Abstract
A 97-year-old woman presented with 4-day history of vesicular rash, initially at the feet but then spread up to the thighs bilaterally, abdomen and trunk. The initial differentials included bullous pemphigus and cellulitis by the emergency department. She was then managed as bullous pemphigus by the acute medical team and started on high-dose steroids, with no other differentials considered. When her care was taken over by the general medical team, varicella zoster virus (VZV) infection was suspected. After confirmation by the dermatology team regarding the clinical diagnosis and the positive VZV DNA swabs, she was started on acyclovir.
Keywords: infections, skin, geriatric medicine, varicella zoster, vesicular rash
Background
To our knowledge, we present the oldest reported case of primary varicella zoster infection in an immunocompetent patient. Due to the age of the patient, the condition was incorrectly diagnosed as pemphigus leading to inappropriate steroid administration. Although there has been a previously reported case, it was not regarding a patient as old as ours.1
In our patient, the diagnosis was confirmed by skin swab showing varicella zoster virus (VZV) and the clinical impression of two senior consultants, a geriatrician and a dermatologist. Unfortunately, a result for VZV IgM was not available, but it is well established in the literature that this does not differentiate between chickenpox and herpes zoster (HZ), as it can be positive in both conditions. The diagnosis is therefore clinical.1
Chickenpox, although rare in this age group, is an important diagnosis to consider. It is well known that older patients are at increased risk of HZ, but also chickenpox due to reduced cell-mediated immunity (CMI). As with our patient, there is generally a good response to acyclovir treatment, even in the presence of complications.2 There are serious sequelae, however, including postherpetic neuralgia, meningoencephalitis, pneumonitis, myelitis and ocular disorders. Furthermore, the incidence of stroke due to VZV vasculopathy is greater in those with recent history of HZ. Primary varicella zoster is also strongly associated with ischaemic stroke in immunocompetent children and adults.3 After VZV reaches the arterial adventitia, it infects all layers of the cerebral arteries, resulting in the characteristic pathology of granulomatous arteritis and progressive intimal thickening via disruption of the internal elastic lamina. Early diagnosis and treatment of varicella zoster (chickenpox) in the elderly may therefore be important in improving prognosis and outcome.
Case presentation
A 97-year-old woman presented to the emergency department with 1-day history of fever and vomiting and a new onset rash. The rash had appeared 4 days earlier, initially on both feet but then spread up to the thighs bilaterally, abdomen and trunk. The rash was itchy. Her medical history included Raynaud’s disease, Barrett’s oesophagus, rectal prolapse and right hip replacement. She lived alone, was an ex-smoker and requiring twice daily carers. Her medications were nifedipine LA 30 mg once a day, quinine sulfate 200 mg once a night, ferrous fumarate 210 mg twice a day and alendronic acid 70 mg once a week.
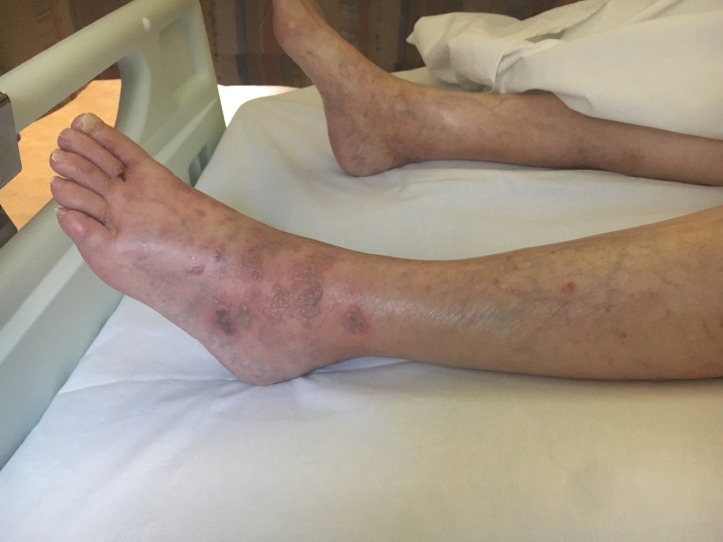
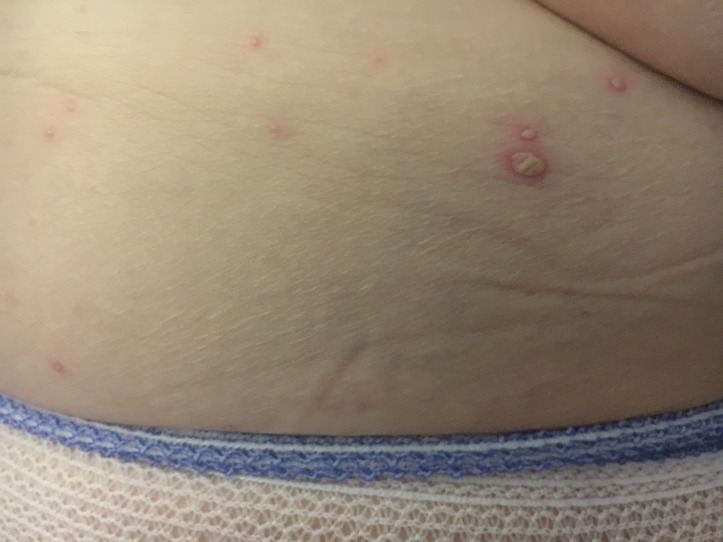
