Abstract
The food-related behavior of functional dyspepsia has been attracting more interest of late. This pilot study aims to provide evidence of the physiological, emotional, and attentional aspects of food processing in functional dyspepsia patients. The study was performed in 15 functional dyspepsia patients and 17 healthy controls after a standard breakfast. We measured autonomic nervous system activity using skin conductance response and heart rate variability, emotional response using facial electromyography, and visual attention using eyetracking during the visual stimuli of food/non-food images. In comparison to healthy controls, functional dyspepsia patients showed a greater craving for food, a decreased intake of food, more dyspeptic symptoms, lower pleasantness rating of food images (particularly of high fat), decreased low frequency/high frequency ratio of heart rate variability, and suppressed total processing time of food images. There were no significant differences of skin conductance response and facial electromyography data between groups. The results suggest that high level cognitive functions rather than autonomic and emotional mechanisms are more liable to function differently in functional dyspepsia patients. Abnormal dietary behavior, reduced subjective rating of pleasantness and visual attention to food should be considered as important pathophysiological characteristics in functional dyspepsia.
Introduction
Functional dyspepsia (FD) is defined as a disorder with unexplained symptoms such as postprandial fullness, early satiation, epigastric pain and burning that originate in the gastroduodenal region1,2. So far, our knowledge of pathophysiological abnormalities in FD has been limited to functional abnormalities in the gastrointestinal tract (visceral hypersensitivity, abnormal accommodation, delayed gastric emptying, and gastric dysmotility), and only a small number of studies have investigated the psychological characteristics of FD patients and revealed the crucial role of anxiety, depression, and somatization3–5.
More recent studies investigating the role of dietary habit and nutritional intake in FD patients suggest that fat ingestion is a factor in symptom triggering6–8. Although it is already known that FD patients tolerate only small amounts of food, evidence on the extent of nutritional intake of daily meals remains inconclusive9. One of the limitations of previous studies in FD patients was that a food diary or questionnaire was used to measure their dietary habits, possibly causing a recall bias10–12. In studies using dietary interventions in FD patients, specific amounts of solid or liquid type meals were served to determine the meal-related dyspeptic symptom, gastric accommodation, or hormonal changes13–16. Furthermore, the psychophysiological response and cognitive processing of food stimuli in FD patients are not well established despite the fact that these are important determinants in the pathophysiology of eating disorders such as anorexia nervosa, binge eating disorder, and obesity17,18. Since a close relationship between the exacerbated FD symptoms and meal ingestion has been reported13–16, chronic negative experience of eating may cause abnormal behavioral and cognitive response, i.e., avoidance or an aversive response rather than a positive approach to food stimuli.
Visual food stimuli have been used to investigate food-related behavior in patients with obesity19, anorexia nervosa20, and binge eating disorder21. Food images are generally used as pleasure or incentive stimuli, eliciting a positive response or causing an attentional bias22. Since previous episodes of the stimuli (food) and conditioning of the stimuli with somatic symptoms may affect the attention to stimuli22,23, visual attention to food in FD patients might be reduced in comparison to healthy controls (HC).
Eye tracking, which measures the gaze parameters such as initial fixation and total duration of fixation on images24, is well suited to the investigation of initial saliency and the later cognitive processing of images. In addition, autonomic nervous system function and facial muscle activity can provide further support for altered homeostatic and emotional changes during food image processing in FD patients. Skin conductance response (SCR) and heart rate variability (HRV) have been used as the parameter of the arousal level of the sympathetic branch of autonomic nervous system and of the balance of sympathetic and parasympathetic activity, respectively. Electromyography (EMG) of facial muscles measuring the intensity of the contraction of the corrugator supercilii and zygomaticus major muscle has been used to quantify negative and positive facial emotional response, respectively25–27.
In the current study, we aimed to determine the physiological/emotional response and visual attention of FD patients to food images. We evaluated the physiological, emotional, and attentional response of FD patients to high fat food, low fat food, and non-food images after taking an ad-libitum breakfast. We hypothesized that, in comparison to HC, 1) FD patients consume a smaller amount of food, but have higher dyspeptic symptoms afterwards; 2) FD patients show negative valence and increased arousal level to food images, particularly to high fat food images; 3) FD patients show decreased visual attention to food images, especially to high fat food images.
Results
Sample characteristics
Sample characteristics and scores of questionnaires are presented in Table 1. Beck Depression Inventory (BDI), Food Craving Questionnaire (FCQ)-state score, age of HC group, and body mass index (BMI) of FD group were not normally distributed (P < 0.05). No significant differences in age and BMI between groups (P > 0.5) was shown. Eleven FD patients showed both postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) (9 females), 3 patients (all females) had PDS only, and 1 patient (male) had EPS only. FD patients showed a significantly higher Nepean Dyspepsia Index (NDI)_Symptom score (P < 0.001) and a lower NDI_QOL score (P < 0.001) than HC group. FD patients also showed significantly higher depression and anxiety levels (P < 0.01) and higher FCQ-state score (P < 0.05) than HC. The total and subscale scores in Eating Disorders Examination questionnaire (EDE-Q) and Fat Preference Questionnaire (FPQ) did not differ significantly between groups.
Table 1.
