholar]
- 2.Walter GH, Chandrasekaran S, Collins PJ, Jagadeesan R, Mohankumar S, Alagusundaram K, Ebert PR, Daglish GJ, Nayak MK, Mohan S, et al. The grand challenge of food security: general lessons from a comprehensive approach to protecting stored grain from insect pests in Australia and India. Indian J Entomol. 2016;78:7–16. [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations. FAOSTAT statistics database [Internet] Rome: FAO; 2017. [cited 2017 Jul 25]. Available from: http://www.fao.org/worldfoodsituation/csdb/en/ [Google Scholar]
- 4.Food and Agriculture Organization. Feeding the world, eradicating hunger: executive summary. World summit on food security [Internet] Rome: FAO; 2009. [cited 2017 Jul 25]. Available from: http://www.fao.org/fileadmin/templates/wsfs/Summit/WSFS_Issues_papers/WSFS_Background_paper_Feeding_the_world.pdf. [Google Scholar]
- 5.Christensen CM, Kaufmann HH. Grain storage: the role of fungi in quality loss. Minneapolis (MN): University of Minnesota Press; 1969. [Google Scholar]
- 6.National Academy of Sciences. Post-harvest food losses in developing countries: a bibliography. Washington, D.C.: National Academy of Sciences; 1978. [Google Scholar]
- 7.Sinha RN. The stored-grain ecosystem. In: Jayas DS, White ND, Muir WE, editors. Stored-grain ecosystems. New York: Marcel Dekker; 1995. pp. 1–32. [Google Scholar]
- 8.Magan N, Aldred D. Post-harvest control strategies: minimizing mycotoxins in the food chain. Int J Food Microbiol. 2007;119:131–139. doi: 10.1016/j.ijfoodmicro.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Levinson H, Levinson A. Control of stored food pests in the ancient orient and classical antiquity. J Appl Entomol. 1998;122:137–144. [Google Scholar]
- 10.Mannaa M, Kim KD. Microbe-mediated control of mycotoxigenic grain fungi in stored rice with focus on aflatoxin biodegradation and biosynthesis inhibition. Mycobiology. 2016;44:67–78. doi: 10.5941/MYCO.2016.44.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannaa M, Oh JY, Kim KD. Microbe-mediated control of Aspergillus flavus in stored rice grains with a focus on aflatoxin inhibition and biodegradation. Ann Appl Biol. 2017;171:376–392. [Google Scholar]
- 12.Mannaa M, Oh JY, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology. 2017;45:213–219. doi: 10.5941/MYCO.2017.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magan N, Lacey J. Effect of temperature and pH on water relations of field and storage fungi. Trans Br Mycol Soc. 1984;82:71–81. [Google Scholar]
- 14.Magan N, Lacey J. Effect of water activity, temperature and substrate on interactions between field and storage fungi. Trans Br Mycol Soc. 1984;82:83–93. [Google Scholar]
- 15.Fleurat-Lessard F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins: an update. J Stored Prod Res. 2017;71:22–40. [Google Scholar]
- 16.Lacey J. Pre-and post-harvest ecology of fungi causing spoilage of foods and other stored products. J Appl Microbiol. 1989;67:11S–25S. doi: 10.1111/j.1365-2672.1989.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 17.Flannigan B. Primary contamination of barley and wheat grain storage fungi. Trans Br Mycol Soc. 1978;71:37–42. [Google Scholar]
- 18.Miller JD. Fungi and mycotoxins in grain: implications for stored product research. J Stored Prod Res. 1995;31:1–16. [Google Scholar]
- 19.Oh JY, Jee SN, Nam Y, Lee H, Ryoo MI, Kim KD. Populations of fungi and bacteria associated with samples of stored rice in Korea. Mycobiology. 2007;35:36–38. doi: 10.4489/MYCO.2007.35.1.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh JY, Kim EN, Ryoo MI, Kim KD. Morphological and molecular identification of Penicillium islandicum isolate KU101 from stored rice. Plant Pathol J. 2008;24:469–473. [Google Scholar]
