Abstract
During a survey of fungal diversity of the order Hypocreales in Korea, two Acremonium isolates, CNUFC-1YSRS2-4 and CNUFC-GSNPF3-1, were isolated from soils collected on a bank of the Yeongsan River, Naju, and in a forest on the Mt. Daegak located on Sinsi Island, Gunsan, South Korea, respectively. Based on the morphological characteristics and sequence analysis of the internal transcribed spacer and D1/D2 domains of 28S ribosomal DNA, the isolates CNUFC-1YSRS2-4 and CNUFC-GSNPF3-1 were identified as A. variecolor and A. persicinum, respectively. These 2 species represent novel Hypocreales isolates in Korea.
Keywords: Acremonium persicinum, Acremonium variecolor, Diversity, Hypocreales
The anamorph Acremonium Link is the largest genus within the order Hypocreales. The genus Acremonium, formerly called Cephalosporium, includes approximately 100 species; these organisms are known to be saprobic on dead plants or soil dwellers [1]. In 1971, the taxon Acremonium fulfilled the criteria of the standard taxonomical nomenclature and was designated as a separate genus [2]. Some of Acremonium species are known to be opportunistic pathogens of humans and animals [3], causing eumycetoma, onychomycosis, and hyalohyphomycosis. Infections by Acremonium in humans are rare but could result in clinical manifestations of hyalohyphomycosis, such as arthritis, osteomyelitis, peritonitis, endocarditis, pneumonia, cerebritis, and subcutaneous infection [4]. Of note, many species of this genus have been identified as producers of useful metabolites. Cephalosporins, which belong to the β-lactam class of antibiotics, were derived from Acremonium strictum W. Gams (syn. Cephalosporium acremonium Corda). In Korea, anti-inflammatory sesquiterpenoids from a sponge-derived Acremonium sp. were reported [5]. In addition, phthalide and isocoumarin derivatives have been reported to be produced by Acremonium sp. isolated from a mangrove [6]. Acremonium cellulolyticus is known to be a possible cellulase producer [7,8].
In terms of taxonomy, this genus is morphologically simple; thus, classification at the species level is difficult. Their morphological features include septate hyphae with simple, tapered, lateral phialides produced singly or in groups and unicellular, globose-to-cylindrical conidia, which are mostly aggregated in slimy heads at the apex of the phialide [9]. Nonetheless, their taxonomy has not been firmly resolved due to the absence of clear-cut morphological differences at the species level and the absence of reliable sequences in public databases [10,11].
In 2011, Summerbell et al. [12] presented the results on the phylogenetic analyses of the D1/D2 domain of large subunit and small subunit ribosomal DNA (rDNA) to classify the majority of Acremonium species. In that study, some species of Acremonium were reclassified into the genus Gliomastix and included into Sarocladium.
Only 5 species of Acremonium, namely A. strictum, A. acutatum, A. cellulolyticus, A. zonatum, and A. sclerotigenum, have been recorded in Korea since 2006 [9,13,14,15,16], but A. cellulolyticus (GenBank accession No. AB474749) was transferred to the genus Talaromyces by Fujii et al. [17]. In the present study, we sought to investigate the morphological characteristics of Acremonium isolates obtained from soil samples in Korea and to determine phylogenetic positions of A. variecolor and A. persicinum.
MATERIALS AND METHODS
Extraction of fungal strains from soil samples
The strains used in this study were Acremonium species isolated from soil samples collected on the Yeongsan riverside in Naju (GPS 35.0022967, 126.6832094) and on Sinsi Island (Sinsido) in Gunsan (GPS 35.818669, 126.473644), Korea. The soil samples were placed in conical tubes and kept at ambient temperature until cultured. Fungi were isolated by the serial dilution plating method. Briefly, 1 g of soil was mixed with 9 mL of sterile distilled water and shaken for 15 min at room temperature; serial dilutions ranging from 10−3 to 10−5 were then prepared. An aliquot of 0.1 mL from each dilution was transferred onto potato dextrose agar (PDA; 39 g PDA in 1 L of deionized water; Becton, Dickinson and Co., Sparks, MD, USA) and incubated at 25℃ for 3–7 days.
Hyphal tips were transferred to new PDA plates under a stereomicroscope. To obtain each pure culture, individual colonies of varied morphologies were transferred to PDA plates. Pure isolates were maintained in PDA slant tubes and stored in 20% glycerol at −80℃ in the Environmental Microbiology Laboratory Fungarium, Chonnam National University, Gwangju, Korea.