Baseline characteristics of the study sample.
| Healthy controls | FD patients | P value | |
|---|---|---|---|
| Gender (m/f) | 5/12 | 3/12 | — |
| Subgroup | — | PDS: 2/12, EPS: 3/9 | — |
| Age (year) | 37.65 ± 4.02 | 41 ± 4.72 | NS* |
| BMI (kg/m2) | 24.95 ± 0.73 | 23.27 ± 1.19 | NS* |
| NDI_Symptom | 10.56 ± 1.90 | 70.62 ± 9.51 | P < 0.001 |
| NDI_QOL | 46.8 ± 1.35 | 23.62 ± 2.33 | P < 0.001 |
| EDE-Q Total | 1.25 ± 0.26 | 1.05 ± 0.29 | NS |
| Restraint scale | 1.07 ± 0.28 | 0.84 ± 0.23 | NS |
| Eating concern | 0.33 ± 0.14 | 0.31 ± 0.15 | NS |
| Weight concern | 1.31 ± 0.32 | 1.13 ± 0.34 | NS |
| Shape concern | 1.58 ± 0.35 | 1.45 ± 0.33 | NS |
| BDI-II | 3.94 ± 1.61 | 9.77 ± 2.44 | P < 0.01* |
| STAI_state | 31.06 ± 1.64 | 43.46 ± 3.21 | P < 0.01 |
| STAI_trait | 31.81 ± 2.06 | 44.54 ± 3.34 | P < 0.01 |
| FCQ_state | 31.94 ± 3.09 | 42.93 ± 3.31 | P < 0.05* |
| FCQ_trait | 83.94 ± 7.28 | 92.08 ± 9.90 | NS |
| FPQ_TASTE (%) | 55.89 ± 5.26 | 65.98 ± 4.81 | NS |
| FPQ_FREQ (%) | 52.10 ± 5.05 | 57.26 ± 4.83 | NS |
| FPQ_DIFF (%) | 3.79 ± 1.73 | 8.72 ± 2.23 | NS |
Mean ± standard error; Higher score indicates more severe symptom (NDI_symptom), depression (BDI-II), anxiety (STAI), craving for food (FCQ), better quality of life (NDI_QOL), and higher frequency of eating concerns (EDE-Q).
BDI: Beck depression inventory (range: 0–63); BMI: body mass index; EDE-Q: Eating disorder examination questionnaire (range: 0–6); EPS: epigastric pain syndrome; f: female; FCQ: Food cravings questionnaire (range: 15–75 for state, 39–234 for trait); FD: functional dyspepsia; FPQ: Fat preference questionnaire; FPQ_TASTE: how much better high fat food tastes, FPQ_FREQ: how much high fat food eaten more often, FPQ_DIFF: high fat restriction (FPQ_TASTE-FPQ_FREQ); m: male; NDI: Nepean dyspepsia index (range: 0–195); NS: statistically not significant; PDS: postprandial distress syndrome; QOL: quality of life (range: 0–124); STAI: State trait anxiety inventory (range: 0–80).
P value: two sample t-test FD vs HC.
*P value: Mann Whitney-U test FD vs HC.
Food/energy intake and FD symptoms
Following overnight fasting, FD patients ate significantly less bread than HC group (FD: 61.6 ± 5.07 g; HC: 76.71 ± 6.95 g, P < 0.05). Albeit FD patients consumed less fat (FD: 9.25 ± 1.12 g; HC: 10.38 ± 1.63 g), carbohydrate (FD: 51.85 ± 6.61 g; HC: 62.53 ± 6.60 g), and protein (FD: 8.56 ± 1.64 g; HC 9.27 ± 1.36 g) than HC group, these differences were statistically not significant. On the whole, patients in the FD group consumed significantly less total energy than HC group (FD: 332.19 ± 37.77 kcal; HC 389.76 ± 38.03 kcal, P < 0.05).
FD symptom ratings of baseline, and at three time points after ingestion (Post1, Post2, Post3) are described in Supplementary Table 1. Hunger rating decreased immediately after breakfast, and increased subsequently in both groups. Appetite also decreased initially and increased gradually afterwards, but FD patients had a significantly lower appetite at Post3 than HC (P < 0.05). Abdominal fullness in the FD group was only slightly higher than HC before breakfast, but significantly higher immediately after breakfast (P < 0.05). Significant main effects of group (FD > HC) were found for abdominal pain (P < 0.05), discomfort, burning, and bloating symptoms (P < 0.01).
Experiment 1. Measurement of physiological response
Physiological response and pleasantness ratings of food and non-food images in FD patients and HC are summarized in Supplementary Table 2.
Pleasantness rating
Analysis of variance (ANOVA) for the 5 image sets showed a significant main effect of image (P < 0.001). In accordance with the post-hoc analysis (Bonferroni corrected), pleasantness of negative images was significantly lower than of any other images (P < 0.001). Pleasantness of high fat food images was significantly lower than of positive images (P < 0.001). In both groups, low fat food images and positive images were rated significantly higher than neutral images (P < 0.001). Subsequent analysis on high fat and low fat food images showed significant main effects of group and image (P < 0.05). Pleasantness ratings of food images in FD were significantly lower than in HC, and pleasantness of high fat food images was rated significantly lower than that of low fat food images in FD (P < 0.05).