On examination, she was feverish at 38.6°C with a heart rate of 84 beats/min and blood pressure of 162/76. She had a widespread maculopapular rash with small pustules, which were coalescing in parts, covering her trunk, back of ears and both lower limbs (figures 1 and 2). There were a few scattered excoriation marks over the torso and limbs. Cardiovascular, respiratory and abdominal examinations showed no significant findings.
Figure 1.
Rash on foot and torso with vesicles present.
Figure 2.
Vesicular rash over back.
Investigations
The initial investigations revealed white cell count 7.3×109/L, neutrophils 5.4×109/L and lymphocytes 1.3×109/L. There were no significant acute changes in her renal and liver function tests with a urea 8.2 mmol/L, creatinine 87 μmol/L, alkaline phosphatase 175 U/L, gamma glutamyl transferase 21 U/L, alanine transferase 12 IU/L and bilirubin 5 μmol/L. The C-reactive protein was 105 mg/L (normal 0–5 mg/L). Her chest X-ray showed no signs of a community-acquired pneumonia. Urinalysis was negative for leucocytes, nitrites, glucose and ketones, but weakly positive for blood and protein. Urine microscopy and culture only identified a heavy mixed growth, demonstrating a likely contaminant.
Differential diagnosis
The differential diagnoses proposed by the emergency medicine team included cellulitis and bullous pemphigoid before handing over to the acute medical team. On admission to the medical ward, the acute medical team made a provisional diagnosis of bullous pemphigoid. She was started on high-dose steroids in the form of 40 mg prednisolone orally per day.
Following 48 hours, she was transferred to a Geriatric/General Medical ward and her management was handed over to the team in charge. On assessment by the ward team, it was felt that the features of her rash were more in keeping with chickenpox rather than pemphigoid.
Other differentials by the ward team included drug eruptions as it fulfilled the clinical features of confluent erythema, high fever and blistering. There was no evidence of mucous membrane erosions, skin necrosis or angioedema, however, nor had the patient been commenced on any new medications prior to the onset of the rash. Similarly, disseminated herpes simplex infection was felt unlikely, as there was no mucosal involvement. Erythema multiforme minor was also considered, but as this was a vesicular rash the possibility was considered to be unlikely.
Outcome and follow-up
Although on direct questioning, she confirmed that she had contacted chickenpox as a child; nonetheless, the itchy pustular coalescing rash was convincing. Skin swabs from the rash for VZV and serology were requested. Prednisolone was discontinued and acyclovir at a dose of 400 mg intravenously three times a day was started empirically. A second opinion was also requested from the dermatology department who confirmed the likely clinical diagnosis of chickenpox. Her skin swab and serology (IgG) were both positive for VZV. Unfortunately, the laboratory was not able to process VZV IgM. Although her rash was resolving and she subjectively felt better, she developed new atrial fibrillation and suffered a myocardial infarction (troponin 45 ng/L). She subsequently developed ischaemic cardiomyopathy, and an echocardiogram showed the left ventricular ejection fraction running at <20%. Despite attempts to control her heart failure, she deteriorated and passed away 16 days into her admission.