Morphological analyses
To obtain samples for microscopic examination and growth rate determination, isolates CNUFC-1YSRS2-4 and CNUFC-GSNPF3-1 were cultured on each of the 3 media: PDA, malt extract agar (MEA; 33.6 g MEA in 1 L of deionized water; Becton, Dickinson and Co.), and oatmeal agar (OA; 1.5% oatmeal and 1.5% agar in 1 L of deionized water; Junsei, Tokyo, Japan). The plates were incubated at 25℃ in the dark for 7 days. Samples were mounted in a drop of distilled water on a glass slide and were examined under an Olympus BX51 microscope with DIC optics (Olympus, Tokyo, Japan). Fine structures of the fungi were analyzed by scanning electron microscopy (Hitachi S4700 field emission scanning electron microscope; Hitachi, Tokyo, Japan). Samples were fixed in 2.5% paraformaldehyde-glutaraldehyde buffer with 0.05M phosphate (pH 7.2) (Junsei) for 2 hr and washed in cacodylate buffer (Junsei). Cellular membranes were preserved by fixing the samples in 1% osmium tetroxide (diluted in cacodylate buffer; Electron Microscopy Sciences, Hatfield, PA, USA) for 1 hr, by washing again in cacodylate buffer, dehydrating in a graded series of ethanol solutions (Emsure, Darmstadt, Germany) and isoamyl acetate (Junsei), and by drying in a fume hood. Finally, samples were covered with gold in a sputter coater and examined at Korea Basic Science Institute, Gwangju, Korea.
DNA extraction, PCR, and sequencing
Total genomic DNA was directly extracted from mycelia using the Solg Genomic DNA Prep Kit for fungi (SolGent, Daejeon, Korea). The internal transcribed spacers (ITS1 and ITS2) and the 5.8S gene were amplified with primers ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) by the method of Cano et al. [18]. D1/D2 domains of the 28S rRNA gene were amplified with primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) according to the method of Gilgado et al. [20]. The PCR amplification mixture (total volume 20 µL) contained 10 ng of a fungal DNA template, 5 pmol/µL each primer, and the Accupower PCR Premix (Taq DNA polymerase, dNTPs, buffer, and a tracking dye; Bioneer Corp., Daejeon, Korea). The PCR products were purified by means of the Accuprep PCR Purification Kit (Bioneer Corp.). DNA sequencing was performed on an ABI 3700 Automated DNA sequencer (Applied Biosystems Inc., Foster City, CA, USA).