SCR
ANOVA analysis for 5 image sets resulted in a significant main effect of image (P < 0.001). Post-hoc analysis (Bonferroni corrected) showed that, in both groups, SCR standardized ratio for negative images was significantly higher than for other images (vs neutral, positive, high fat images, each P < 0.001; vs low fat images P < 0.01). There were no significant differences between groups for either ANOVA.
EMG corrugator supercilii
ANOVA analysis for 5 image sets showed a significant main effect of image (P < 0.001). Post-hoc analysis (Bonferroni corrected) showed that the EMG response to negative images was significantly higher than to any other image (positive, high fat food, low fat food images, all P < 0.001; neutral image P < 0.01). There were no significant differences between groups from either ANOVA.
EMG zygomaticus major
ANOVA analysis for 5 image sets showed that there was a significant main effect of image (P < 0.001) and interaction of group*image (P < 0.05). Post-hoc analysis (Bonferroni corrected) showed that the zygomaticus major muscle EMG response was significantly higher to high fat food images than to negative (P < 0.01) and low fat food images (P < 0.05). EMG signal was significantly higher to positive images than to negative, neutral, and low fat food images (all P < 0.001). No differences were found between groups from the 2 × 5 ANOVA. A 2 × 2 ANOVA analysis for high fat and low fat images showed a significant main effect of image (P < 0.01). EMG activation was lower in FD patients than in HC and significantly higher for high fat food images than for low fat food images in HC (P < 0.01).
HRV SDNN
2 × 5 ANOVA analysis showed that there was a main effect of group (p < 0.05), and FD patients showed higher SDNN (Standard Deviation of Normal-to-Normal interbeat intervals reflecting the overall variation) values than HC group.
HRV HF
No significant main effect was registered for either the group for the images of amplitude of high frequency band (HF) value.
HRV LF/HF ratio
2 × 5 ANOVA analysis showed that there was a significant main effect of group (P < 0.05), and FD patients showed significantly lower LF/HF ratio (the ratio of amplitude of low frequency band (LF) and amplitude of high frequency band (HF) values) than HC group.
Experiment 2. Eye tracking experiment
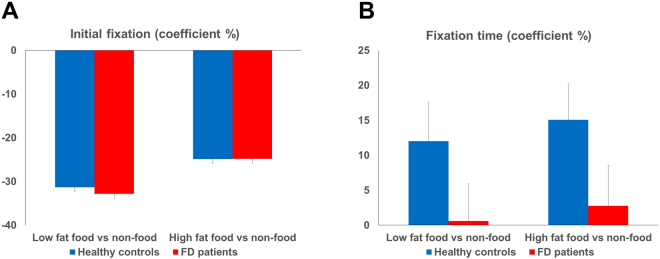
Initial fixation (coefficient %)
There were no significant differences according to the ANOVA (high fat: FD −24.78 ± 7.53, HC −24.87 ± 6.19; low fat: FD −32.80 ± 5.62, HC −31.33 ± 3.86, Fig. 1A)
Figure 1.
Coefficient percentage (%) of initial fixation and total fixation time (ms) in FD and healthy controls. Mean and standard error of coefficient % of initial fixation (A) and total fixation duration (B) on low fat food and high fat food images compared to paired non-food images in FD patients and healthy controls. There were no significant differences of initial fixation between groups and images. Total fixation time was significantly lower in FD patients than in HC for both high and low fat food images (P < 0.05).
Fixation duration (coefficient %)
There was a significant main effect of group and both high and low fat food images were fixated significantly less often by FD patients than by HC (high fat: FD 2.77 ± 5.18, HC 15.07 ± 5.16; low fat: FD 0.60 ± 5.34, HC 12.01 ± 5.53; P < 0.05, Fig. 1B).
Anticipated symptom rating
There was a significant main effect of group on anticipated symptom rating, with FD patients showing higher ratings to high fat food images used in Experiment 2 than HC (P < 0.001). Post-hoc analysis (Bonferroni corrected) showed that FD patients anticipated significantly higher pain and burning sensation than HC group (P < 0.05, P < 0.01, respectively) and that there were no differences in fullness and satiation between groups. With regard to the low fat food images, none of the symptoms differed between groups (Supplementary Table 3).
Correlation analysis
Correlation analysis revealed significant negative correlations between the fat intake and BDI-II (non-parametric Spearman correlation, r = −0.62), fat intake and FPQ_DIFF (r = −0.93), energy intake and FPQ_DIFF (Pearson correlation, r = −0.95, P < 0.05) in FD patients.
Discussion
We investigated the physiological responses and visual attention to food and non-food images in FD patients and healthy controls. Food craving, depression, and anxiety scores were significantly higher in FD patients than in HC. Despite lower total food/energy (kcal) consumption than HC group, FD patients reported more symptoms of bloating, nausea, vomiting, abdominal pain, abdominal discomfort, and burning sensation after food intake. Furthermore, FD patients showed significantly less pleasantness ratings for both high and low fat food images than HC group. Although there was no difference in the initial orientation bias between groups, FD patients had a significantly lower total attentional processing time of food images versus non-food images than HC group. The depression score with the consumption of fat, and fat restriction score with fat/total energy intake were negatively correlated in FD patients only.
