Abstract
Hyperemesis gravidarum is not uncommon. Its pathogenies is multifactorial but not fully understood. We present a case of a middle class, Caucasian pregnant woman aged 24 years with coexisting type 1 diabetes, who had severe hyperemesis gravidarum from the sixth week of pregnancy and was resistant to all standard and off-the-label treatments raising questions about the pathogenesis of hyperemesis gravidarum. She was managed with a multidisciplinary approach and was supported with total parenteral nutrition till she had an emergency caesarean section in the 29th week of pregnancy. Her vomiting stopped as soon as a small for gestational age but otherwise healthy male baby was delivered.
Keywords: pregnancy, diabetes
Background
Nausea and vomiting of pregnancy (NVP) affects roughly 80% of pregnant women.1 It usually starts between fourth and seventh weeks of pregnancy and resolves by the 20th week of pregnancy.2 In most cases, NVP is usually mild and self-remitting. However, in a small proportion of pregnant women, the symptoms can be extremely disabling, both physically and mentally.
Hyperemesis gravidarum is defined as severe nausea and vomiting causing weight loss of 5% or more of prepregnancy weight, dehydration and electrolyte imbalance. The incidence of hyperemesis gravidarum is 0.3%–3.6%.1 3 Although there is an association between hyperemesis gravidarum and human chorionic gonadotropin (hCG) levels, the underlying mechanism is still not fully understood.
We present a case of a young woman with type 1 diabetes in her second pregnancy, who had severe hyperemesis gravidarum during both of her pregnancies. Her case was very challenging to manage as her nausea and vomiting were resistant to all available standard and off-the-label treatments.
Case presentation
A middle class, Caucasian woman aged 24 years gravida 2, para 1 (G2 P1) was admitted to hospital with severe nausea and vomiting at 6 weeks gestation. She was diagnosed with type 1 diabetes at the age of 8 years, for which she was on the basal-bolus insulin regimen. She also had severe hyperemesis gravidarum in her previous pregnancy, which started at 16 weeks of pregnancy and continued through the 30th week of pregnancy, when it was decided to do a planned caesarean section because of the severity of her symptoms.
Investigations
She was investigated for any other potential cause that could be causing or contributing to nausea and vomiting. This included thyroid function tests, serum calcium, lipid profile and random cortisol levels, ruling out any endocrine causes for her symptoms. Also, she was reviewed by the surgical team around the time of onset of nausea and vomiting to ensure that no surgical cause such as acute cholecystitis, intestinal obstruction, etc was resulting in uncontrolled nausea and vomiting.
Treatment
In the index pregnancy, she was initially treated with regular intravenous antiemetics including metoclopramide, prochlorperazine, cyclizine and ondansetron, along with intravenous hydration and variable rate insulin infusion (VRII). However, her symptoms did not subside. She was then started on intravenous hydrocortisone from the sixth week of pregnancy, but unfortunately, after some initial response, there was no significant improvement in her persistent nausea and vomiting and steroids were slowly weaned off over next 4 weeks. After obtaining consent from both partners, she was also given a trial of off-the-label mirtazapine. This was given as an oral dose of 30 mg of mirtazapine once daily, but this failed as well, as there was no improvement in her symptoms and she refused to take more doses after the second day. She even considered termination of pregnancy in view of the severity of physical and mental strain on her health. However, she eventually decided to keep the pregnancy. From initial presentation, the patient spent almost all of her time in the hospital, only managing a few days at home. A trial with subcutaneous ondansetron syringe driver was not successful for her to spend some time at home. A peripherally inserted central catheter was inserted as the patient required continuous insulin infusion throughout her pregnancy.