Phylogenetic analyses
Fungal sequences (Table 1) were initially aligned in Clustal_X v.2.1 [21] and BioEdit v.7.2.6 software [22]. Phylogenetic analyses were conducted in MEGA 7.0.26 with the default settings [23]. Phylogenetic trees were constructed by the maximum likelihood method with the ITS rDNA sequences and a combined dataset of the ITS and D1/D2 sequences. Acremonium butyri (J. F. H. Beyma) W. Gams served as an outgroup in the phylogenetic tree of the ITS sequences, whereas Acremonium implicatum (J. C. Gilman & E. V. Abbott) W. Gams and Sarocladium bacillisporum (Onions & G. L. Barron) Summerb., served as an outgroup in the combined tree of ITS regions and D1/D2 datasets. The sequence identity was determined by means of the National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool for nucleotides (BLASTn).
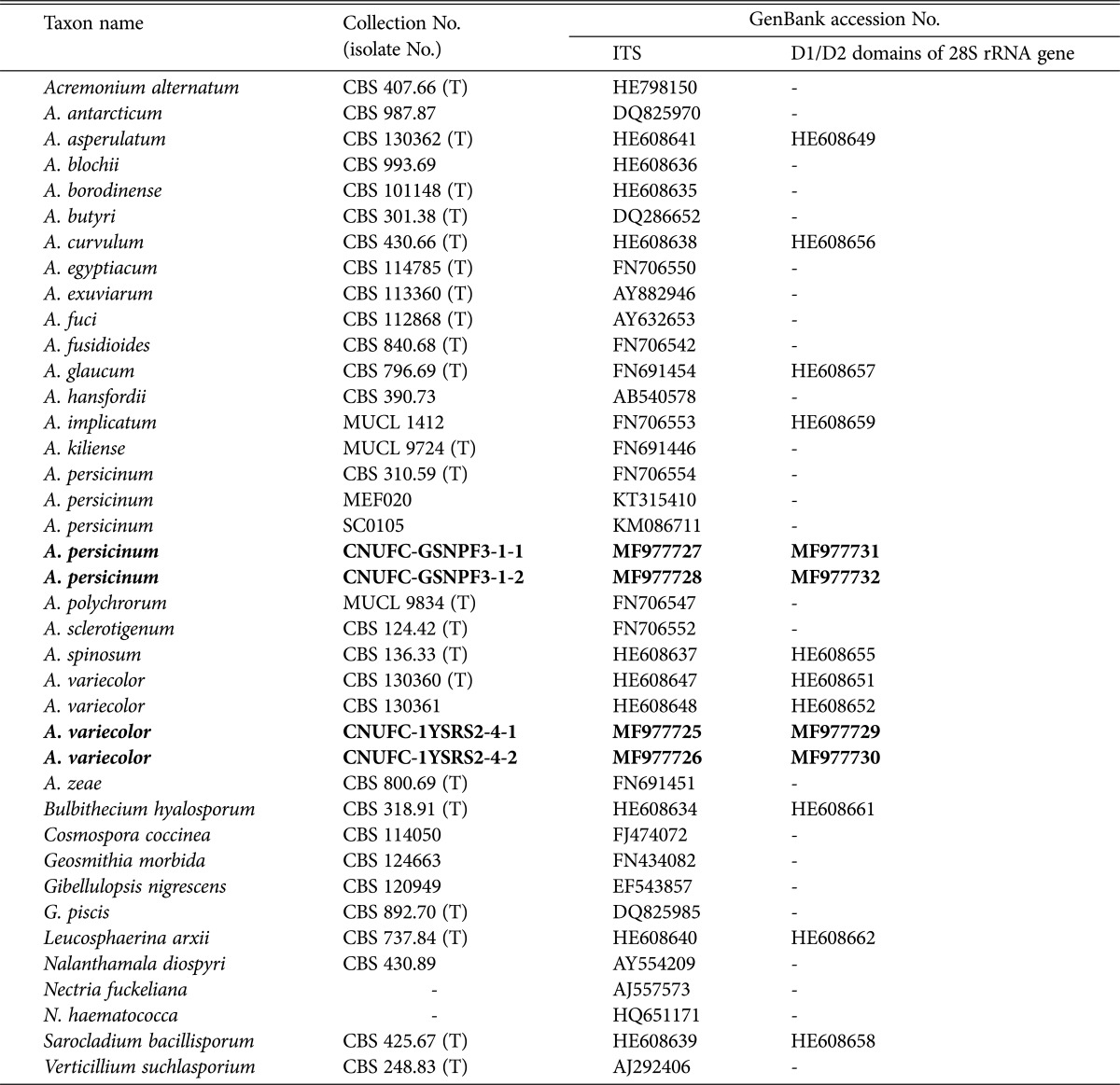
Table 1. Information on the sequences of Acremonium and related genera used in the present study.
Bold letters represent strains used in this study.
ITS, internal transcribed spacer; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; T, ex-type strain; MUCL, Mycothéque de l'Université Catholique de Louvain, Louvain-la Neuve, Belgium; MEF and SC, strain or voucher number absent; CNUFC, Environmental Microbiology Laboratory Fungarium, Chonnam National University, Gwangju, South Korea.
RESULTS
Phylogenetic analysis
The ITS and 28S sequences of strains CNUFC-1YSRS2-4-1, CNUFC-1YSRS2-4-2, CNUFC-GSNPF3-1-1, and CNUFC-GSNPF3-1-2 were deposited in the NCBI database under accession numbers shown in Table 1.
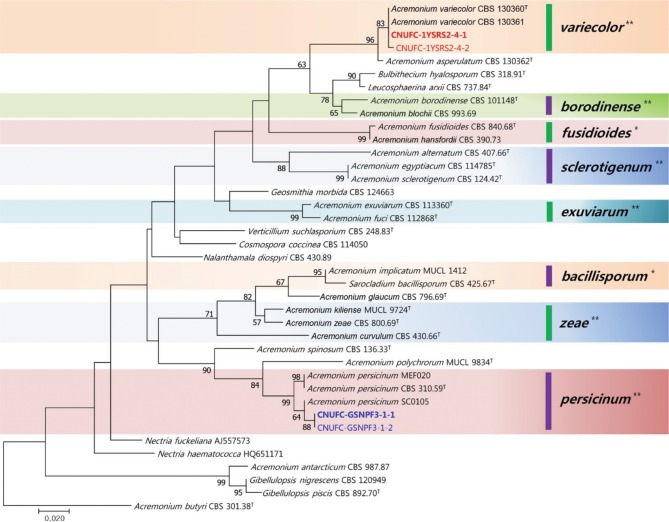
A BLASTn search revealed that the ITS rDNA sequences of CNUFC-1YSRS2-4-1 and CNUFC-GSNPF3-1-1 have high sequence identities of 99.1% (472/476 bp) and 98.3% (520/529 bp) with A. variecolor (GenBank accession No. LN714516) and A. persicinum (KF993390), respectively. The phylogenetic tree of the ITS region with high bootstrap values (96% and 99%) confirmed that the CNUFC-1YSRS2-4-1 and CNUFC-GSNPF3-1-1 isolates are A. variecolor and A. persicinum, respectively (Fig. 1).