In this study, FD patients showed higher meal-induced FD symptoms after consuming less food and energy than healthy controls. It is noteworthy that the pain and burning sensation in FD patients decreased immediately after meal ingestion and then gradually increased again. These results suggest that food ingestion can not only aggravate but also temporarily alleviate FD symptoms. According to a previous study14, the intensity of each FD symptom increased significantly following meal ingestion. These inconsistent results may be due to the different composition of meals and instructions (“eat everything” vs “eat as much as you want”), and sample characteristics. Furthermore, we observed that FD patients also suffered from FD symptoms (pain, discomfort, burning, bloating) even when they were in a fasted state. FD patients are known to eat more frequently, but they take smaller portions and are unable to finish a normal meal portion. This may be due to dynamic changes of symptoms in a state of hunger or fullness.
As often reported in earlier studies4,28–30, FD patients had significantly higher anxiety and depression levels than HC group. In the current study we detected a negative correlation between the depression score and the amount of fat intake in FD patients. Chronic food restriction induced depression-like behavior in rats31 and high fat diet showed an anti-depressive effect in mice32. Fat intake was negatively correlated to depression score33 and a cohort study in the population without depression found that trans unsaturated fatty acids intake was positively related to the incidence of depression34. Although a clear conclusion cannot be drawn from correlation analyses, the results may show the mutual influences of a psychological state and eating behavior in FD patients.
High HRV and decreased sympathetic activation in FD patients were observed regardless of picture category. This could therefore be an intrinsic characteristic of FD patients rather than a response to external stimuli. Furthermore, the emotional response during the visual stimulation of food and non-food cues did not differ significantly between groups. This can be interpreted along with the eye tracking results. FD patients showed a tendency of initial attention (a marker of an immediate attention-grabbing effect of images) similar to that of HC group and a lower total attention processing time (fixation duration, a marker of a total attention-grabbing effect) for food images. While visual food images may not immediately induce negative valence and avoidance responses, a late cognitive processing of the images by higher cognitive function may cause the avoidance response to food images in FD patients while processing food images. While still hypothetical, it is nonetheless plausible that high level cognitive functions rather than autonomic and emotional mechanisms can operate differently in FD patients. Furthermore, a decreased fixation duration on food images in FD patients is not in line with earlier findings in patients with obesity and binge eating disorder35,36 (where increased duration on food images was reported) and is similar to the results from anorexia nervosa patients37. This might indicate a positive or negative perception of food cues in eating-related diseases.
The reduced pleasantness of and attentional bias to visual food stimuli in FD patients could be considered in future psychotherapeutic intervention and research. Various treatment options have been proposed for FD, such as H. pylori eradication, prokinetic agents, acid suppressive medications, and antidepressants. Nevertheless, a standardized treatment strategy for FD patients has yet to be established and cognitive behavioral therapy remains an unexplored area38,39. A new therapy that includes self-restraint response to food, emotional management, and eating behavior modification could be considered for patients who do not respond to conventional therapies. Furthermore, how FD patients perceive, encode, store, and recall the value of food and how food memories influence their food-related decision making might be interesting topics for future studies in FD mechanisms.
In the interviews conducted before the study, almost all FD patients complained about the changes in their eating behavior and their poor quality of life. Most patients avoided symptom-related foods, such as fatty foods, bread, pasta, or alcohol. The list of foods avoided varied from person to person. Fatty foods aggravated the symptoms in some patients, whereas others remained unaffected. Nevertheless, the high fat restriction score was significantly related to the lower intake of fat and total energy in FD patients only, and they anticipated more severe symptoms from high fat food than HC. Previous negative memory of the aftereffect of eating may affect the anticipation of symptoms by learning of stimuli and somatic symptoms. This could be extended to the restriction of food intake, reduced pleasantness rating and the attentional avoidance of food images22,23.
Limitations of this study were the use of food images instead of real food and subjective ratings of symptoms and pleasantness. Unlike real food, food images lack odor and texture information. Furthermore, it was challenging to identify one particular symptom-related food item in each patient. Perceptual characteristics such as color, contrast, or brightness of image categories may differ and may influence the participants’ responses. This may be the basis for the similar autonomic and emotional responses to high fat and low fat images in our study. Secondly, our sample size was not large enough to conduct further subgroup analysis and we did not examine any differences between PDS and EPS, patients with severe and mild FD/depression/anxiety symptoms. The small sample size in this study limits the interpretation of our findings. Nevertheless, the investigation of eating behavior using standardized meals and of physiological responses using visual food cues provides new insight into pathomechanism in FD patients. Further studies with a larger sample size are necessary to expand our knowledge of the food-related behavior, cognitive, emotional, and physiological responses in FD patients.
In conclusion, we observed an increased food craving, decreased food intake, food ingestion-induced aggravation of FD symptoms, reduced food image processing time, and reduced perception of food-related pleasantness in FD patients. The effectiveness of conventional therapies in FD patients might be enhanced by the modification of psychological responses to food.
Methods
Participants
15 FD patients (3 male, aged 41 ± 4.72 years) and 17 age- and BMI-matched HC (5 male, aged 39.65 ± 4.02 years) were included in the study. They were all right-handed and the age range was 18–75 years and BMI (weight/height2) range 19–29 kg/m2. FD patients were diagnosed on the basis of ROME III criteria40 and an unsuspicious endoscopy documented in their medical records. Participants with visual impairment, severe psychiatric illness, intake of antidepressants or antipsychotics, and any food allergy or intolerance were excluded. The study was approved by the ethics committee of the Medical Faculty, Tübingen University, Germany (041/2016BO2). All participants provided informed consent and all experiments were conducted ethically according to the principles of the Declaration of Helsinki.