At 16 weeks gestation, she was reviewed by gastroenterology team who offered her total parenteral nutrition (TPN). After initial refusal by the patient, this was commenced from 20th week of pregnancy, and continued until the end of her pregnancy at 29 weeks. Once the rate of TPN infusion was established, the VRII was switched to twice-a-day levemir insulin. Her glycaemic control was quite decent despite steroids and her haemoglobin A1c (HbA1c) was 46 mmol/mol towards the end of pregnancy (normal range 20–41 mmol/mol). She lost some weight in the first trimester, which she compensated for towards the end of her pregnancy with a total weight gain of 2.5 kg between the 5th and 29th weeks of gestation.
Outcome and follow-up
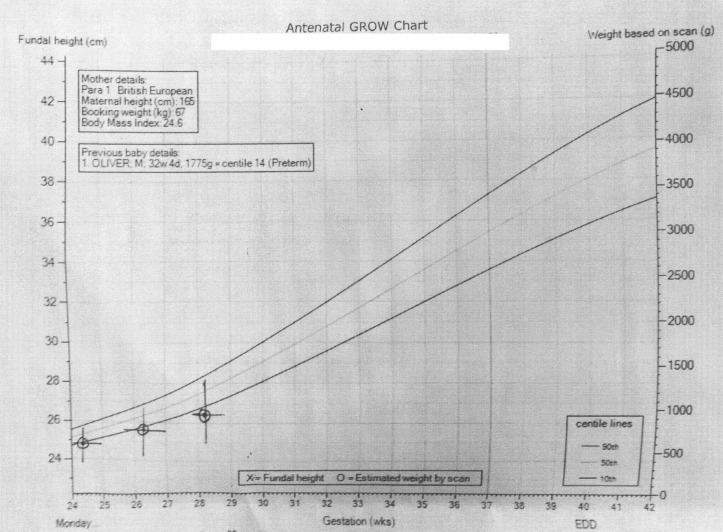
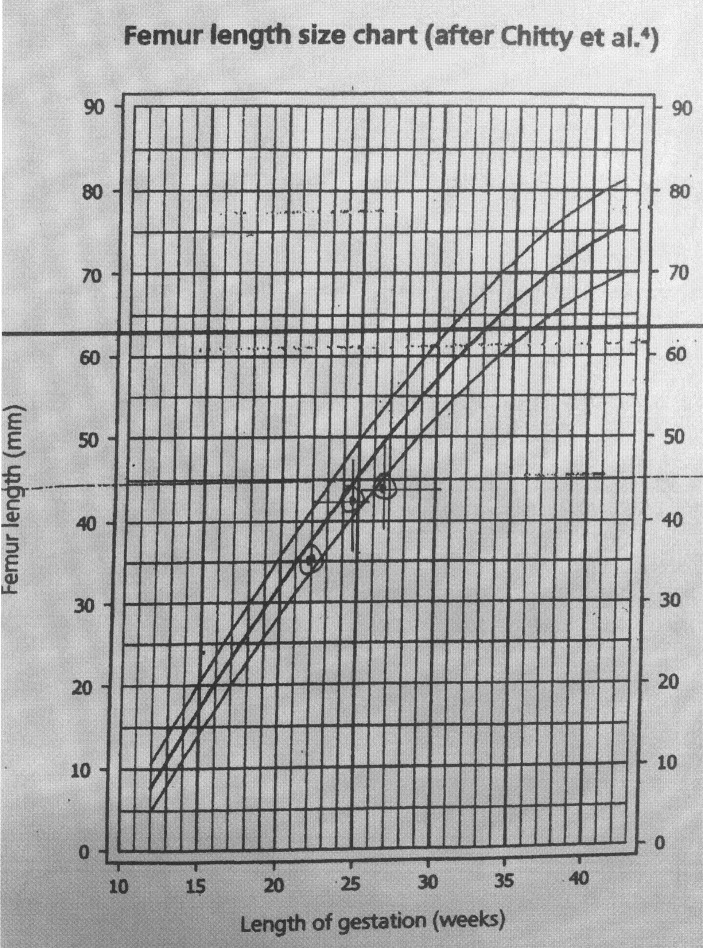
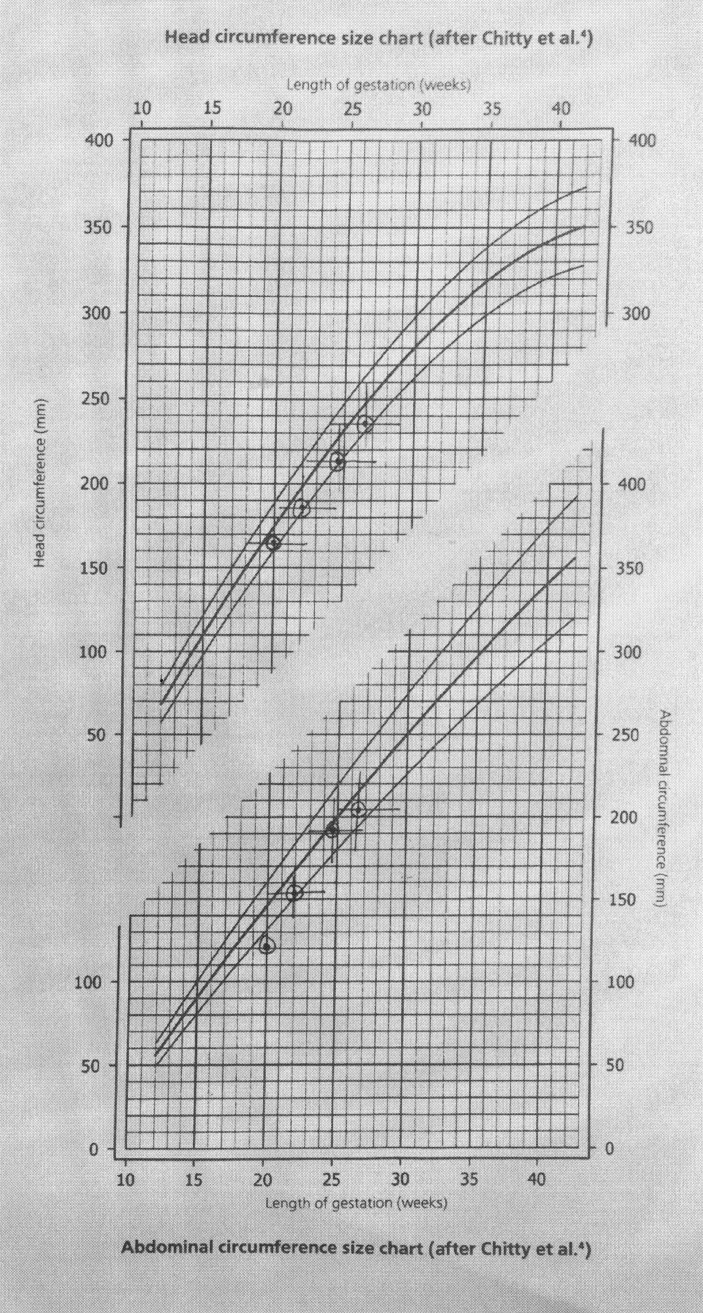
The fetal growth remained below 10th centile as shown in the growth charts (figures 1, 2 and 3). During the 29th week of pregnancy, the umbilical artery Doppler showed reduced end-diastolic flow and raised pulsatility index. Subsequently, the patient reported reduced fetal movements. Her cardiotocography (CTG) was unsatisfactory with unprovoked deceleration and non-reassuring trace. She was given magnesium sulfate for fetal neuroprotection and delivered by caesarian section. A healthy male baby was delivered weighing 1102 g with Apgar score of 8 and 9 at 1 and 5 min, respectively. Blood gases pH were 7.30 and 7.37 for arterial and venous samples and base excess was 2.5 and 2.4 mEq/L, respectively. Her vomiting stopped as soon as a small for gestational age but otherwise healthy male baby was delivered.
Figure 1.
Fetal weight growth chart.
Figure 2.
