Abstract
We characterized two endophyte fungi from the leaves of Astragalus membranaceus in Korea. The isolated strains were identified on the basis of the morphological characters and sequences analysis of the internal transcribed spacer and large subunit regions of the rDNA and β-tubulin gene. To the best of our knowledge, this is the first report of Diaporthe oncostoma and Diaporthe infecunda in Korea, and we have provided descriptions and figures.
Keywords: Astragalus mebranaceus, Diaporthe infecunda, Diaporthe oncostoma, Endophytic fungi
Diaporthe Nitschke, including the asexual state of Phomopsis (Sacc.) Bubak, is the one of the largest genera of Ascomycota and is composed of up to 2,000 species names [1]. Diaporthe species have been reported to be endophytes and saprobes [2], but also, they are economically important plant pathogens with a wide range of host plants worldwide [3]. Diaporthe species are ubiquitous endophytes of many host plants [4]. Endophytic fungi colonize plant tissues, without causing any apparent disease symptoms [5,6]. Although the fungi are potentially pathogenic, their pathogenicity was suppressed during the host plant is healthy [5]. They form a symbiotic relationship with a wide range of host plants worldwide and provide benefits to the host plants [7]. They help in seed germination [8], provide pathogen resistance to the host plants, and deter herbivory by producing various enzymes and secondary metabolites [6,9,10].
Astragalus membranaceus Bunge is a perennial herb that belongs to the family Fabaceae and is mainly grown in China and Korea [11,12]. Its roots contain various biologically active substances that has been used as a cardiotonic, diuretic, and vasodilator [13,14]. Recently, substances extracted from A. membranaceus have shown anticancer effects [15] and boosted the immune system [16]. We isolated endophytic fungi colonizing A. membranaceus and identified them on the basis of morphological characteristics and rDNA and β-tubulin gene sequence analysis. In this study, we have reported two Diaporthe species isolated from the roots of A. membranaceus that have not been reported in Korea.
Sampling
Leaves of A. membranaceus were collected from a cultivated field in Jecheon, Korea (37°15′N, 128°18′E). Healthy leaves without any disease symptoms were selected and transported to the laboratory in polyethylene bags within 24 hr of sampling.
Isolation of endophytic fungi
The leaf samples were cleaned with tap water and surface-sterilized with 30% H2O2 solution. The leaf samples were cut into 5 × 5-mm squares and placed in petri dishes containing potato dextrose agar (PDA). The petri dishes were sealed and incubated at 25℃ and examined periodically. The hyphae growing out from the root fragments were transferred to new petri dishes with PDA and incubated at 25℃. The isolates were stored in 20% glycerol at −80℃ at the Mycology Laboratory of Korea National University of Education, Cheongju, Korea, and deposited as a glycerol stock at the Culture Collection of National Institute of Biological Resources, Incheon, Korea.
Morphological characterization
The growth characteristics of the fungal colonies, such as color and diameter, were recorded after incubation on PDA at 25℃ under dark conditions for 7 days. The conidiophores and conidia were examined under a light microscope (AXIO Imager A1; Carl Zeiss, Oberkochen, Germany).
Phylogenetic analysis
Genomic DNA was extracted from the fungal mycelia by using the Exgene Plant SV mini kit (GeneAll, Seoul, Korea), according to the manufacturer's protocols. The internal transcribed spacer (ITS) region of rDNA was amplified using primers ITS1F and ITF4 [17] and the large subunit (LSU) region of rDNA was amplified using primers LR0R and LR16 [18]. In addition, primers Bt2a and Bt2b was used to amplify β-tubulin (TUB) gene [19]. The PCR conditions were as follows: 94℃ for 5 min, followed by 30 cycles of 94℃ for 30 sec, 50℃ (ITS), 44℃ (LSU) or 58℃ (TUB) for 30 sec, 72℃ for 1 min, and final extension at 72℃ for 5min. The amplicons were sequenced by SolGent (Daejeon, Korea). The sequences were deposited in NCBI GenBank and compared with those available in GenBank via BLAST. Phylogenetic analysis was conducted using the neighbor-joining method in MEGA6 [20].
Diaporthe oncostoma (Duby) Fuckel, Jahrb. Nassau. Ver. Naturkd. 23–24: 205 (1870) (Fig. 1).