- 21.Oh JY, Sang MK, Lee H, Ryoo MI, Kim KD. First detection of Penicillium fellutanum from stored rice in Korea. Res Plant Dis. 2011;17:216–221. [Google Scholar]
- 22.Oh JY, Sang MK, Oh JE, Lee HJ, Ryoo MI, Kim KD. Microbial population, aflatoxin contamination and predominant Aspergillus species in Korean stored rice. Plant Pathol J. 2010;26:121–129. [Google Scholar]
- 23.Wilson DM, Mubatanhema W, Jurjevic Z. Biology and ecology of mycotoxigenic Aspergillus species as related to economic and health concerns. Adv Exp Med Biol. 2002;504:3–17. doi: 10.1007/978-1-4615-0629-4_2. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis V, Magan N. Environmental conditions affecting mycotoxins. In: Magan N, Olsen M, editors. Mycotoxins in food: detection and control. Cambridge: Woodhead Publishing Ltd.; 2004. pp. 174–189. [Google Scholar]
- 25.Hocking AD, Pitt JI. Water relations of some Penicillium species at 25°C. Trans Br Mycol Soc. 1979;73:141–145. [Google Scholar]
- 26.World Health Organization. Basic food safety for health workers [Internet] Geneva: World Health Organization; 1999. [cited 2017 Jul 25]. Available from: http://apps.who.int/iris/bitstream/10665/65992/1/WHO_SDE_PHE_FOS_99.1. [Google Scholar]
- 27.Cole RJ, Cox RH. Handbook of toxic fungal metabolites. New York: Academic Press; 1981. [Google Scholar]
- 28.Pitt JI. In: Champ BR, Highley E, Hocking AD, Pitt JI, editors. Penicillium toxins; Fungi and mycotoxins in stored products: Proceedings of an International Conference; 1991 Apr 23-26; Bangkok, Thailand. Canberra: Australian Centre for International Agricultural Research; 1991. pp. 99–103. [Google Scholar]
- 29.Peraica M, Radić B, Lucić A, Pavlović M. Toxic effects of mycotoxins in humans. Bull World Health Organ. 1999;77:754–766. [PMC free article] [PubMed] [Google Scholar]
- 30.Agrios G. Plant pathology. 5th ed. Boston (MA): Elsevier Academic Press; 2005. [Google Scholar]
- 31.Kushiro M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. JSM Mycotoxins. 2015;65:19–23. [Google Scholar]
- 32.Lutsky II, Mor N. Alimentary toxic aleukia (septic angina, endemic panmyelotoxicosis, alimentary hemorrhagic aleukia): t-2 toxin-induced intoxication of cats. Am J Pathol. 1981;104:189–191. [PMC free article] [PubMed] [Google Scholar]
- 33.Azziz-Baumgartner E, Lindblade K, Gieseker K, Rogers HS, Kieszak S, Njapau H, Schleicher R, McCoy LF, Misore A, DeCock K, et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ Health Perspect. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamachari KA, Bhat RV, Nagarajan V, Tilak TB. Hepatitis due to aflatoxicosis: an outbreak in western India. Lancet. 1975;305:1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- 35.Wagacha JM, Muthomi JW. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol. 2008;124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Liu GT, Qian YZ, Zhang P, Dong ZM, Shi ZY, Zhen YZ, Miao J, Xu YM. Relationships between Alternaria alternata and oesophageal cancer. IARC Sci Publ. 1991;(105):258–262. [PubMed] [Google Scholar]
- 37.Flieger M, Wurst M, Shelby R. Ergot alkaloids: sources, structures and analytical methods. Folia Microbiol. 1997;42:3–30. doi: 10.1007/BF02898641. [DOI] [PubMed] [Google Scholar]
- 38.Thompson C, Henke SE. Effects of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J Wildl Dis. 2000;36:172–179. doi: 10.7589/0090-3558-36.1.172. [DOI] [PubMed] [Google Scholar]
- 39.Magan N, Medina A, Aldred D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011;60:150–163. [Google Scholar]
- 40.Paterson RR, Lima N. How will climate change affect mycotoxins in food? Food Res Int. 2010;43:1902–1914. [Google Scholar]