Procedure
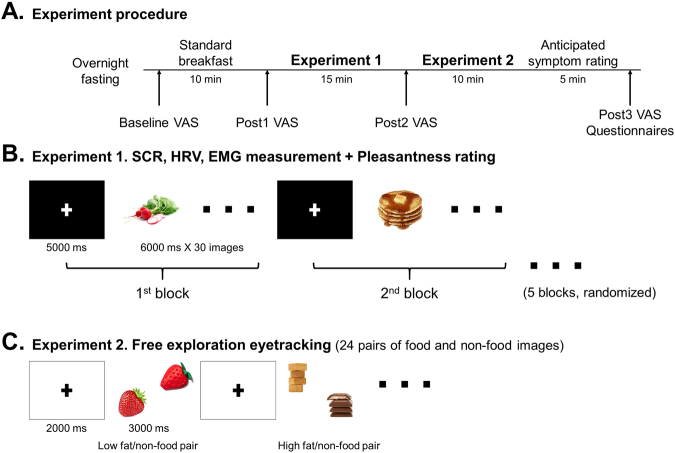
The study was conducted at the University Clinic, Tübingen, Germany. Participants were asked to fast from 10 pm of the evening prior to the study. The study commenced at 8 a.m. the following morning. The participants began by rating their physical condition such as hunger, appetite, abdominal fullness, satiation, nausea, vomiting, abdominal pain, abdominal discomfort, burning, and bloating symptoms (baseline) on a visual analogue scale (VAS; 0 = not at all, 10 = very much). They were then served a standard breakfast consisting of bread (2 slices, 110 g), butter (36 g), jam (46 g), milk (1.5% fat, 500 ml), orange juice (500 ml), and water (total calorie 402.09 kcal, fat 14.52 g, carbohydrate 53.61 g, protein 12.98 g). The participants could eat as much as they wished within 10 minutes. VAS ratings were assessed again immediately after breakfast (Post1), between Experiment 1 and Experiment 2 (Post2, 20–25 minutes after the meal), and at the end of the experiment (Post3, 45–50 minutes after the meal, Fig. 2A). The remaining food from each participant was weighed and calorie intake was calculated.
Figure 2.
Experimental protocol. (A) Experimental procedure of the study. (B) Illustration of Experiment 1 including skin conductance response, heart rate, electromyography measurements, and pleasantness rating to food and non-food images. Randomized order of 5 blocks of images (neutral, positive, negative, high fat, and low fat food images, n = 30, 6000 ms for each image) with fixation cross (5000ms) between each block were presented. (C) Schematic presentation of the eye tracking experiment using free exploration paradigm. Low fat food and non-food pairs and high fat food and non-food pairs (n = 12, respectively) were presented for 3000 ms with 2000 ms of fixation cross between pairs. Location of the images (1st–4th quadrant) was balanced.
Experiment 1. Emotional and physiological response to food and non-food images
Skin conductance response was measured with two electrodes attached to the index and middle finger of the left hand. Three electrodes were placed on the chest region to measure the electrocardiography (ECG) signal. For facial EMG measurement, three electrodes were attached on both the corrugator supercilii and zygomaticus major muscles on the left side of the face41. The data were recorded with a Biopac MP36 system and Acknowledge software ver. 4.1 (Biopac Systems Inc., Goleta, USA).
Five fixed-order sets of image stimuli (neutral household items, positive, negative, high fat, and low fat food images, n = 30, respectively), were presented in a randomized order for 180 seconds (6 seconds for each image) followed by a 5-second rest with visual cross fixation between each set. Subjective pleasantness to each image was measured by pressing a button from 1 to 10 on a keyboard (1 = very unpleasant, 10 = very pleasant). Participants were also requested not to move or talk while the measurements were being carried out (Fig. 2B).
Experiment 2. Visual attention to food and non-food images
After Experiment 1, gaze data were recorded with the eye tracking system iView X Hi-Speed (SensoMotoric Instruments GmbH, Berlin, Germany). Each participant received a standardized 13-point calibration procedure to ensure optimal gaze data quality. Following calibration, 24 pairs of images (different from those used in Experiment 1) composed of food (high fat n = 12, low fat n = 12) and non-food images (household items, n = 24), were randomly presented. Each pair of stimuli images was presented for 3 seconds and a fixation cross at the center of the screen was shown for 2 seconds between each pair. Participants were requested to freely explore the presented pictures and to fixate the cross when shown (Fig. 2C).
After the eye tracking experiment, the anticipated FD symptoms (postprandial fullness, early satiation, abdominal pain, and burning sensation) for each food image (high fat n = 12, low fat n = 12) were assessed using VAS (0 = not at all, 10 = very much) during the visual stimuli of food images without response time restriction. At the conclusion of the study, each participant’s dyspepsia symptom intensity and disease-related quality of life were assessed using the NDI42. Depression and anxiety levels were evaluated using the BDI-II43 and State-Trait Anxiety Inventory (STAI)44, respectively. Furthermore, the EDE-Q45, FCQ46, and FPQ47 were used to identify eating behavior.
