Abstract
Orchidaceous plants have symbiotic relationships with endophytic fungi, including mycorrhizal fungi, which play important roles in the seed germination and growth of the host plants. In this study, endophytic fungal communities isolated from the roots of Cephalanthera longibracteata collected from three different sites in Korea were analyzed, and it was determined whether fungal communities were preferentially correlated with the sites. The fungal isolates were identified by sequence analysis of the internal transcribed spacer regions of rDNA. In total, 30 species of endophytic fungi, including two species of mycorrhizal fungi belonging to the genus Tulasnella, were identified. Leptodontidium orchidicola showed the highest frequency and was isolated from all root samples. Species diversity and richness were not significantly different among sites. However, the community structure of the endophytic fungi significantly differed among sites, suggesting that the site characteristics affected the community composition of the endophytic fungi colonizing the roots of C. longibracteata. Our findings will aid in developing methods involving the use of symbiotic fungi for orchid conservation and restoration in native habitats.
Keywords: Cephalanthera longibracteata, Fungal endophytes, Leptodontidium orchidicola, Mycorrhizas, Orchids
Cephalanthera longibracteata is a well-known orchid species that is endangered in Korea [1]. This wild terrestrial orchid species grows in shaded areas in forests and is seriously endangered because of excessive overcollection, habitat destruction, and climate change. Therefore, conservation and restoration of this endangered orchid are regarded as focus areas in research pertaining to plant ecosystems and diversity [2].
Most orchidaceous plants have a symbiotic relationship with endophytic fungi [3]. Orchid mycorrhizal fungi form special structures called pelotons in the root cortex [4], enhance plant uptake of inorganic nutrients such as nitrogen and phosphorus [5], and provide carbon to the host plant during the early phase of germination and seedling growth [6]. Nonmycorrhizal endophytic fungi are nonpathogenic fungi that colonize host plants and play a role in improving resistance to pathogens by producing secondary metabolites [7]. Fusarium spp. isolated from surface-sterilized seeds of Cypripedium reginae facilitate plant seed germination in vitro [8], suggesting that these endophytic fungi may also affect seed germination of these plants in nature.
The host specificity of root symbiotic fungi in orchids has been controversial since long [4]. Some studies have shown that mycorrhizal fungi isolated from wild orchids have host specificity [9,10], while others reported that their specificity is low [11]. However, only a few studies have compared the host specificities of endophytic fungi (including nonmycorrhizal endophytes) colonizing orchids.
In this study, the endophytic fungal communities colonizing the roots of C. longibracteata were analyzed to determine whether they were preferentially correlated with different regions. Our results would provide important fundamental data for developing methods involving the use of symbiotic fungi for orchid conservation and restoration in native habitats.
MATERIALS AND METHODS
Root sampling and fungal isolation
Sampling areas for C. longibracteata roots were selected from three sites in Korea: Mt. Hambaek (37°16' N, 128°91' E) in Jeongseon, Gangwon-do; Mt. Gaya (36°70' N, 126°61' E) in Seosan, Chungcheongnam-do; and Mt. Baekjok (36°59' N, 127°58' E) in Cheongju, Chungcheongbuk-do. Three C. longibracteata root specimens were collected at each site. The roots of the collected orchids were surface-sterilized using a method modified from that reported by Richardson et al. [12], within 24 hr of sampling. After the healthy roots were washed with running tap water, they were surface-sterilized with 70% ethanol, 3% NaClO, and streptomycin and chloramphenicol solution, and rinsed with sterilized distilled water. After water was removed from the root surface, the root was cut into 5-mm-long segments. In total, 40 segments were selected from each sample and four segments were placed on a petri dish containing 1% water agar. Hyphal tips from root segments were transferred onto potato dextrose agar (PDA). After isolation, mycelia were subcultured on PDA for identification. Morphological characteristics of the fungi were examined using an AXIO Imager A1 light microscope (Carl Zeiss, Jena, Germany).