- 41.Paterson RR, Lima N. Further mycotoxin effects from climate change. Food Res Int. 2011;44:2555–2566. [Google Scholar]
- 42.Van Egmond HP. Worldwide regulations for mycotoxins. Adv Exp Med Biol. 2002;504:257–269. doi: 10.1007/978-1-4615-0629-4_27. [DOI] [PubMed] [Google Scholar]
- 43.Henry SH, Bosch FX, Troxell TC, Bolger PM. Reducing liver cancer: global control of aflatoxin. Science. 1999;286:2453–2454. doi: 10.1126/science.286.5449.2453. [DOI] [PubMed] [Google Scholar]
- 44.Benford D, Boyle C, Dekant W, Fuchs R, Gaylor DW, Hard G, McGregory DB, Pitt JI, Plestina R, Shephard G, et al. Ochratoxin A. Safety evaluation of certain mycotoxins in food. WHO Food Additives Series 47. FAO Food and Nutrition Paper [Internet] Geneva: World Health Organization IPCS; 2001. [cited 2017 Jul 25]. Available from: http://www.inchem.org/documents/jecfa/jecmono/v47je01.htm. [Google Scholar]
- 45.Shapira R, Paster N. Control of mycotoxins in storage and techniques for their decontamination. In: Magan N, Olsen M, editors. Mycotoxins in food: detection and control. Cambridge: Woodhead Publishing Ltd.; 2004. pp. 190–223. [Google Scholar]
- 46.Pose G, Patriarca A, Kyanko V, Pardo A, Pinto VF. Effect of water activity and temperature on growth of Alternaria alternata on a synthetic tomato medium. Int J Food Mircobiol. 2009;135:60–63. doi: 10.1016/j.ijfoodmicro.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Young AB, Davis ND, Diener UL. Effect of temperature and moisture on tenuazonic acid production by Alternaria tenuissima. Phytopathology. 1980;70:607–609. [Google Scholar]
- 48.Northolt MD, Bullerman LB. Prevention of mold growth and toxin production through control of environmental conditions. J Food Prot. 1982;45:519–526. doi: 10.4315/0362-028X-45.6.519. [DOI] [PubMed] [Google Scholar]
- 49.Sorenson WG, Hesseltine CW, Shotwell OL. Effect of temperature on production of aflatoxin on rice by Aspergillus flavus. Mycopathol Mycol Appl. 1967;33:49–55. [Google Scholar]
- 50.Marín S, Sanchis V, Sáenz R, Ramos AJ, Vinas I, Magan N. Ecological determinants for germination and growth of some Aspergillus and Penicillium spp. from maize grain. J Appl Microbiol. 1998;84:25–36. doi: 10.1046/j.1365-2672.1997.00297.x. [DOI] [PubMed] [Google Scholar]
- 51.Ramos AJ, Labernia N, Marín S, Sanchis V, Magan N. Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int J Food Microbiol. 1998;44:133–140. doi: 10.1016/s0168-1605(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 52.Northolt MD, Van Egmond HP, Paulsch WE. Ochratoxin A production by some fungal species in relation to water activity and temperature. J Food Prot. 1979;42:485–490. doi: 10.4315/0362-028X-42.6.485. [DOI] [PubMed] [Google Scholar]
- 53.Marín S, Magan N, Bellí N, Ramos AJ, Canela R, Sanchis V. Two-dimensional profiles of fumonisin B1 production by Fusarium moniliforme and Fusarium proliferatum in relation to environmental factors and potential for modelling toxin formation in maize grain. Int J Food Microbiol. 1999;51:159–167. doi: 10.1016/s0168-1605(99)00115-4. [DOI] [PubMed] [Google Scholar]
- 54.Lee HB, Patriarca A, Magan N. Alternaria in food: ecophysiology, mycotoxin production and toxicology. Mycobiology. 2015;43:93–106. doi: 10.5941/MYCO.2015.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marín S, Sanchis V, Ramos AJ, Vinas I, Magan N. Environmental factors, in vitro interactions, and niche overlap between Fusarium moniliforme, F. proliferatum, and F. graminearum, Aspergillus and Penicillium species from maize grain. Mycol Res. 1991;102:831–837. [Google Scholar]