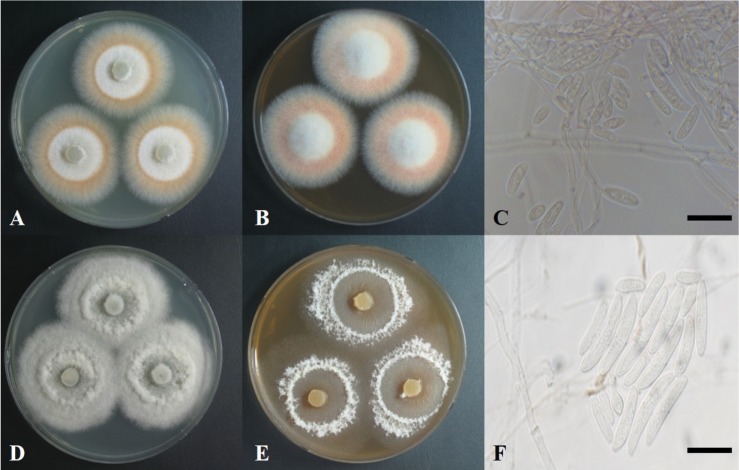
Fig. 1. Colonies of Diaporthe oncostoma grown on potato dextrose agar (PDA) (A), malt extract agar (MEA) medium (B), conidia (C); Diaporthe infecunda grown on PDA (C), MEA medium (E), conidia (F) (scale bars: C, F = 20 µm).
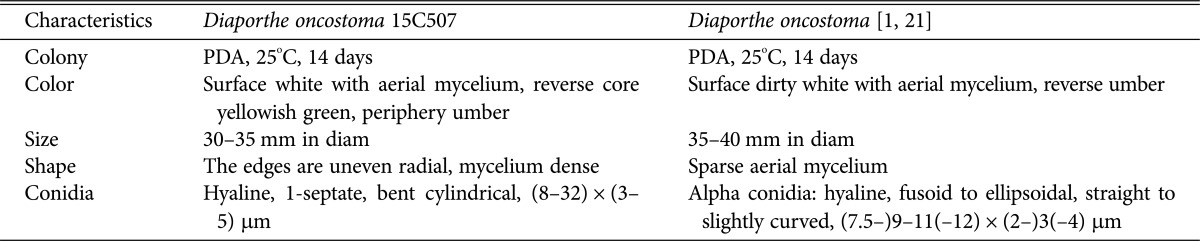
The colony grown on PDA at 25℃ for 14 days was 35–40 mm in diameter (Fig. 1A, Table 1). The surface of the colony was flat and smooth with no exudate. Dense mycelial growth was observed, with aerial growth at the margin. The color of the colony was white at the center and reddish brown at the margin. The reverse side was yellowish green at the center and reddish brown at the margin. The colony on malt extract agar (MEA) for 7 days was 30–35 mm (Fig. 1B). The surface of the colony was convexly raised at the center. The conidium was hyaline, glassy cylindrical with a septum, and was bent in a half-moon shape (Fig. 1C). Its size was (8–32) × (3–5) µm.
Table 1. Comparison of morphological characteristics of Diaporthe oncostoma 15C507 with original descriptions.
PDA, potato dextrose agar.
Specimen examined: Korea, Chungcheongbuk-do, Jecheon-si, 37°15′N, 128°18′E, 14 Aug 2015, isolated from healty leaves of Astragalus membranaceus, strain 15C507 (GenBank No. MF547406, NIBR No. NIBRFG0000499911).