Discussion
VZV causes two distinct disease processes: chickenpox (varicella) and shingles (HZ). It is a unique alphaherpes virus due to the transmission being airborne, leading to a typical winter-spring seasonality pattern of varicella, with primary infection predominantly affecting children aged under 10 years.4 Although infection results in lifelong immunity, there are five distinct genotypes of the virus and multiple strains.5 Therefore, acquiring chickenpox from one strain may not always confer protection from infection by another strain.6 7
Infection is via the respiratory tract, with incubation period of 10–21 days, at which point it arrives at the skin causing the typical vesicular rash. Innate, humoral and CMI are needed, with the latter playing an essential role in eliminating intracellular pathogens, as demonstrated by severity of disease in T cell-compromised patients.8 During a varicella infection, CD4+ T cells release interferon gamma (IFN-γ), which stimulates CD8+ T cells and upregulate the expression of major histocompatibility complex class II (MHC-II) on infected cells, which enables CD4+ T cells to lyse them within the skin.9 Historically, it is known that protection against acquiring primary chickenpox infection is mediated by antibodies, as evidenced by the efficacy of passive immunisation with specific anti-VZV immunoglobulin to exposed neonates.10 There is recent evidence, however, that T cell-specific response and VZV-specific IFN-γ responses are integral part in managing severity of disease.11
All herpes viruses have the ability to establish latency. VZV does this in the dorsal root ganglia and the cranial root ganglia. When reactivated, it is transported within the sensory axons to infect epithelial cells; therefore, rashes are only within the dermatome innervated by a single sensory nerve. T cell immunity is essential for maintenance of latency, demonstrated by the incidence of HZ correlated with lymphoma and bone marrow transplant patients.12 13
Reduction in T cell-mediated immunity occurs with age, hence the reason for old age as a risk factor for HZ. At a median age of 59 years, re-exposure to the virus (from a primary varicella infection) does not produce significant rise in IFN-γ producing CD4+ and CD8+ T cells.14 In these cases, it is not clear if the poor response is due to the immune system or inadequate exposure. In confirmed primary varicella infection, however, clinical disease severity (>500 vesicles) and varicella zoster viral load have been shown to increase significantly in advancing age.14 Furthermore, an inverse correlation has been established between rapid IFN-γ production, surrogate for functional T cell response, and viral load.12 Although poor T cell-mediated response occurs with advancing age, patients who are cytomegalovirus (CMV) IgG positive have demonstrated a higher T cell percentage than those who were CMV IgG negative regardless of age.14
Primary varicella infection does not always manifest as a vesicular rash, however, with varicella pneumonitis being a severe complication of the infection.15 Smoking has been demonstrated to be a significant risk factor for developing pneumonitis.15 16 When Lin et al reviewed varicella hospitalisations over a 10-year period, haemorrhagic pneumonitis was a complication particularly in adults aged over 20 years.17 Another feature affecting the respiratory system is the late sequelae of chickenpox pneumonitis, widespread micronodular calcification due to granulomatous inflammation and scarring leading to pulmonary nodules.18 Although usually associated with previous varicella pneumonitis, incidental pulmonary nodules have been reported in a patient who previously presented with varicella myelitis. Diagnosis was confirmed on histopathology VZV DNA PCR.19
Early recognition and treatment of primary varicella infection in elderly patients is essential, considering the sequelae of ischaemic stroke and pneumonitis. Prompt initiation of intravenous acyclovir in primary varicella infection has been associated with clinical improvement is several case series.15 Advancing age is a risk factor for severity of clinical disease and development of haemorrhagic pneumonitis, which justifies recommendations by Gogos et al to use oral acyclovir chemoprophylaxis in high-risk populations with chickenpox.20
Learning points.
Primary varicella zoster should not be ruled out as a differential diagnosis based on patient’s age alone.
Presentation is similar, and often mistaken for, pemphigoid disease and should therefore always be considered as a differential in these cases.
Varicella zoster is associated with significant disease, including strokes and varicella pneumonitis, so should be identified and treated early.
Primary varicella zoster is a clinical diagnosis, with confirmation by varicella zoster virus DNA PCR, but IgM does not differentiate between chickenpox and herpes zoster.
Acknowledgments
The authors would like to acknowledge the hard working staff at Princess Royal University Hospital, delivering high quality patient care and putting patient safety as a priority.