Materials and apparatus
Experiment 1
Positive and negative images were selected from the International affective picture system (IAPS)48. Taking the diversity of food and color-matching between food and non-food images into consideration, we selected neutral household items and food images from food image databases49. Food items for the high fat food images included cake, pancake, croissant, hamburger, cheese, pie, ham, sausage, white chocolate, peanuts, and meat. Food items for the low fat food included salad, asparagus, pea, cucumber, fruit, and rice. Images were presented and subjective pleasantness rating was recorded with Presentation® (version 16.5, www.neurobs.com). Physiological signals were recorded with a Biopac MP36 system and Acknowledge software 4.1 (Biopac Systems Inc., Goleta, USA). SCR, ECG and EMG signals were sampled at 1 kHz. For SCR, a low pass filter of 10 Hz, for ECG a bandpass filter between 0.5 and 35 Hz, and for EMG a bandpass filter between 30 and 250 Hz were applied.
Experiment 2
A validated image set for the eye tracking experiment was used in this study36. The food and non-food stimuli were subjectively matched in color and quantitatively for brightness and contrast (paired t-test, for both P > 0.8). The complexity, valence, and arousal levels of the images were rated in an earlier study50. Eye movements were recorded with the IViewX Hi-Speed and IViewX 2.8 software (SensoMotoric Instruments, Berlin, Germany) and sampling rate was set at 500 Hz.
Data processing
Experiment 1
We analyzed the raw SCR signal using Ledalab software (www.ledalab.de). Raw SCR was smoothed and event-related activation was extracted when the signal exceeded 0.01muS. For the computation of the standardized ratio, the total amplitude of SCR of each block (neutral, positive, negative, high, and low fat cues) was divided by the total amplitude to normalize individual differences.
Rectified EMG was derived from raw EMG data, while integrated EMG was defined as the area under the curve of the rectified EMG signal. Muscle activation was located every 30ms automatically and visually ascertained. To calculate the standardized ratio, we divided the whole EMG signal from all muscle activations located in each block by the total amplitude (Acknowledge software 4.1, Biopac Systems Inc., Goleta, USA).
ECG signals during the visual stimuli of 5 sets of images (neutral, positive, negative, high fat, and low fat food images) were analyzed with Kubios HRV software (version 2.2, http://kubios.uku.fi/)51. QRS complexes were detected based on the Pan-Tompkins algorithm. The algorithm uses slope, amplitude, and width information of the QRS and comprises of two learning phases (detection threshold and RR-interval limit) and a detection phase (recognition of QRS)52. Missing/additional or ectopic/arrhythmic beats were visually checked and RR intervals (time interval between successive R-waves) which differed from the local average more than 0.25 seconds were identified as artifacts. Artifacts were replaced with interpolated values. SDNN (Standard Deviation of Normal-to-Normal interbeat intervals reflecting the overall variation) was computed from the artifacts-corrected series of consecutive RR intervals of each image block (6000ms). For frequency domain analysis, a linear observation model was used to detrend low frequency aperiodic trend components. Trend components were defined as ‘Ztrend = observation matrix*regression parameters + observation error’ and removed using the smoothness priors method (high-pass filter with a cut-off frequency: Lambda 500, f = 0.035 Hz). Frequency bands were set at 0.04–0.15 Hz for low frequency and at 0.15–0.4 Hz for high frequency. Non-parametric Fast Fourier Transform (FFT)-based power spectrum estimation was applied to calculate power spectral density of each frequency band following the cubic spline interpolation of the detrended RR series. Amplitude of low frequency band (LF), amplitude of high frequency band (HF), and the ratio of LF and HF values during the visual stimuli of images (6000ms) were calculated by using FFT algorithm and log transformed.
Experiment 2
The raw gaze data was analyzed using BeGaze 3.0 software (SensoMotoric Instruments GmbH, Berlin, Germany). The areas of interest (AOIs) were defined for food and non-food images and fixation cross. The initial fixation position was defined by the geographical position of gaze on AOIs and fixation duration was calculated as the sum of the duration time of fixation inside each AOI53. Any trials in which participants did not fixate on the cross at the onset of the trial were ruled out. Two variables were defined to test the hypothesis: 1) the coefficient percentage (%) of initial fixation on food versus non-food images: (number of initial fixations on food images–number of initial fixations on non-food images)/(number of initial fixations on food images + number of initial fixations on non-food images)*100(%), 2) the coefficient percentage (%) of total fixation duration on food versus non-food images: (fixation duration on food images–fixation duration on non-food images)/(fixation duration on food images + fixation duration on non-food images)*100(%). All authors had access to the study data and reviewed and approved the final manuscript.