- 56.Magan N, Lacey J. Ecological determinants of mould growth in stored grain. Int J Food Microbiol. 1988;7:245–256. doi: 10.1016/0168-1605(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 57.Squire RA. Ranking animal carcinogens: a proposed regulatory approach. Science. 1981;214:877–880. doi: 10.1126/science.7302565. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 56. Lyon: IARC Press; 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; pp. 397–444. [Google Scholar]
- 59.Groopman JD, Kensler TW. The light at the end of the tunnel for chemical-specific biomarkers: daylight or headlight? Carcinogenesis. 1999;20:1–11. doi: 10.1093/carcin/20.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect. 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrlich KC, Kobbeman K, Montalbano BG, Cotty PJ. Aflatoxin-producing Aspergillus species from Thailand. Int J Food Microbiol. 2007;114:153–159. doi: 10.1016/j.ijfoodmicro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Pildain MB, Frisvad JC, Vaamonde G, Cabral D, Varga J, Samson RA. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int J Syst Evol Microbiol. 2008;58(Pt 3):725–735. doi: 10.1099/ijs.0.65123-0. [DOI] [PubMed] [Google Scholar]
- 63.Scheidegger KA, Payne GA. Unlocking the secrets behind secondary metabolism: a review of Aspergillus flavus from pathogenicity to functional genomics. J Toxicol. 2003;22:423–459. [Google Scholar]
- 64.Marsh SF, Payne GA. Scaning EM studies on the colonization of dent corn by Aspergillus flavus. Phytopathology. 1984;74:557–561. [Google Scholar]
- 65.Hill RA, Wilson DM, McMillian WW, Widstrom NW, Cole RJ, Sanders TH, Blankenship PD. Ecology of the Aspergillus flavus group and aflatoxin formation in maize and groundnut. In: Lacey J, editor. Trichothecenes and other mycotoxins. Chichester: Wiley & Sons; 1985. pp. 79–95. [Google Scholar]
- 66.Klich MA. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycoscience. 2007;48:71–80. [Google Scholar]
- 67.Abdel-Hadi A, Schmidt-Heydt M, Parra R, Geisen R, Magan N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J R Soc Interface. 2012;9:757–767. doi: 10.1098/rsif.2011.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousa W, Ghazali FM, Jinap S, Ghazali HM, Radu S. Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. J Food Sci. 2013;78:M56–M63. doi: 10.1111/j.1750-3841.2012.02986.x. [DOI] [PubMed] [Google Scholar]
- 69.Northolt MD, Van Egmond HP, Paulsch WE. Differences between Aspergillus flavus strains in growth and aflatoxin B1 production in relation to water activity and temperature. J Food Prot. 1977;40:778–781. doi: 10.4315/0362-028X-40.11.778. [DOI] [PubMed] [Google Scholar]
- 70.Ogundero VW. Temperature and aflatoxin production by Aspergillus flavus and A. parasiticus strains from Nigerian groundnuts. J Basic Microbiol. 1987;27:511–514. doi: 10.1002/jobm.3620270910. [DOI] [PubMed] [Google Scholar]
- 71.Kheiralla ZH, Hassanin NI, Amra H. Effect of incubation time, temperature and substrate on growth and aflatoxin production. Int Biodeterior Biodegradation. 1992;30:17–27. [Google Scholar]
- 72.Medina A, Gilbert MK, Mack BM, OBrian GR, Rodríguez A, Bhatnagar D, Payne G, Magan N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int J Food Microbiol. 2017;256:36–44. doi: 10.1016/j.ijfoodmicro.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Fedorova ND, Montalbano BG, Bhatnagar D, Cleveland TE, Bennett JW, Nierman WC. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol Lett. 2011;322:145–149. doi: 10.1111/j.1574-6968.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- 74.Faraj MK, Smith JE, Harran G. Interaction of water activity and temperature on aflatoxin production by Aspergillus flavus and A. parasiticus in irradiated maize seeds. Food Addit Contam. 1991;8:731–736. doi: 10.1080/02652039109374031. [DOI] [PubMed] [Google Scholar]