Fig. 1. A phylogenetic tree based on the maximum likelihood method analysis of the internal transcribed spacer rDNA region for Acremonium variecolor CNUFC-1YSRS2-4-1, A. variecolor CNUFC-1YSRS2-4-2, A. persicinum CNUFC-GSNPF3-1-1, and A. persicinum CNUFC-GSNPF3-1-2. Acremonium butyri served as an outgroup. Numbers at the nodes indicate the bootstrap values (> 50%) from 1,000 replications. The scale bar indicates the number of substitutions per position. The thick colored clade lines with the asterisk (*) were constructed by means of a phylogenetic system described by Summerbell et al. [12], whereas the clade lines marked with the asterisk (**) are suggested by the present authors.
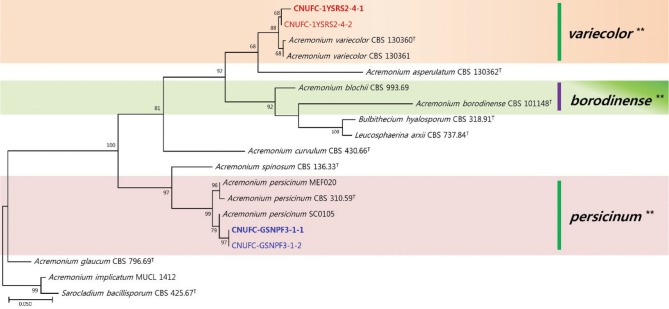
In the analysis of the D1/D2 domains of the 28S rDNA sequences, CNUFC-1YSRS2-4-1 and CNUFC-GSNPF3-1-1 strains showed 100% (488/488 bp) and 99.5% (544/547 bp) sequence identities with A. variecolor (LN714516) and A. persicinum (AB920179), respectively. Moreover, in a combined analysis of the ITS and D1/D2 sequence regions, the 2 strains within the order Hypocreales, like other Acremonium species, showed well-supported branch values (Fig. 2).
Fig. 2. A phylogenetic tree based on application of the maximum likelihood method to multiple loci including internal transcribed spacer region and D1/D2 rDNA sequence for Acremonium variecolor CNUFC-1YSRS2-4-1, A. variecolor