Statistical analysis
All statistical analyses were performed with IBM SPSS statistics 24.0 (IBM Corp. New York, USA). Normal distribution of data was assessed by Lilliefors-corrected Kolmogorov-Smirnov test. For normally distributed data, independent two sample t-tests were used to assess any differences between study groups (FD and HC). For data showing non-normal distribution, we used the non-parametric Mann Whitney-U test. A two-way repeated measures ANOVA with the groups (FD and HC) and the time factors (baseline, Post1, Post2, Post3) was used to identify the changes in meal-induced FD symptom ratings. A two-way ANOVA with the group (FD and HC) and type of image (2 × 5: neutral, positive, negative, high fat food, low fat food; 2 × 2: high fat, low fat food) factors were applied to the pleasantness rating, SCR, EMG, HRV variables, eye tracking data, and anticipated FD symptom rating. Two-tailed partial Pearson correlation analysis or non-parametric Spearman partial correlation were also computed between BMI, fat and total energy intake, NDI_Symptom and NDI_QOL (quality of life) scores, BDI-II, STAI, FCQ, FPQ scores, and the eye-tracking variables while controlling for age and BMI. The statistical significance level was set at α = 0.05 and a Bonferroni correction was applied to account for multiple testing if necessary.
Data availability
All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Writing assistance
English correction, funded by the European Union’s Seventh Framework Programme under REA grant agreement No. 607652 (NeuroGUT).
Ethics approval
The study was approved by the ethics committee of the Medical Faculty, Tübingen University, Germany (041/2016BO2).
Clinical trial registration
ClinicalTrials.gov number NCT02727556, date of registration March 21, 2016.
Electronic supplementary material
Acknowledgements
The research leading to these results received funding from the People Programme of the European Union’s Seventh Framework Programme under REA grant agreement No. 607652 (NeuroGUT), the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement No. 607310 (Nudge-it). We also acknowledge support by the Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen, Germany.
Author Contributions
study concept and design: I.S.L., H.P., K.G., K.S., P.E.; acquisition of data: I.S.L.; analysis and interpretation of data: I.S.L., H.P., K.G., K.S., P.E.; drafting of the manuscript: I.S.L.; critical revision of the manuscript for important intellectual content: I.S.L., H.P., P.E.; statistical analysis: I.S.L.; obtained funding: H.P., P.E.; administrative, technical, or material support: H.P., P.E.; study supervision: H.P., P.E.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-19112-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. Journal of gastrointestinal and liver diseases: JGLD. 2006;15:237–241. [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ. Functional dyspepsia–symptoms, definitions and validity of the Rome III criteria. Nature reviews. Gastroenterology & hepatology. 2013;10:134–141. doi: 10.1038/nrgastro.2013.14. [DOI] [PubMed] [Google Scholar]
- 3.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nature reviews. Gastroenterology & hepatology. 2013;10:158–167. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 4.Haag S, et al. Impairment of health-related quality of life in functional dyspepsia and chronic liver disease: the influence of depression and anxiety. Alimentary pharmacology & therapeutics. 2008;27:561–571. doi: 10.1111/j.1365-2036.2008.03619.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Oudenhove L, et al. Abuse history, depression, and somatization are associated with gastric sensitivity and gastric emptying in functional dyspepsia. Psychosom Med. 2011;73:648–655. doi: 10.1097/PSY.0b013e31822f32bf. [DOI] [PubMed] [Google Scholar]
- 6.Khodarahmi M, Azadbakht L. Dietary fat intake and functional dyspepsia. Advanced biomedical research. 2016;5:76. doi: 10.4103/2277-9175.180988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goktas Z, et al. Nutritional habits in functional dyspepsia and its subgroups: a comparative study. Scandinavian journal of gastroenterology. 2016;51:903–907. doi: 10.3109/00365521.2016.1164238. [DOI] [PubMed] [Google Scholar]
- 8.Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51(Suppl 1):i54–57. doi: 10.1136/gut.51.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinle-Bisset C, Azpiroz F. Dietary and lifestyle factors in functional dyspepsia. Nature reviews. Gastroenterology & hepatology. 2013;10:150–157. doi: 10.1038/nrgastro.2012.246. [DOI] [PubMed] [Google Scholar]
- 10.Pilichiewicz AN, Horowitz M, Holtmann GJ, Talley NJ, Feinle-Bisset C. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2009;7:317–322. doi: 10.1016/j.cgh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho RV, Lorena SL, Almeida JR, Mesquita MA. Food intolerance, diet composition, and eating patterns in functional dyspepsia patients. Digestive diseases and sciences. 2010;55:60–65. doi: 10.1007/s10620-008-0698-8. [DOI] [PubMed] [Google Scholar]
- 12.Mullan A, et al. Food and nutrient intakes and eating patterns in functional and organic dyspepsia. European journal of clinical nutrition. 1994;48:97–105. [PubMed] [Google Scholar]
- 13.Kazemi M, Eshraghian A, Hamidpour L, Taghavi S. Changes in serum ghrelin level in relation to meal-time in patients with functional dyspepsia. United European gastroenterology journal. 2015;3:11–16. doi: 10.1177/2050640614563373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisschops R, et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. 2008;57:1495–1503. doi: 10.1136/gut.2007.137125. [DOI] [PubMed] [Google Scholar]
- 15.Yamawaki H, et al. Improvement of meal-related symptoms and epigastric pain in patients with functional dyspepsia treated with acotiamide was associated with acylated ghrelin levels in Japan. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2016;28:1037–1047. doi: 10.1111/nmo.12805. [DOI] [PubMed] [Google Scholar]