- 75.Cuero RG, Smith JE, Lacey J. Interaction of water activity, temperature and substrate on mycotoxin production by Aspergillus flavus, Penicillium viridicatum and Fusarium graminearum in irradiated grains. Trans Br Mycol Soc. 1987;89:221–226. [Google Scholar]
- 76.Zhang F, Guo Z, Zhong H, Wang S, Yang W, Liu Y, Wang S. RNA-Seq-based transcriptome analysis of aflatoxigenic Aspergillus flavus in response to water activity. Toxins (Basel) 2014;6:3187–3207. doi: 10.3390/toxins6113187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georgianna DR, Payne GA. Genetic regulation of aflatoxin biosynthesis: from gene to genome. Fungal Genet Biol. 2009;46:113–125. doi: 10.1016/j.fgb.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Yu J, Bhatnagar D, Cleveland TE. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004;564:126–130. doi: 10.1016/S0014-5793(04)00327-8. [DOI] [PubMed] [Google Scholar]
- 79.Nierman WC, Yu J, Fedorova-Abrams ND, Losada L, Cleveland TE, Bhatnagar D, Bennett JW, Dean R, Payne GA. Genome sequence of Aspergillus flavus NRRL 3357, a strain that causes aflatoxin contamination of food and feed. Genome Announc. 2015;3:e00168–e00115. doi: 10.1128/genomeA.00168-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Brian GR, Georgianna DR, Wilkinson JR, Yu J, Abbas HK, Bhatnagar D, Cleveland TE, Nierman W, Payne GA. The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia. 2007;99:232–239. doi: 10.3852/mycologia.99.2.232. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Hadi A, Carter D, Magan N. Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B1 production during storage of peanuts under different water activity levels. J Appl Microbiol. 2010;109:1914–1922. doi: 10.1111/j.1365-2672.2010.04820.x. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt-Heydt M, Abdel-Hadi A, Magan N, Geisen R. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int J Food Microbiol. 2009;135:231–237. doi: 10.1016/j.ijfoodmicro.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt-Heydt M, Rüfer CE, Abdel-Hadi A, Magan N, Geisen R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010;26:241–246. doi: 10.1007/s12550-010-0062-7. [DOI] [PubMed] [Google Scholar]
- 84.Gallo A, Solfrizzo M, Epifani F, Panzarini G, Perrone G. Effect of temperature and water activity on gene expression and aflatoxin biosynthesis in Aspergillus flavus on almond medium. Int J Food Microbiol. 2016;217:162–169. doi: 10.1016/j.ijfoodmicro.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 85.Bernáldez V, Córdoba JJ, Magan N, Peromingo B, Rodríguez A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. Food Sci Technol. 2017;83:283–291. [Google Scholar]
- 86.Liu BH, Chu FS. Regulation of aflR and its product, AflR, associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1998;64:3718–3723. doi: 10.1128/aem.64.10.3718-3723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang PK. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol Genet Genomics. 2003;268:711–719. doi: 10.1007/s00438-003-0809-3. [DOI] [PubMed] [Google Scholar]
- 88.Chang PK. Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J Biotechnol. 2004;107:245–253. doi: 10.1016/j.jbiotec.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Yu J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins (Basel) 2012;4:1024–1057. doi: 10.3390/toxins4111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Price MS, Yu J, Nierman WC, Kim HS, Pritchard B, Jacobus CA, Bhatnagar D, Cleveland TE, Payne GA. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol Lett. 2006;255:275–279. doi: 10.1111/j.1574-6968.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 91.Li F, Yoshizawa T. Alternaria mycotoxins in weathered wheat from China. J Agric Food Chem. 2000;48:2920–2924. doi: 10.1021/jf0000171. [DOI] [PubMed] [Google Scholar]