Phylogenetic analysis
Genomic DNA was extracted from the fungal mycelium by using the DNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA). The ITS1F and ITS4 primers were used to amplify the internal transcribed spacer (ITS) region of rDNA [13]. The PCR protocol was as follows: predenaturation at 94℃ for 5min, followed by 30 cycles of denaturation at 94℃ for 30 sec, annealing at 50℃ for 30 sec, and elongation at 72℃ for 1 min. PCR product purification and sequencing were performed by Solgent (Daejeon, Korea). Phylogenetic analysis was performed using neighbor-joining methods with MEGA6 [14].
Data analysis
The relative abundance and frequency of endophytic fungi isolated from the orchids collected from the same site were determined. The similarity index and Shannon's diversity index were calculated and compared among sites using one-way ANOVA (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
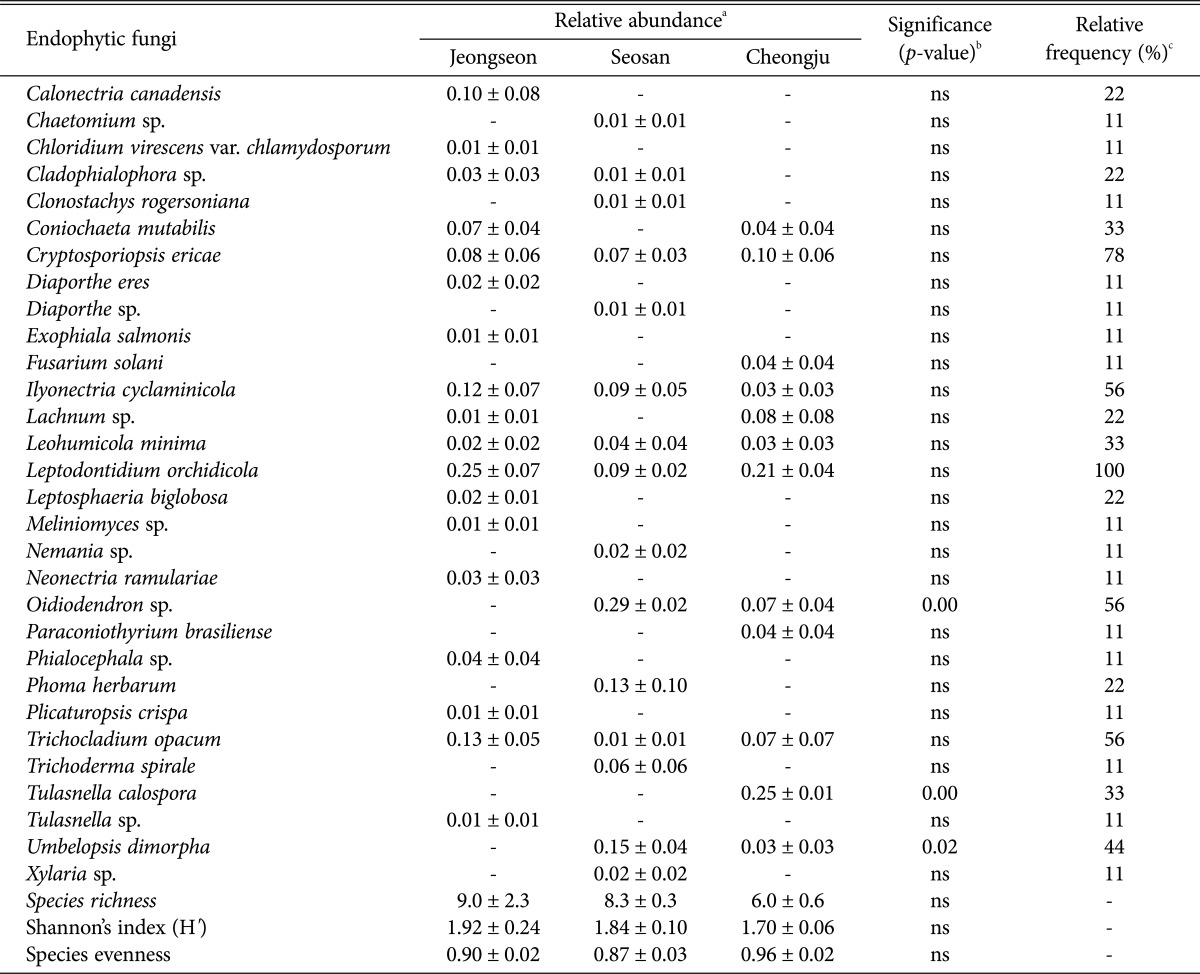
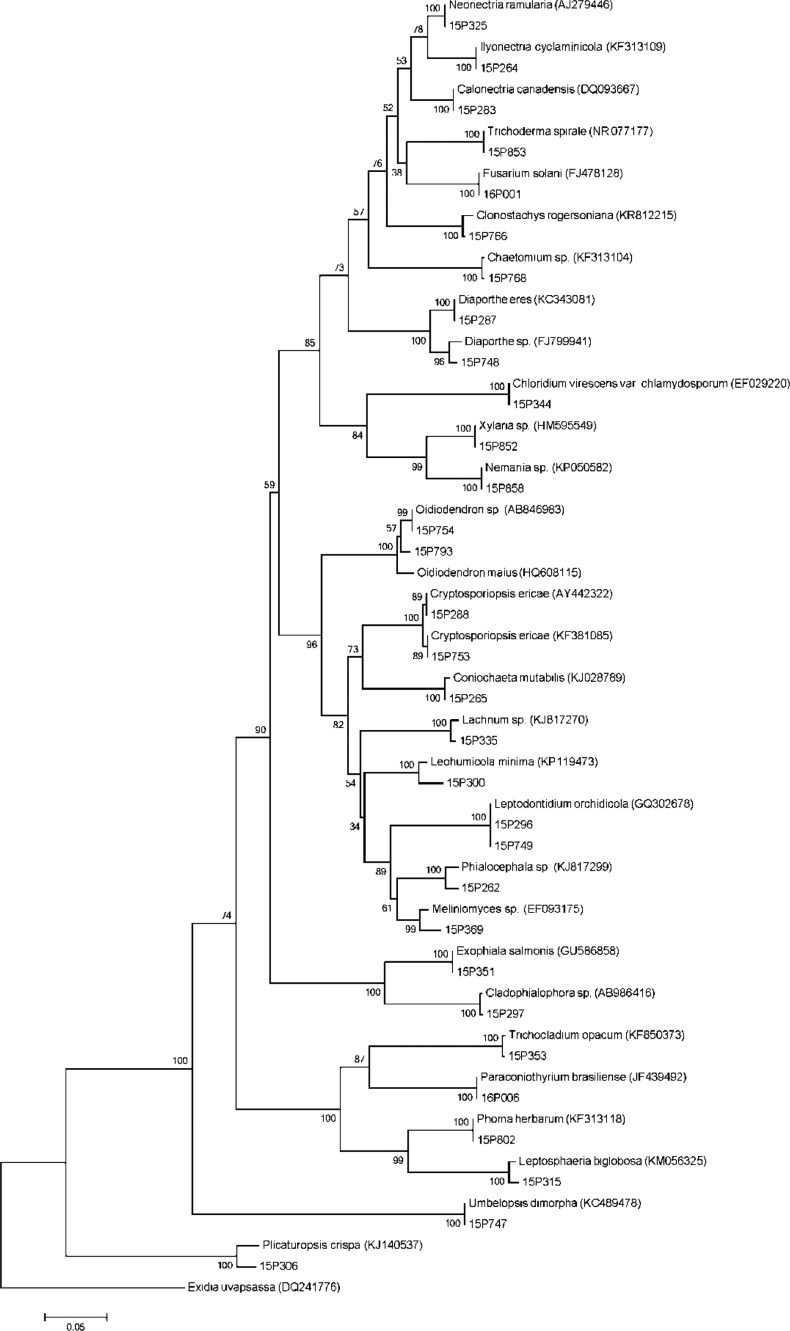
In this study, we analyzed the endophytic fungal communities colonizing the roots of C. longibracteata to ascertain whether these communities were preferentially correlated with different regions. In total, 30 endophytic fungal species were identified in this study (Table 1, Fig. 1). Seventeen species of endophytic fungi, including a mycorrhizal fungal species, Tulasnella, were isolated from the roots of C. longibracteata collected in Jengseon. Although the dominant species slightly differed among regions, Leptodontidium orchidicola had the highest relative frequency in all individuals. L. orchidicola was first isolated from orchid roots and reported as a new species by Currah et al. [15]. It was isolated from terrestrial orchids such as Coeloglossum viride and Platanthera hyperborea [16], as well as from Cypripedium japonicum and Cypripedium macranthum [17]. Phialocephala fortinii is the endophytic fungal species the most frequently isolated from terrestrial orchids [18]. It is a dark septate endophyte that creates hyphal connections between plant roots and transports photosynthetic products through these connections [19]. P. fortinii promotes seed germination and seedling growth of Dactylorhiza praetermissa, a terrestrial orchid, indicating that this species is an endophytic fungus that also plays an important role in the early growth of orchids [20].