Femur length growth chart. Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 4. Femur length. Br J Obstet Gynaecol 1994 Feb;101(2):132-5.
Figure 3.
Head and abdominal circumference growth chart. Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 2. Head measurements. Br J Obstet Gynaecol 1994Jan;101(1):35-43. Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 3. Abdominal measurements. Br J Obstet Gynaecol 1994Feb;101(2):125-31.
Discussion
Nausea and vomiting are not uncommon in pregnancy. In most of the cases, it does not require hospital admissions, and self-resolves by the second half of pregnancy. Its severe form, hyperemesis gravidarum, which is associated with weight loss, however, can sometimes be very challenging especially in a patient with type 1 diabetes, due to requirement of intravenous insulin resulting in frequent hospital admissions.
The pathogenesis of hyperemesis gravidarum is multifactorial. Multiple hypotheses have been postulated but still, the exact mechanism is unclear. The risk factors described for hyperemesis gravidarum include young age, black or Asian ethnicity, socioeconomic deprivation, nulliparity, female fetus, multiple pregnancy, history of hyperemesis gravidarum in a previous pregnancy, hypercholesterolaemia, thyroid and parathyroid dysfunction and type 1 diabetes.4
It is believed that various endocrine and metabolic factors are associated with hyperemesis gravidarum. The most implicated factor is hCG, as the peak of NVP and hCG both occur around 9–12 weeks of gestation. It is believed that there is an important role of placental prostaglandin E2 on gastric smooth muscle. Prostaglandin E2 causes relaxation of the stomach and delayed gastric motility. hCG stimulates the prostaglandin release from the placenta. This is evidenced by the high serum prostaglandin levels found during periods of nausea and vomiting.5 hCG has cross-reactivity to the thyroid-stimulating hormone receptors and causes thyroid dysfunction during pregnancy and may also have a role in hyperemesis gravidarum pathogenesis. The success of steroids in achieving improvement in symptoms as reported in many cases,6 7 could be due to inhibition of prostaglandin synthesis.
Oestrogen and progesterone also have a role in the pathogenesis of hyperemesis gravidarum. Oestrogen is believed to increase production of nitric oxide which in turn causes relaxation of smooth muscle of gastrointestinal tract. Progesterone also decreases smooth muscle contractility and also has an effect on gastric emptying.8
Interestingly, some studies have shown that Helicobacter pylori infection is associated with hyperemesis gravidarum as well. A systematic review and meta-analysis of case-control studies found that exposure to H. pylori is associated with risk of hyperemesis gravidarum9; however, the mechanism is poorly understood and there are no such guidelines established anywhere, which encourage H. pylori serology testing and eradication therapy in the preconception period or during the pregnancy. More precise and larger studies are required to establish the association with H. pylori and effectiveness of its eradication. Even if proved, it is worth mentioning that there will be doubt over the safety of current treatment options used for H. pylori eradication during pregnancy, unless it is used before pregnancy in women with history of severe hyperemesis gravidarum. As the authors became aware of this association at the time of writing the article, this hypothesis was not tested in this patient; however, this can obviously be tested in the subsequent pregnancy.
There are indeed some psychological factors associated with hyperemesis gravidarum as evidenced by the successful use of mirtazapine in some cases.10 Mirtazapine acts on noradrenergic, serotonergic, histaminergic and muscarinic receptors to produce antidepressant, anxiolytic, antiemetic, sedative and appetite-stimulating effects and is currently unlicensed for use in pregnancy. Its limited success in some cases holds promise to expand treatment options for hyperemesis gravidarum in future.
We believe that despite the various explanations for severity of symptoms in hyperemesis gravidarum, our current knowledge remains limited. This is the reason why the available standard treatment options can be unsuccessful in certain cases like our case.