- 92.Magan N, Cayley GR, Lacey J. Effect of water activity and temperature on mycotoxin production by Alternaria alternata in culture and on wheat grain. Appl Environ Microbiol. 1984;47:1113–1117. doi: 10.1128/aem.47.5.1113-1117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kosiak B, Torp M, Skjerve E, Andersen B. Alternaria and Fusarium in Norwegian grains of reduced quality: a matched pair sample study. Int J Food Microbiol. 2004;93:51–62. doi: 10.1016/j.ijfoodmicro.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 94.Azcárate MP, Patriarca A, Terminiello L, Fernández Pinto V. Alternaria toxins in wheat during the 2004 to 2005 Argentinean harvest. J Food Prot. 2008;71:1262–1265. doi: 10.4315/0362-028x-71.6.1262. [DOI] [PubMed] [Google Scholar]
- 95.Meronuck RA, Steele JA, Mirocha CJ, Christensen CM. Tenuazonic acid, a toxin produced by Alternaria alternata. Appl Microbiol. 1972;23:613–617. doi: 10.1128/am.23.3.613-617.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller ME, Urban K, Köppen R, Siegel D, Korn U, Koch M. Mycotoxins as antagonistic or supporting agents in the interaction between phytopathogenic Fusarium and Alternaria fungi. World Mycotoxin J. 2014;8:311–321. [Google Scholar]
- 97.Bezuidenhout SC, Gelderblom WC, Gorst-Allman CP, Horak RM, Marasas WF, Spiteller G, Vleggaar R. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem Commun. 1988;11:743–745. [Google Scholar]
- 98.Marasas WF, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, Missmer SA, Cabrera J, Torres O, Gelderblom WC, et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 99.Sanchis V, Abadias M, Oncins L, Sala N, Viñas I, Canela R. Occurrence of fumonisins B1 and B2 in corn-based products from the Spanish market. Appl Environ Microbiol. 1994;60:2147–2148. doi: 10.1128/aem.60.6.2147-2148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sedmíková M, Reisnerova H, Dufková Z, Bárta I, Jílek F. Potential hazard of simultaneous occurrence of aflatoxins B1 and ochratoxin A. Vet Med. 2001;46:169–174. [Google Scholar]
- 101.Frisvad JC, Frank JM, Houbraken JA, Kuijpers AF, Samson RA. New ochratoxin A producing species of Aspergillus section Circumdati. Stud Mycol. 2004;50:23–43. [Google Scholar]
- 102.Lee HB, Magan N. Impact of environment and interspecific interactions between spoilage fungi and Aspergillus ochraceus on growth and ochratoxin production in maize grain. Int J Food Microbiol. 2000;61:11–16. doi: 10.1016/s0168-1605(00)00385-8. [DOI] [PubMed] [Google Scholar]
- 103.Pardo E, Marín S, Sanchis V, Ramos AJ. Prediction of fungal growth and ochratoxin A production by Aspergillus ochraceus on irradiated barley grain as influenced by temperature and water activity. Int J Food Microbiol. 2004;95:79–88. doi: 10.1016/j.ijfoodmicro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Wearing AH, Burgess LW. Water potential and the saprophytic growth of Fusarium roseum “Graminearum. ” Soil Biol Biochem. 1979;11:661–667. [Google Scholar]
- 105.Madhyastha S, Marquardt RR, Abramson D. Effect of ochratoxin producing fungi on the chemical composition of wheat and barley. J Food Qual. 1993;16:287–299. [Google Scholar]
- 106.Häggblom P. Production of ochratoxin A in barley by Aspergillus ochraceus and Penicillium viridicatum: effect of fungal growth, time, temperature, and inoculum size. Appl Environ Microbiol. 1982;43:1205–1207. doi: 10.1128/aem.43.5.1205-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cairns-Fuller V, Aldred D, Magan N. Water, temperature and gas composition interactions affect growth and ochratoxin A production by isolates of Penicillium verrucosum on wheat grain. J Appl Microbiol. 2005;99:1215–1221. doi: 10.1111/j.1365-2672.2005.02695.x. [DOI] [PubMed] [Google Scholar]