Table 1. Relative abundances for endophytic fungi isolated from roots of Cephalanthera longibracteata.
ns, not significant.
aRelative abundance indicates the percent ratio of the isolate numbers for each fungal species to the total numbers of isolates in each study site.
bp-values were obtained by ANOVA to compare the means of the relative abundances of each fungal species among sites. ns, not significant at α = 0.05.
cRelative frequency indicates the percent percentages of samples from which each fungal species was isolated to the total number of samples.
Fig. 1. Neighbor-joining tree based on analysis of sequences of internal transcribed spacer sequence of endophytic fungi isolated from roots of Cephalanthera longibracteata. Exidia uvapassa was used as an outgroup.
Fifteen species of endophytic fungi were isolated from C. longibracteata collected in Seosan; mycorrhizal species were not isolated. At this site, fungal species belonging to Oidiodendron showed the highest relative frequency in the orchid roots. Fungi belonging to the genus Oidiodendron are known as ericoid mycorrhizal fungi, which form a symbiotic relationship with the roots of ericaceous plants [21]. In particular, Oidiodendron maius was found to form mycorrhizae in the roots of Rhododendron fortunei, an ericaceous plant, and to increase the absorption of nitrogen from the soil [22]. This fungal species has been isolated from the roots of Cypripedium acaule [23] and from C. macranthum in Korea [17].
Twelve species of endophytic fungi, including Tulasnella calospora, which is a mycorrhizal fungus, were isolated from C. longibracteata in Cheongju. T. calospora is the mycorrhizal fungal species the most frequently isolated from terrestrial orchids and has been isolated from several native orchid species in Korea, including Cymbidium goeringii, Neolindleya camtschatica, Oreorchis patens, Spiranthes sinensis, and Cephalanthera falcata [24,25]; it was first isolated from C. longibracteata.
Shannon's diversity index and evenness index for endophytic fungi isolated from the roots of C. longibracteata did not significantly differ among different study sites (p > 0.05 for both). L. orchidicola was isolated at 100% frequency from all three sites, and its relative abundance was also high at each site, suggesting that this species is closely related to the orchid. However, the relative abundances of Oidiodendron sp., Umbelopsis dimorpha, and T. calospora significantly differed (p < 0.05) among sites, suggesting that the communities of endophytic fungi symbiotic with C. longibracteata were site-dependent.
The mycorrhizal fungus T. calospora was isolated at a high frequency from orchids collected from Chungcheongbuk-do, suggesting site-specificity of the fungi colonizing the orchid. However, further studies involving DNA sequencing would be required to identify fungal communities colonizing orchid roots.