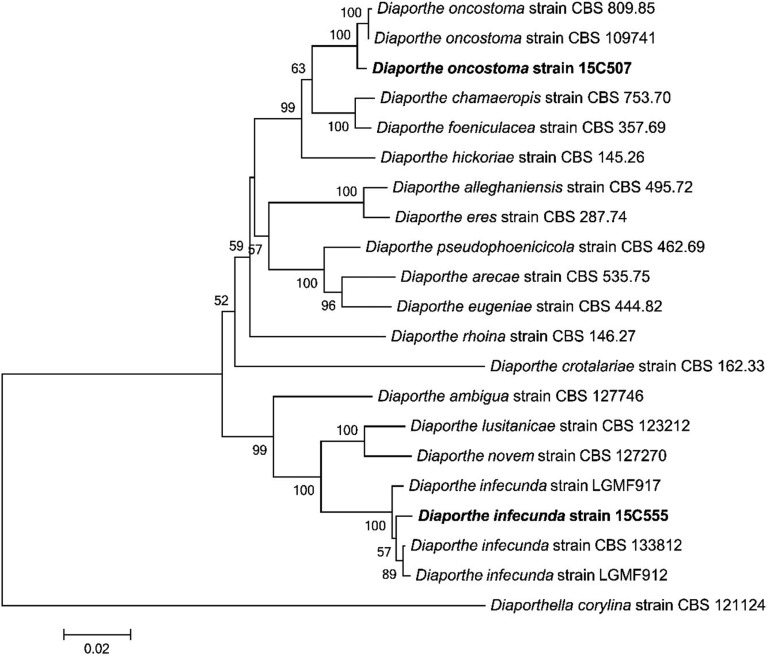
Note: Fuckel [21] has simply characterized the ascospores of this species in the original description. Gomes et al. [1] described D. oncostoma specimens isolated from dead branches or leaves of Robinia pseudoacacia in France, Germany, and Russia and reported two types of conidia, bent oblong alpha conidia and fusiform beta conidia; however, only alpha conidia were found. This species has been considered a saprotrophic or weak parasitic fungus, but also, reported as a pathogen that causes stem canker in R. pseudoacacia in Russia and Greece [22]. Based on a Blast search of NCBI GenBank database, the closest matches for the ITS, LSU, and TUB sequences of D. oncostoma 15C507 were a fungal strain CBS 100454 [1] isolated from leaf spot of R. pseudoacacia in Germany (KC343160 with 97% similarity, KC343160 with 98% similarity and KC344128 with 98% similarity, respectively). The association with Diaporthe was confirmed by the phylogenetic inference of combined sequences of ITS, LSU, and TUB. The isolate 15C507 was clustered together with D. oncostoma CBS100454 (Fig. 2).
Fig. 2. Neighbor-joining tree of Diaporthe oncostoma, Diaporthe infecunda isolates based on a combined alignment of sequences of internal transcribed spacer, large subunit region of rDNA, and β-tubulin. Diaporthella corylina was used as an outgroup. Bootstrap values > 50% are provided above branches. The scale bar indicates the expected number of substitutions per nucleotide.
Diaporthe infecunda R. R. Gomes, C. Glienke & Crous, Persoonia 31: 1 (2013) (Fig. 1).
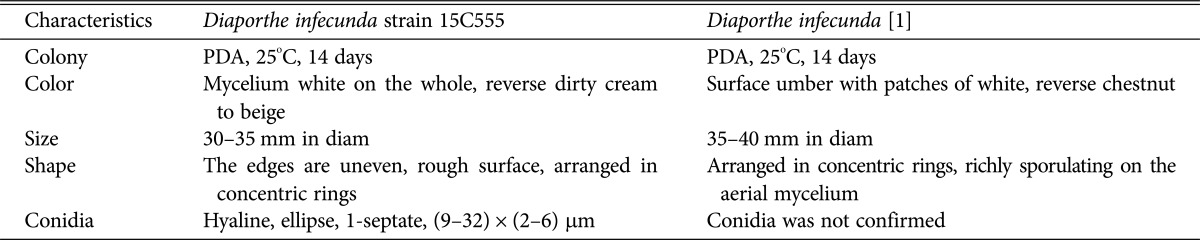
The colony grown on PDA at 25℃ for 14 days was 40–45 mm in diameter (Fig. 1D, Table 2). The surface of the colony was rough with no exudate. Loose mycelial growth was observed. The colony was white and convexly raised at the center. The reverse side was hyaline at the center and dark cream or beige at the margin. The colony grown on MEA for 7 days was 30–35 mm in diameter (Fig. 1E). The surface of the colony was flat, hyaline at the center, and white at the margin. The conidia was hyaline, glassy oblong with a septum (Fig. 1F) and its size was (9–32) × (2–6) µm.
Table 2. Comparison of morphological characteristics of Diaporthe infecunda 15C555 with original discriptions.
PDA, potato dextrose agar.
Specimen examined: Korea, Chungcheongbuk-do, Jecheon-si, 37°15′N, 128°18′E, 8 Aug 2014, isolated from healthy leaves of Astragalus membranaceus, 15C555 (GenBank No. MF547407, NIBR No. NIBRFG0000499912).