Footnotes
Contributors: AMDN conducted the literature search and created the manuscript. NM, MF and AA were all directly involved in the management of the case. All authors reviewed and edited the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gunawardena I, Attygalle T. Re-infection with primary varicella zoster in older people. Age Ageing 2008;37:235 10.1093/ageing/afn005 [DOI] [PubMed] [Google Scholar]
- 2.Malavige GN, Jones L, Kamaladasa SD, et al. . Viral load, clinical disease severity and cellular immune responses in primary varicella zoster virus infection in Sri Lanka. PLoS One 2008;3:e3789 10.1371/journal.pone.0003789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amlie-Lefond C, Gilden D. Varicella zoster virus: a common cause of stroke in children and adults. J Stroke Cerebrovasc Dis 2016;25:1561–9. 10.1016/j.jstrokecerebrovasdis.2016.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol 1993;4:222–30. 10.1097/00013542-199310000-00006 [DOI] [Google Scholar]
- 5.Gershon A, Silverstein S. Varicella zoster virus : Richman D, Whitley R, Hayden F, Clinical virology. 3rd Washington, DC: ASM Press, 2009:451–73. [Google Scholar]
- 6.Taha Y, Scott FT, Parker SP, et al. . Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis 2006;43:1301–3. 10.1086/508539 [DOI] [PubMed] [Google Scholar]
- 7.Quinlivan M, Hawrami K, Barrett-Muir W, et al. . The molecular epidemiology of varicella-zoster virus: evidence for geographic segregation. J Infect Dis 2002;186:888–94. 10.1086/344228 [DOI] [PubMed] [Google Scholar]
- 8.Sartori AM. A review of the varicella vaccine in immunocompromised individuals. Int J Infect Dis 2004;8:259–70. 10.1016/j.ijid.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Gershon AA, Gershon MD, Breuer J, et al. . Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010;48:S2–7. 10.1016/S1386-6532(10)70002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller E, Cradock-Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster virus. Lancet 1989;2:371–3. 10.1016/S0140-6736(89)90547-3 [DOI] [PubMed] [Google Scholar]
- 11.Arvin AM, Pollard RB, Rasmussen LE, et al. . Cellular and humoral immunity in the pathogenesis of recurrent herpes viral infections in patients with lymphoma. J Clin Invest 1980;65:869–78. 10.1172/JCI109739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata A, Asanuma H, Rinki M, et al. . Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002;347:26–34. 10.1056/NEJMoa013441 [DOI] [PubMed] [Google Scholar]
- 13.Ogunjimi B, Van den Bergh J, Meysman P, et al. . Multidisciplinary study of the secondary immune response in grandparents re-exposed to chickenpox. Sci Rep 2017;7:1077 10.1038/s41598-017-01024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross CA, McDaid R. Specific IgM antibody in serum of patients with herpes zoster infections. Br Med J 1972;4:522–3. 10.1136/bmj.4.5839.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Daher N, Magnussen R, Betts RF. Varicella pneumonitis: clinical presentation and experience with acyclovir treatment in immunocompetent adults. Int J Infect Dis 1998;2:147–51. 10.1016/S1201-9712(98)90117-5 [DOI] [PubMed] [Google Scholar]
- 16.Ellis ME, Neal KR, Webb AK. Is smoking a risk factor for pneumonia in adults with chickenpox? Br Med J 1987;294:1002 10.1136/bmj.294.6578.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin F, Hadler JL. Epidemiology of primary varicella and herpes zoster hospitalizations: the pre-varicella vaccine era. J Infect Dis 2000;181:1897–905. 10.1086/315492 [DOI] [PubMed] [Google Scholar]
- 18.Khan AN, Al-Jahdali HH, Allen CM, et al. . The calcified lung nodule: What does it mean? Ann Thorac Med 2010;5:67–79. 10.4103/1817-1737.62469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schvoerer E, Frechin V, Warter A, et al. . Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol 2003;41:4904–5. 10.1128/JCM.41.10.4904-4905.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogos CA, Bassaris HP, Vagenakis AG. Varicella pneumonia in adults. a review of pulmonary manifestations, risk factors and treatment. Respiration 1992;59:339–43. 10.1159/000196084 [DOI] [PubMed] [Google Scholar]