CNUFC-1YSRS2-4-2, A. persicinum CNUFC-GSNPF3-1-1, and A. persicinum CNUFC-GSNPF3-1-2. Acremonium implicatum and Sarocladium bacillisporum served as outgroups. Numbers at the nodes indicate the bootstrap values (> 50%) from 1,000 replications. The bar indicates the number of substitutions per position. The thick colored clade lines with the asterisk (**) are suggested by the authors.
Taxonomy of CNUFC-1YSRS2-4-1
Acremonium variecolor Giraldo, Guarro, Gené & Cano, Mycologia 104: 1463 (2012) (Table 2, Fig. 3).
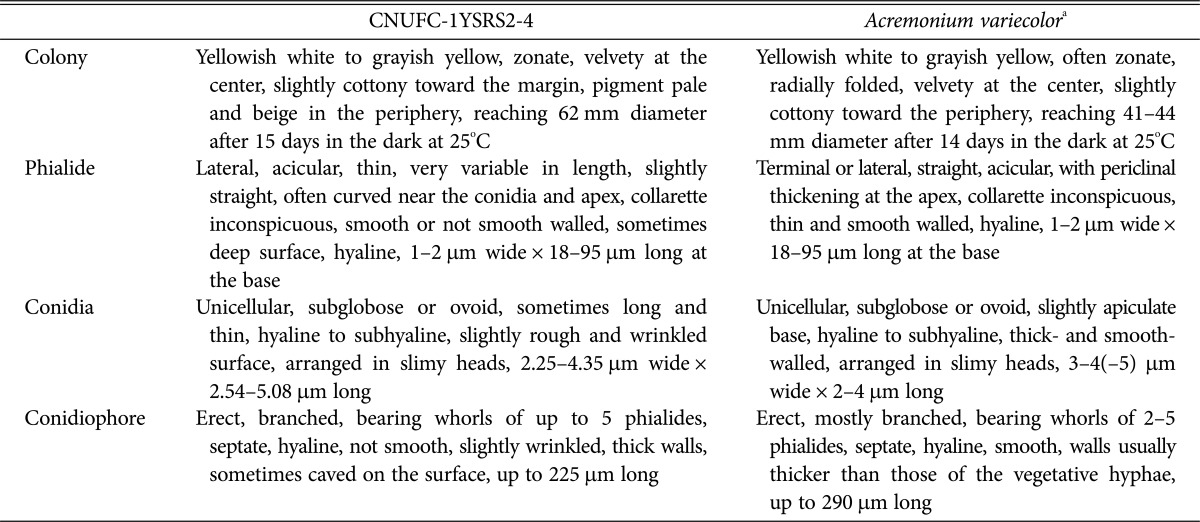
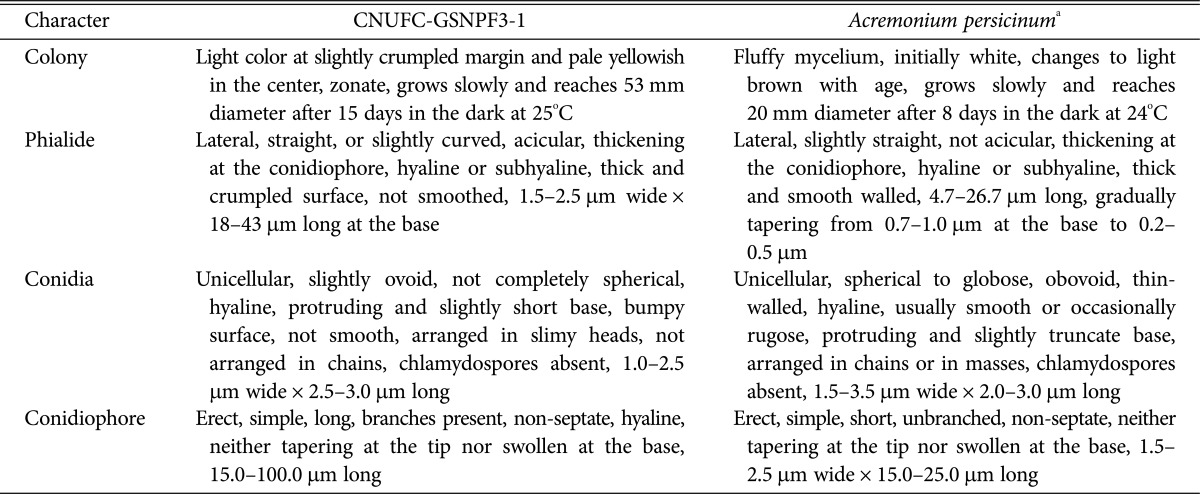
Table 2. Morphological characteristics of CNUFC-1YSRS2-4 and of the reference species Acremonium variecolor when grown on the potato dextrose agar medium at 25℃.
a From the description by Giraldo et al. [19].
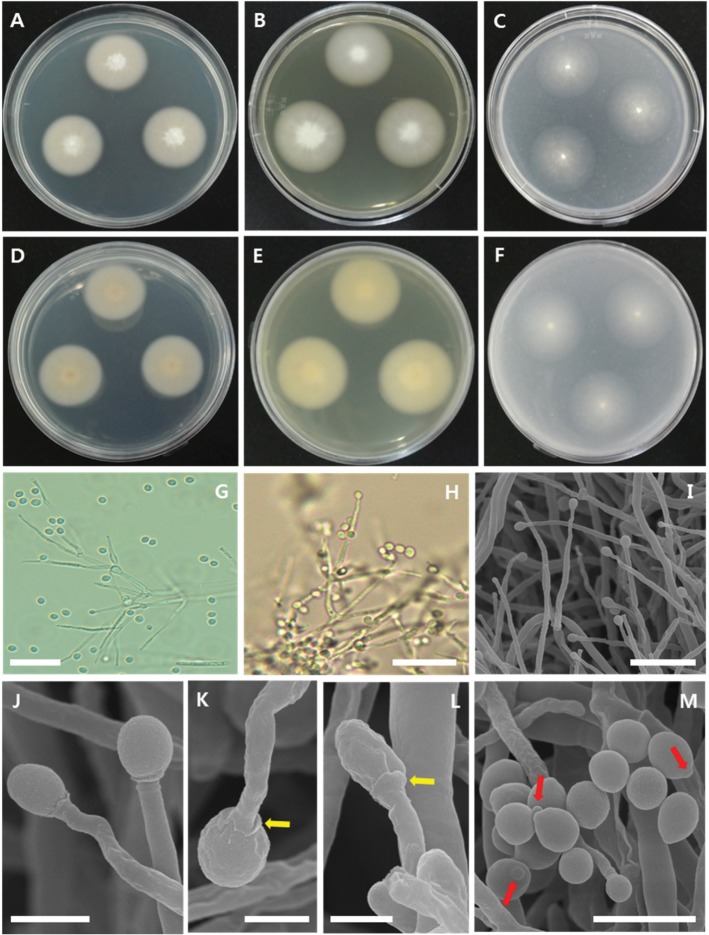
Fig. 3. Morphology of Acremonium variecolor CNUFC-1YSRS2-4-1. A–F, Colonies on potato dextrose agar (A, D), malt extract agar (B, E), or oatmeal agar (C, F) (A–C, obverse view; D–F, reverse view); G–I, Branched conidiophores, an acicular phialide, and oval conidia; J–L, A curved or straight phialide and globose to subglobose or ovoid conidia supported by a calyxlike structure (arrows); M, Conidia detached from slimy heads and conidia with an apiculate base (arrows) (scale bars: G, H = 20 µm, I = 10 µm, J = 2.5 µm, K, L = 1.5 µm, M = 5 µm).