Note: Gomes et al. [1] first described this species isolated from the leaves of Schinus terebinthifolius and Maytenus ilicifolia, which are medicinal plants found in Brazil. This fungus has been reported as an endophyte and is sterile. Based on a BLAST search of NCBI GenBank database, the closest matches for the ITS and LSU and TUB sequences of D. infecunda 15C507 were a endophytic strains LGMF917 for ITS and LSU and CBS133812 for TUB [1] isolated from leaf of Schinus terebinthifolius in Brazil (KC343129 with 99% similarity, KC343126 with 99% similarity, and KC344094 with 98% similarity, respectively). The association with Diaporthe was confirmed by the phylogenetic affinity of combined sequences of ITS, LSU, and TUB. The isolate 15C555 was clustered together with D. infecunda KGMF917 (Fig. 2).
A. membranaceus is an important medicinal plant in Korea. However, endophytic fungi for this plant have not been studied in Korea. In addition, some studies report that endophytic fungi produce secondary metabolites with anti-inflammatory activities. Therefore, two Diaporthe species isolated from A. membranaceus and described as new record can contribute to the knowledge of diversity of endophytic fungi in Korea and further efforts to identify endophytic fungi in medicinal plants will allow a better understanding of the relationship between medicinal plants and their endophytic fungi.
ACKNOWLEDGEMENTS
This work was supported by the Project on Survey and Discovery of Indigenous Species of Korea funded by NIBR of the Ministry of Environment (MOE), Republic of Korea.
References
- 1.Gomes RR, Glienke C, Videira SI, Lombard L, Groenewald JZ, Crous PW. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udayanga D, Liu X, McKenzie EH, Chukeatirote E, Bahkali AH, Hyde KD. The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011;50:189. [Google Scholar]
- 3.Santos JM, Phillips AJ. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009;34:111–125. [Google Scholar]
- 4.Botella L, Diez JJ. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011;47:9–18. [Google Scholar]
- 5.Carroll G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology. 1988;69:2–9. [Google Scholar]
- 6.Weber D. Endophytic fungi, occurrence and metabolites. In: Anke T, Weber D, editors. The Mycota. XV. Physiolofy and genetics. Berlin: Springer; 2009. pp. 153–195. [Google Scholar]
- 7.Kai W, Zhiwei Z. Occurrence of arbuscular mycorrhizas and dark septate endophytes in hydrophytes from lakes and streams in southwest China. Int Rev Hydrobiol. 2006;91:29–37. [Google Scholar]
- 8.Clay K. Effects of fungal endophytes on the seed and seedling biology of Lolium perenne and Festuca arundinacea. Oecologia. 1987;73:358–362. doi: 10.1007/BF00385251. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 10.Kusari S, Hertweck C, Spiteller M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Choi IS, Kim SY, Choi BH. A taxonomic revision of Astragalus L. (Fabaceae) in Korea. Korean J Plant Taxon. 2015;45:227–238. [Google Scholar]
- 12.Song IB, Heo K. Phylogenetic study of east-asia Astragalus L. based on morphological characters. Korean J Plant Taxon. 2014;44:191–201. [Google Scholar]
- 13.Yin Y, Heo SI, Jung MJ, Wang MH. Antioxidant and antidiabetic effects of various sections of Astragalus membranaceus. Korean J Parmacogn. 2009;40:1–5. [Google Scholar]
- 14.Jalsrai A, Grecksch G, Becker A. Evaluation of the effects of Astragalus mongholicus Bunge saponin extract on central nervous system functions. J Ethnopharmacol. 2010;131:544–549. doi: 10.1016/j.jep.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Cho WC, Leung KN. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007;252:43–54. doi: 10.1016/j.canlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Song BK, Lee EJ, Kim HK, Jin SD, Kim SJ, Kim DH. Effects of Astrgali radix on the function of murine immunocytes in vivo and in vitro. Korean J Herbol. 1998;13:115–128. [Google Scholar]
- 17.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuckel L. Symbolae mycologicae: Beitrage zur Kenntniss der Rheinischen Pilze. 23-24. Wiesbaden: Jahrb Nassau Ver Naturkd; 1870. pp. 1–459. [Google Scholar]
- 22.Vajna L. Diaporthe oncostoma causing stem canker of black locust in Hungary. Plant Pathol. 2002;51:393. [Google Scholar]