The extra complexity in our case was that the patient had type 1 diabetes. As she was unable to eat and drink, she required frequent hospital admissions for variable rate insulin infusion. Also, the risk of hypoglycaemia and diabetic ketoacidosis is increased in such cases. Hyperemesis gravidarum in such patients can become even more difficult to manage if there is coexistent gastroparesis due to type 1 diabetes. Luckily, our patient did not have gastroparesis but still had to spend most of her pregnancy in hospital, which was debilitating for her and her family. All the available treatment options were exhausted, without any success, which was frustrating for the patient and family, as well as the medical team. Her glycaemic control, however, improved while she was an inpatient. Despite being on steroids, her HbA1c improved in pregnancy, and she did not have any episode of diabetic ketoacidosis. A multidisciplinary approach involving obstetricians, endocrinologists, gastroenterologists, dieticians and mental health team is important in providing holistic care pathways for such patients covering medical, obstetric, psychological and social aspects during this stressful time as was the case with our patient resulting in safe and timely delivery and eventually resolution of debilitating maternal symptoms.
Learning points.
The pathogenesis of hyperemesis gravidarum is still not fully understood and the underlying mechanism cannot be explained by one factor only.
Complicated cases of hyperemesis gravidarum should be managed by a multidisciplinary team involving obstetricians, endocrinologists (for patients with diabetes), gastroenterologists, dietitians and psychiatrists.
More studies are required to explore the role of Helicobacter pylori in the causation of hyperemesis gravidarum and if there are any benefits with regard to primary or secondary prevention in subsequent pregnancies.
The role of other treatment options like antipsychotic drugs for hyperemesis gravidarum needs further attention.
Footnotes
Contributors: Conception and planning: MFA, MB. Literature search: NJ, MFA. Writing the report: MFA, NJ, MB.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Einarson TR, Piwko C, Koren G. Quantifying the global rates of nausea and vomiting of pregnancy: a meta analysis. J Popul Ther Clin Pharmacol 2013;20:171–83. [PubMed] [Google Scholar]
- 2.Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract 1993;43:245–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews A, Haas DM, O’Mathúna DP, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2014;3:CD007575. [DOI] [PubMed] [Google Scholar]
- 4.Fiaschi L, Nelson-Piercy C, Tata LJ. Hospital admission for hyperemesis gravidarum: a nationwide study of occurrence, reoccurrence and risk factors among 8.2 million pregnancies. Hum Reprod 2016;31:1675–84. 10.1093/humrep/dew128 [DOI] [PubMed] [Google Scholar]
- 5.North RA, Whitehead R, Larkins RG. Stimulation by human chorionic gonadotropin of prostaglandin synthesis by early human placental tissue. J Clin Endocrinol Metab 1991;73:60–70. 10.1210/jcem-73-1-60 [DOI] [PubMed] [Google Scholar]
- 6.Taylor R. Successful management of hyperemesis gravidarum using steroid therapy. QJM 1996;89:103–8. 10.1093/qjmed/89.2.103 [DOI] [PubMed] [Google Scholar]
- 7.Bondok RS, El Sharnouby NM, Eid HE, et al. Pulsed steroid therapy is an effective treatment for intractable hyperemesis gravidarum. Crit Care Med 2006;34:2781–3. 10.1097/01.CCM.0000242156.15757.70 [DOI] [PubMed] [Google Scholar]
- 8.London V, Grube S, Sherer DM, et al. Hyperemesis Gravidarum: A review of recent literature. Pharmacology 2017;100:161–71. 10.1159/000477853 [DOI] [PubMed] [Google Scholar]
- 9.Sandven I, Abdelnoor M, Nesheim BI, et al. Helicobacter pylori infection and hyperemesis gravidarum: a systematic review and meta-analysis of case-control studies. Acta Obstet Gynecol Scand 2009;88:1190–200. 10.3109/00016340903284927 [DOI] [PubMed] [Google Scholar]
- 10.Guclu S, Gol M, Dogan E, et al. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298–300. 10.1007/s00404-005-0007-0 [DOI] [PubMed] [Google Scholar]