Description: Colonies were initially white and then changed to brown, reaching 62 mm in diameter after 15 days in culture at 25℃ on PDA. The colony reverse was pale yellow and regularly zonate. Conidiophores were erect, mostly branched, bearing whorls of up to 5 phialides. Phialides were straight or acicular, measuring 1.0–2.0 µm wide by 18.0–95.0 µm long at the base. Conidia were subglobose or ovoid, measuring 2.25–4.35 µm wide × 2.54–5.08 µm long.
Taxonomy of CNUFC-GSNPF3-1-1
Acremonium persicinum (Nicot) W. Gams, Cephalosporium-artige Schimmelpilze: 75 (1971) (Table 3, Fig. 4).
Table 3. Morphological characteristics of CNUFC-GSNPF3-1 and of the reference species Acremonium persicinum on potato dextrose agar medium at 24–25℃.
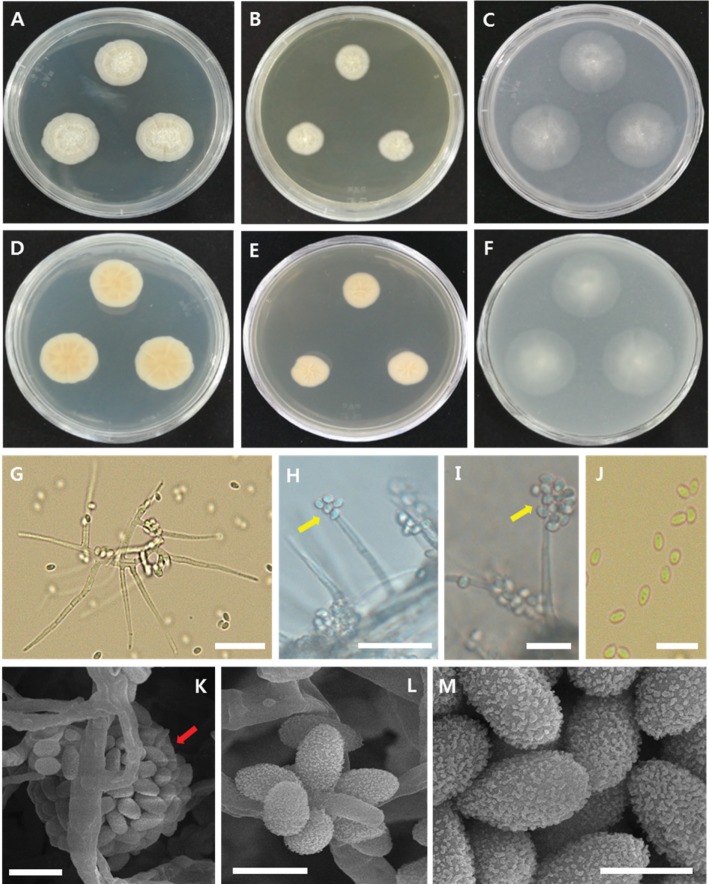
Fig. 4. Morphological characteristics of Acremonium persicinum CNUFC-GSNPF3-1-1. A–F, Colonies on potato dextrose agar (A, D), malt extract agar (B, E), or oatmeal agar (C, F) (A–C, obverse view; D–F, reverse view); G, A long and sharp phialide with conidia; H, I, Oval conidia arranged in slimy heads (arrows) on the phialide; J, Conidia; K, Conidia clustered in the slimy head (arrow); L, Arranged conidia with rough walls in the slimy head; M, Oval conidia with rough walls (scale bars: G, H = 20 µm, I, J = 10 µm, K, L = 2.5 µm, M = 2 µm).
= Paecilomyces persicinus Nicot, Bulletin de la Société Mycologique de France 74: 222 (1958).
= Cephalosporium purpurascens Sukapure & Thirum, Mycologia 55: 563 (1963).
Description: Colonies were initially white and then changed to light pinkish with age, reaching 53 mm in diameter after 15 days in culture at 25℃ on PDA. The middle of the colony obverse was initially dark white and became pale and formed a crumpled shape as the colony grew toward the edge. On MEA and OA, the colony reverse was light yellow. Conidiophores were erect, simple, or branched. Phialides were lateral and acicular, measuring 1.5–2.5 µm wide × 18–43 µm long at the base. Conidia were oval, measuring 1.0–2.0 µm wide × 2.5–3.0 µm long.