- 108.Hagler WM, Jr, Towers NR, Mirocha CJ, Eppley RM, Bryden WL. Zearalenone: mycotoxin or mycoestrogen? In: Summerell BA, Leslie JF, Backhouse D, Bryden WL, Burgess LW, editors. Fursarium: Paul E. Nelson Memorial Symposium. St. Paul (MN): APS Press; 2001. pp. 321–331. [Google Scholar]
- 109.Magan N, Lacey J. Water relations of some Fusarium species from infected wheat ears and grain. Trans Br Mycol Soc. 1984;83:281–285. [Google Scholar]
- 110.Versonder RF, Ellis JJ, Kwolek WF, DeMarini DJ. Production of vomitoxin on corn by Fusarium graminearum NRRL 5883 and Fusarium roseum NRRL 6101. Appl Environ Microbiol. 1982;43:967–970. doi: 10.1128/aem.43.4.967-970.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sutton JC, Baliko W, Funnell HS. Relation of weather variables to incidence of zearalenone in corn in southern Ontario. Can J Plant Sci. 1980;60:149–155. [Google Scholar]
- 112.Eugenio CP, Christensen CM, Mirocha CJ. Factors affecting production of the mycotoxin F-2 by Fusarium roseum. Phytopathology. 1970;60:1055–1057. doi: 10.1094/phyto-60-1055. [DOI] [PubMed] [Google Scholar]
- 113.Ryu D, Bullerman LB. Effect of cycling temperatures on the production of deoxynivalenol and zearalenone by Fusarium graminearum NRRL 5883. J Food Prot. 1999;62:1451–1455. doi: 10.4315/0362-028x-62.12.1451. [DOI] [PubMed] [Google Scholar]
- 114.Hope R, Magan N. Two-dimensional environmental profiles of growth, deoxynivalenol and nivalenol production by Fusarium culmorum on a wheat-based substrate. Lett Appl Microbiol. 2003;37:70–74. doi: 10.1046/j.1472-765x.2003.01358.x. [DOI] [PubMed] [Google Scholar]
- 115.Jayas DS, Jeyamkondan S. PH-postharvest technology: modified atmosphere storage of grains meats fruits and vegetables. Biosyst Eng. 2002;82:235–251. [Google Scholar]
- 116.Landers KE, Davis ND, Diener UL. Influence of atmospheric gases on aflatoxin production by Aspergillus flavus in peanuts. Phytopathology. 1967;57:1086–1090. [PubMed] [Google Scholar]
- 117.Shih CN, Marth EH. Aflatoxin produced by Aspergillus parasiticus when incubated in the presence of different gases. J Milk Food Technol. 1973;36:421–425. [Google Scholar]
- 118.Lillehoj EB, Milburn MS, Ciegler A. Control of Penicillium martensii development and penicillic acid production by atmospheric gases and temperatures. Appl Microbiol. 1972;24:198–201. doi: 10.1128/am.24.2.198-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paster N, Lisker N, Chet I. Ochratoxin A production by Aspergillus ochraceus Wilhelm grown under controlled atmospheres. Appl Environ Microbiol. 1983;45:1136–1139. doi: 10.1128/aem.45.3.1136-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paster N, Barkai-Golan R, Calderon M. Control of T-2 toxin production using atmospheric gases. J Food Prot. 1986;49:615–617. doi: 10.4315/0362-028X-49.8.615. [DOI] [PubMed] [Google Scholar]
- 121.Paster N, Blumenthal-Yonassi J, Barkai-Golan R, Menasherov M. Production of zearalenone in vitro and in corn grains stored under modified atmospheres. Int J Food Microbiol. 1991;12:157–165. doi: 10.1016/0168-1605(91)90065-w. [DOI] [PubMed] [Google Scholar]
- 122.Samapundo S, De Meulenaer B, Atukwase A, Debevere J, Devlieghere F. The influence of modified atmospheres and their interaction with water activity on the radial growth and fumonisin B1 production of Fusarium verticillioides and F. proliferatum on corn. Part I: The effect of initial headspace carbon dioxide concentration. Int J Food Microbiol. 2007;114:160–167. doi: 10.1016/j.ijfoodmicro.2006.09.005. [DOI] [PubMed] [Google Scholar]