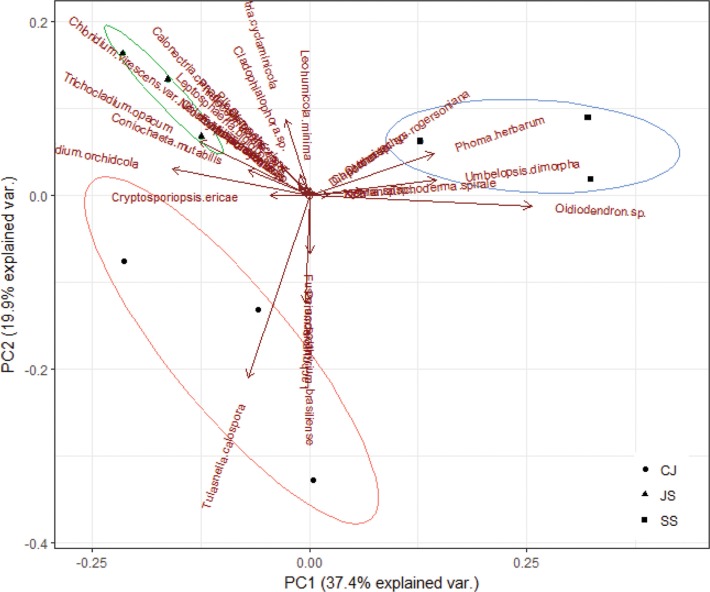
The similarity within fungal communities was considerably higher than that between the communities (Fig. 2). High similarity within communities reflects the site-specificity in relation to the host. The similarity between communities was low, suggesting that the site characteristics were affected, reflecting the results for Oidiodendron sp., U. dimorpha, and T. calospora, which showed significant differences among different sites. In addition, ordination among fungal communities showed that the symbiotic fungi were more specific to the habitat than to their host, suggesting that this species could be established in a new habitat without their specific symbiotic fungi (Fig. 3). Determining factors affecting the spatial distribution and abundance of endangered plants is an important challenge in current conservation biology. Dispersal limitation and recruitment affect plant distribution and abundance [26]. For orchid plants, which depend on mycorrhizal symbiosis for the completion of their life cycle, it has been reported that host specificity of symbiotic fungi and the distribution of suitable fungi have decisive influences on the distribution of the orchid plants [27,28]. By investigating symbiotic fungi that have a significant impact on orchid growth and seed germination and the host specificity of symbiotic fungi, the restorative effect could be improved by supplying the symbiotic fungi specific to orchids [29].
Fig. 2. Similarity index (mean ± SE) within and among communities of endophytic fungi isolated from Cephalanthera longibracteata.
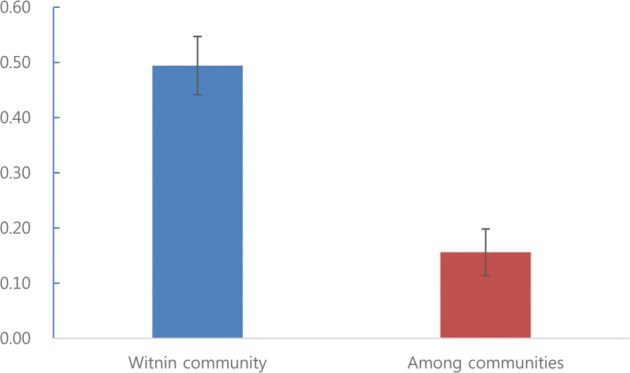
Fig. 3. Principle component analysis for communities of endophytic fungi isolated from Cephalanthera longibracteata. CJ, Cheongju; JS, Jeongseon; SS, Seosan.
Studies on using symbiotic fungi for restoration of orchids initially determine fungal host specificity and verify diversity. Therefore, it is necessary to continue studies on the effect of fungal strain on orchid seed germination and early growth by isolating endophytic fungi symbiotic with various orchids from various sites. The current study confirmed the diversity of endophytic fungi symbiotic with C. longibracteata, and confirmed that site characteristics affect the community composition of these endophytic fungi. In addition, P. fortinii, which is known to promote seed germination [12], was isolated from our specimens; further studies on increasing the seed germination rate should be performed using this fungal strain.