- 16.Amiriani T, et al. Assessment of Gastric Accommodation in Patients with Functional Dyspepsia by 99mTc-Pertechtenate Single Photon Emission Computed Tomography Imaging: Practical but not Widely Accepted. Molecular imaging and radionuclide therapy. 2015;24:105–109. doi: 10.4274/mirt.36854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stojek MMK, MacKillop J. Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: A systematic review. Clinical psychology review. 2017;55:1–11. doi: 10.1016/j.cpr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Soussignan R, Jiang T, Rigaud D, Royet JP, Schaal B. Subliminal fear priming potentiates negative facial reactions to food pictures in women with anorexia nervosa. Psychological medicine. 2010;40:503–514. doi: 10.1017/S0033291709990377. [DOI] [PubMed] [Google Scholar]
- 19.Nijs IM, Franken IH. Attentional Processing of Food Cues in Overweight and Obese Individuals. Current obesity reports. 2012;1:106–113. doi: 10.1007/s13679-012-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaife JC, Godier LR, Reinecke A, Harmer CJ, Park RJ. Differential activation of the frontal pole to high vs low calorie foods: The neural basis of food preference in Anorexia Nervosa? Psychiatry research. 2016;258:44–53. doi: 10.1016/j.pscychresns.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolz I, et al. Subjective craving and event-related brain response to olfactory and visual chocolate cues in binge-eating and healthy individuals. Scientific reports. 2017;7:41736. doi: 10.1038/srep41736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgs S. Cognitive processing of food rewards. Appetite. 2016;104:10–17. doi: 10.1016/j.appet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Folkvord F, Anschutz DJ, Wiers RW, Buijzen M. The role of attentional bias in the effect of food advertising on actual food intake among children. Appetite. 2015;84:251–258. doi: 10.1016/j.appet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Dimberg U. Facial electromyography and emotional reactions. Psychophysiology. 1990;27:481–494. doi: 10.1111/j.1469-8986.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 26.Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;55:685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Soussignan R, Schaal B, Rigaud D, Royet JP, Jiang T. Hedonic reactivity to visual and olfactory cues: rapid facial electromyographic reactions are altered in anorexia nervosa. Biol Psychol. 2011;86:265–272. doi: 10.1016/j.biopsycho.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Richter JE. Stress and psychologic and environmental factors in functional dyspepsia. Scand J Gastroenterol Suppl. 1991;182:40–46. doi: 10.3109/00365529109109536. [DOI] [PubMed] [Google Scholar]
- 29.Haug TT, Svebak S, Wilhelmsen I, Berstad A, Ursin H. Psychological factors and somatic symptoms in functional dyspepsia. A comparison with duodenal ulcer and healthy controls. J Psychosom Res. 1994;38:281–291. doi: 10.1016/0022-3999(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 30.Hill AJ, Weaver CF, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187–197. doi: 10.1016/0195-6663(91)90021-J. [DOI] [PubMed] [Google Scholar]
- 31.Jahng JW, et al. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain research. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- 32.Del Rosario A, McDermott MM, Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br J Nutr. 2012;108:1143–1149. doi: 10.1017/S0007114511006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banikazemi Z, et al. Dietary vitamin E and fat intake are related to Beck’s depression score. Clin Nutr ESPEN. 2015;10:e61–e65. doi: 10.1016/j.clnesp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Villegas A, et al. Dietary fat intake and the risk of depression: the SUN Project. PloS one. 2011;6:e16268. doi: 10.1371/journal.pone.0016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellanos EH, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. International journal of obesity. 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 36.Schag K, et al. Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PloS one. 2013;8:e76542. doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giel KE, et al. Attentional processing of food pictures in individuals with anorexia nervosa–an eye-tracking study. Biological psychiatry. 2011;69:661–667. doi: 10.1016/j.biopsych.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 38.Paul Enck, F. A. et al. Functional dyspepsia. Nature Reviews Disease Primers in press (2017). [DOI] [PubMed]
- 39.Soo, S. et al. Psychological interventions for non-ulcer dyspepsia. The Cochrane database of systematic reviews, CD002301, 10.1002/14651858.CD002301.pub4 (2005). [DOI] [PubMed]
- 40.Douglas, A. D et al. ROME III: The Functional Gastrointestinal Disorders. 3rd edn, (Yale University Section of Digestive Disease: Degnon Associates, 2006).
- 41.Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 42.Talley NJ, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Alimentary pharmacology & therapeutics. 1999;13:225–235. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 43.Beck, A. T., Steer, R. A. & Brown, G. Manual for the Beck Depression Inventory-II. (San Antonio, TX: Psychological Corporation., 1996).
- 44.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 45.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? The International journal of eating disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- 46.Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the state and trait food-cravings questionnaires. Behavior Therapy. 2000;1:151–173. doi: 10.1016/S0005-7894(00)80009-X. [DOI] [PubMed] [Google Scholar]
- 47.Ledikwe JH, et al. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite. 2007;49:74–83. doi: 10.1016/j.appet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lang, P. J., Bradley, M. M. & Cuthbert, B. N. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. (University of Florida, 2008).
- 49.Blechert J, Meule A, Busch NA, Ohla K. Food-pics: an image database for experimental research on eating and appetite. Frontiers in psychology. 2014;5:617. doi: 10.3389/fpsyg.2014.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank S, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain research. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Computer methods and programs in biomedicine. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 53.Instruments, S. BeGaze Manual version 3.0. (SensoMotoric Instruments, 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.