Mycelial growth
The growth varied with strains and media. The average growth rates of CNUFC-1YSRS2-4-1 on the PDA, MEA, and OA media at 25℃ were 53, 73, and 75 mm per 12 days, respectively. The growth rates of CNUFC-GSNPF3-1-1 were 36, 51, and 53 mm after 12 days, respectively. The A. variecolor CNUFC-1YSRS2-4-1 strain grew faster than did the A. persicinum CNUFC-GSNPF3-1-1 strain in the 3 media, and both strains grew the fastest on the OA medium.
DISCUSSION
Recent molecular studies [19] showed that the genus Acremonium is polyphyletic, whose species split into different orders of Sordariomycetes. As a result, significant taxonomic changes for Acremonium and Acremonium-like anamorphs were recently made. To date, many Acremonium species have been reported, but there are no trustworthy classification systems and little sequence data are available in GenBank for multigene analyses. Herein, the phylogenetic positions of A. variecolor and A. persicinum were confirmed based only on a combination of ITS region and large subunit rDNA sequence analysis.
Giraldo et al. [19] used only several loci of ITS, 28S, and actin gene sequences that are available in GenBank to create a phylogenetic tree. Nevertheless, because the act-1 sequences for A. variecolor are lacking in GenBank, it was impossible to apply this type of analysis to the samples obtained here.
Multilocus sequence analyses employed herein revealed that the 2 species undescribed in Korea were well placed within the related clades without the use of act-1 data. This is because there are still unknown and unconfirmed species related to these A. variecolor and A. persicinum species [19].
The A. persicinum strain identified in this study was placed into the persicinum clade within the Gliomastix/Bionectria clade. Moreover, the A. variecolor strain identified herein completely matched the type strain of A. variecolor CBS130360 described by Giraldo et al. [19].
Most Acremonium are saprophytes isolated from diverse dead plant materials and soils. The role of Acremonium species in human infections has long been known. The high prevalence of these species in soil or air may lead to superficial infections [26]. Since Guarro et al. [27] provided an overview of opportunistic fungal infections caused by Acremonium (Cephalosporium) species and discussed the classification of these species along with the diagnosis and treatment of the relevant diseases, much progress in Acremonium research has been made.
The 2 Acremonium species, A. variecolor and A. persicinum, for the first time recorded in Korea in this study, were isolated from soil samples. A. variecolor and A. persicinum were first isolated from forest soil collected at Garganta de Escuaín, Huesca, in Northern Spain and from soil collected at Koyna Valley in Chiplun, India, respectively [19,24].
Acremonium species are generally resistant to antifungal agents. According to Guarro et al. [27], clinical and environmental isolates of Acremonium are also resistant to antifungal agents including azole compounds, but are sensitive to amphotericin B in in vitro antibiotic sensitivity assays.
In this study, the 2 species A. variecolor and A. persicinum produced phialides with conidia arranged in slimy heads, but only A. persicinum produced single conidia or conidia in a chain. By contrast, A. persicinum CNUFC-GSNPF3-1-1 did not produce them.
Although some species of Acremonium have been found previously in Korea [9,13,14,15,16], there is little information regarding descriptions and phylogenetic analyses. Especially, the sequence data on Acremonium cellulolyticus Y-94 (AB474749) retrieved from GenBank reveals that the species name is wrong and was placed into Talaromyces cellulolyticus as mentioned by Fujii and colleagues [15,17].
Further studies are needed, especially regarding the growth of these strains under different environmental conditions and resistance to antifungal agents.
ACKNOWLEDGEMENTS
This work was supported by the Project on Survey and Discovery of Indigenous Species of Korea funded by NIBR, and by the Project on Discovery of Fungi from Freshwater and Collection of Fungarium funded by NNIBR, the Ministry of Environment (MOE), and in part carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (PJ012957), Rural Development Administration, Republic of Korea.