References
- 1.Kim S, Lee K. The orchid in Korea. Seoul: Kyohaksa; 1997. [Google Scholar]
- 2.Swarts ND, Dixon KW. Terrestrial orchid conservation in the age of extinction. Ann Bot. 2009;104:543–556. doi: 10.1093/aob/mcp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant Soil. 2002;244:149–163. [Google Scholar]
- 4.Rasmussen HN. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 5.Dearnaley JD. Further advances in orchid mycorrhizal research. Mycorrhiza. 2007;17:475–486. doi: 10.1007/s00572-007-0138-1. [DOI] [PubMed] [Google Scholar]
- 6.Cameron DD, Leake JR, Read DJ. Mutualistic mycorrhiza in orchids: evidence from plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol. 2006;171:405–416. doi: 10.1111/j.1469-8137.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 7.Carroll G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology. 1988;69:2–9. [Google Scholar]
- 8.Vujanovic V, St-Arnaud M, Barabé D, Thibeault G. Viability testing of orchid seed and the promotion of colouration and germination. Ann Bot. 2000;86:79–86. [Google Scholar]
- 9.Warcup JH. Specificity of mycorrhizal association in some Australian terrestrial orchids. New Phytol. 1971;70:41–46. [Google Scholar]
- 10.Taylor DL, Bruns TD, Szaro TM, Hodges SA. Divergence in mycorrhizal specialization within Hexalectris spicata (Orchidaceae), a nonphotosynthetic desert orchid. Am J Bot. 2003;90:1168–1179. doi: 10.3732/ajb.90.8.1168. [DOI] [PubMed] [Google Scholar]
- 11.Hadley G. Non-specificity of symbiotic interction in orchid mycorrhiza. New Phytol. 1970;69:1015–1023. [Google Scholar]
- 12.Richardson KA, Currah RS, Hambleton S. Basidiomycetous endophytes from the roots of neotropical epiphytic Orchidaceae. Lindleyana. 1993;8:127–137. [Google Scholar]
- 13.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currah RS, Sigler L, Hambleton S. New records and new taxa of fungi from the mycorrhizae of terrestrial orchids of Alberta. Can J Bot. 1987;65:2473–2482. [Google Scholar]
- 16.Zelmer CD. Interactions between northern terrestrial orchids and fungi in nature. Edmonton: University of Alberta; 1994. [Google Scholar]
- 17.Lee BH, Han HK, Kwon HJ, Eom AH. Diversity of endophytic fungi isolated from roots of Cypripedium japonicum and C. macranthum in Korea. Korean J Mycol. 2015;43:20–25. [Google Scholar]
- 18.Currah RS, Zelmer CD, Hambleton S, Richardson KA. Fungi from orchid mycorrhizas. In: Arditti J, Pridgeon AM, editors. Orchid biology. Dordrecht: Springer; 1997. pp. 117–170. [Google Scholar]
- 19.Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature. 1997;388:579–582. [Google Scholar]
- 20.Zimmerman E, Peterson RL. Effect of a dark septate fungal endophyte on seed germination and protocorm development in a terrestrial orchid. Symbiosis. 2007;43:45–52. [Google Scholar]
- 21.Couture M, Fortin JA, Dalpe Y. Oidiodendron griseum Robak: an endophyte of ericoid mycorrhiza in Vaccinium spp. New Phytol. 1983;95:375–380. [Google Scholar]
- 22.Wei X, Chen J, Zhang C, Pan D. A new Oidiodendron maius strain isolated from Rhododendron fortunei and its effects on nitrogen uptake and plant growth. Front Microbiol. 2016;7:1327. doi: 10.3389/fmicb.2016.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunch WD, Cowden CC, Wurzburger N, Shefferson RP. Geography and soil chemistry drive the distribution of fungal associations in lady's slipper orchid, Cypripedium acaule. Botany. 2013;91:850–856. [Google Scholar]
- 24.Youm JY, Han HK, Chung JM, Cho YC, Lee BC, Eom AH. Identification of orchid mycorrhizal fungi isolated from five species of terrestrial orchids in Korea. Korean J Mycol. 2012;40:132–135. [Google Scholar]
- 25.Lee BH, Han HK, Eom AH. Identification of orchid mycorrhizal fungi isolated from terrestrial orchids in Mt. Hambaek, Korea. Korean J Mycol. 2015;43:129–132. [Google Scholar]
- 26.Clark CJ, Poulsen JR, Levey DJ, Osenberg CW. Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am Nat. 2007;170:128–142. doi: 10.1086/518565. [DOI] [PubMed] [Google Scholar]
- 27.McCormick MK, Taylor DL, Juhaszova K, Burnett RK, Jr, Whigham DF, O'Neill JP. Limitations on orchid recruitment: not a simple picture. Mol Ecol. 2012;21:1511–1523. doi: 10.1111/j.1365-294X.2012.05468.x. [DOI] [PubMed] [Google Scholar]
- 28.McCormick MK, Jacquemyn H. What constrains the distribution of orchid populations? New Phytol. 2014;202:392–400. [Google Scholar]
- 29.Reiter N, Whitfield J, Pollard G, Bedggood W, Argall M, Dixon K, Davis B, Swarts N. Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecol. 2016;217:81–95. [Google Scholar]