References
- 1.Domsch KH, Gams W, Anderson TH. Compendium of soil fungi. Eching: IHW-Verlag; 2007. pp. 30–38. [Google Scholar]
- 2.Gams W. Cephalosporium-artige Schimmelpilze (Hyphomycetes) Stuttgart: Gustav Fischer Verlag; 1971. [Google Scholar]
- 3.de Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of clinical fungi. Utrecht/Reus: Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili; 2000. [Google Scholar]
- 4.Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ. Infection due to the fungus Acremonium (Cephalosporium) Medicine (Baltimore) 1991;70:398–409. doi: 10.1097/00005792-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Bao B, Dang HT, Hong J, Lee HJ, Yoo ES, Bae KS, Jung JH. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J Nat Prod. 2009;72:270–275. doi: 10.1021/np8006793. [DOI] [PubMed] [Google Scholar]
- 6.Rukachaisirikul V, Rodglin A, Sukpondma Y, Phongpaichit S, Buatong J, Sakayaroj J. Phthalide and isocoumarin derivatives produced by an Acremonium sp. isolated from a mangrove Rhizophora apiculata. J Nat Prod. 2012;75:853–858. doi: 10.1021/np200885e. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Yano S, Inoue H, Sawayama S. Strain improvement of Acremonium cellulolyticus for cellulase production by mutation. J Biosci Bioeng. 2009;107:256–261. doi: 10.1016/j.jbiosc.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Fujii T, Fang X, Inoue H, Murakami K, Sawayama S. Enzymatic hydrolyzing performance of Acremonium cellulolyticus and Trichoderma reesei against three lignocellulosic materials. Biotechnol Biofuels. 2009;2:24. doi: 10.1186/1754-6834-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Ten L, Lee SY, Back CG, Lee JJ, Lee HB, Jung HY. New recorded species in three genera of the Sordariomycetes in Korea. Mycobiology. 2017;45:64–72. doi: 10.5941/MYCO.2017.45.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarro J, del Palacio A, Gené J, Cano J, González CG. A case of colonization of a prosthetic mitral valve by Acremonium strictum. Rev Iberoam Micol. 2009;26:146–148. doi: 10.1016/S1130-1406(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 11.Perdomo H, Sutton DA, Garcia D, Fothergill AW, Cano J, Gené J, Summerbell RC, Rinaldi MG, Guarro J. Spectrum of clinically relevant Acremonium species in the United States. J Clin Microbiol. 2011;49:243–256. doi: 10.1128/JCM.00793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summerbell RC, Gueidan C, Schroers HJ, de Hoog GS, Starink M, Rosete YA, Guarro J, Scott JA. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud Mycol. 2011;68:139–162. doi: 10.3114/sim.2011.68.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi GJ, Kim JC, Jang KS, Cho KY, Kim HT. Mycoparasitism of Acremonium strictum BCP on Botrytis cinerea, the gray mold pathogen. J Microbiol Biotechnol. 2008;18:167–170. [PubMed] [Google Scholar]
- 14.Oh SY, Nam KW, Yoon DH. Identification of Acremonium acutatum and Trichothecium roseum isolated from grape with white stain symptom in Korea. Mycobiology. 2014;42:269–273. doi: 10.5941/MYCO.2014.42.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari M, Kim S, Kim HS, Lee HB, Lee YS. Sixteen new records of ascomycetes from crop field soil in Korea. Korean J Mycol. 2016;44:271–288. [Google Scholar]
- 16.Lee YS, Jung HY, Lee HB, Kim SH, Shin KS, Eom AH, Kim C, Lee SY Korean Society of Mycology. National list of species of Korea. Ascomycota, Glomeromycota, Zygomycota, Myxomycota, Oomycota. Incheon: National Institute of Biological Resources; 2015. [Google Scholar]
- 17.Fujii T, Hoshino T, Inoue H, Yano S. Taxonomic revision of the cellulose-degrading fungus Acremonium cellulolyticus nomen nudum to Talaromyces based on phylogenetic analysis. FEMS Microbiol Lett. 2014;351:32–41. doi: 10.1111/1574-6968.12352. [DOI] [PubMed] [Google Scholar]
- 18.Cano J, Guarro J, Gene J. Molecular and morphological identification of Colletotrichum species of clinical interest. J Clin Microbiol. 2004;42:2450–2454. doi: 10.1128/JCM.42.6.2450-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldo A, Gené J, Cano J, de Hoog S, Guarro J. Two new species of Acremonium from Spanish soils. Mycologia. 2012;104:1456–1465. doi: 10.3852/11-402. [DOI] [PubMed] [Google Scholar]
- 20.Gilgado F, Cano J, Gené J, Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol. 2005;43:4930–4942. doi: 10.1128/JCM.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 23.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukapure RS, Thirumalachar MJ. Studies of Cephalosporium species from India-II. Bull Torrey Bot Soc. 1966;93:305–311. [Google Scholar]
- 25.Lo Piccolo S, Alfonzo A, Giambra S, Conigliaro G, Lopez-Llorca LV, Burruano S. Identification of Acremonium isolates from grapevines and evaluation of their antagonism towards Plasmopara viticola. Ann Microbiol. 2015;65:2393–2403. [Google Scholar]
- 26.Das S, Saha R, Dar SA, Ramachandran VG. Acremonium species: a review of the etiological agents of emerging hyalohyphomycosis. Mycopathologia. 2010;170:361–375. doi: 10.1007/s11046-010-9334-1. [DOI] [PubMed] [Google Scholar]
- 27.Guarro J, Gams W, Pujol I, Gené I. Acremonium species: new emerging fungal opportunists–in vitro antifungal susceptibilities and review. Clin Infect Dis. 1997;25:1222–1229. doi: 10.1086/516098. [DOI] [PubMed] [Google Scholar]