Abstract
Objective
To examine whether primary reports of randomized clinical trials (RCTs) in 6 high impact, general medical journals reported (1) whether or not a Data Monitoring Committee/Data and Safety Monitoring Board (DMC/DSMB) was used and (2) the composition of the responsibilities of the reported DSMB/DMCs.
Study design and Setting
Systematic review of RCTs published in 2014 in Annals of Internal Medicine, BMJ, NEJM, JAMA, JAMA Internal Medicine, and Lancet.
Results
Of the 294 articles identified, 174 (59%) mentioned using a DMC/DSMB. Of these 174, 126 (72%) indicated at least one responsibility of the DMC/DSMB, 26% listed the names of the DMC/DSMB members, and another 14% listed both their names and affiliations. Only one article stated that a DSMB was not used. The remaining 119 articles did not report whether or not a DMC/DSMB was used, even though 59 had previously stated in a clinical trials registry entry or a published protocol that a DMC/DSMB was to be used.
Conclusions
Considering the major role that DMC/DSMB s play in protecting participant safety, data quality, and interim analyses in RCTs, we recommend that authors of publications of RCTs report whether a DMC/DSMB was used and the responsibilities and members of DMC/DSMBs to increase transparency regarding study conduct.
Keywords: Data and Safety Monitoring Board, Data Monitoring Committee, clinical trial reporting, trial integrity
1. Introduction
Data Monitoring Committees/Data and Safety Monitoring Boards (DMC/DSMBs) are an integral part of randomized clinical trials (RCTs). An external monitoring board was originally proposed in the 1967 Greenberg Report for the purpose of coordinating data from multi-site trials [1]. Today, this concept has evolved to an independent board of experts responsible for protecting participant safety and data quality and overseeing the conduct and interpretation of interim analyses [2,3,4,5,6]. The integrity of the trial can be (or be seen as) compromised when the trial leadership and investigators have access to accumulating results of the trial by treatment group; hence, the independence of the DSMB is central to maintaining trial integrity. It has been suggested that a DMC/DSMB should include, at a minimum, an experienced clinical trial statistician and a clinician with expertise in the medical area being studied [3,4,7,8,9]. In peer-reviewed publications of RCTs, explicitly mentioning whether a DSMB was used and if so, the roles that the DMC/DSMB had in the trial (e.g., monitoring of trial information for evidence of safety, efficacy, or futility; monitoring of data timeliness and quality, monitoring site performance), as well as the names and affiliated institutions of the members, would provide valuable information regarding the clinical trial procedures used to enhance transparency and ensure the quality of the data and safety of the participants.
It is impossible to know which trials included a DMC/DSMB unless this information is reported in peer-reviewed publications or on trial registries such as clinicaltrials.gov. Although regulatory and funding agencies have recommendations regarding which RCTs require a DMC/DSMB, these recommendations vary among, and even within, agencies. For example, the National Institutes of Health (NIH) maintains DSMB policies that differ across institutes [10,11,12,13,14,15]. However, certain trial characteristics increase the likelihood that the Food and Drug Administration (FDA), NIH, and European Medicines Agency (EMA) would require a DSMB, including trials that (1) compare rates of mortality or major morbidity, (2) test invasive treatments or a treatments with potentially high toxicity, (3) include vulnerable participants, or (4) are large, multi-site, and double-blinded [11,13,14,15].
Previous reviews found that use of a DMC/DSMB was reported in 10% to 25% of primary reports of RCTs published in general medical and pediatric journals in time frames ranging from 1990 to 2007 [9,12,16,17,18]. The objective of this study was to investigate whether RCTs published in 6 prominent general medical journals in 2014 clearly stated whether or not a DSMB was used. When a DMC/DSMB was reported, we reviewed the inclusion of details regarding the DSMB’s responsibilities as well as whether the DSMB members’ names and affiliations were provided. Our intention is to encourage clear reporting of whether a DSMB was used in a clinical trial, the composition of the DMC/DSMB, and the DMC/DSMB’s activities; doing so will improve the transparency of approaches used to monitor the efficacy and safety data by independent experts.
2. Methods
2.1 Data Sources and Searches
We conducted a systematic review of primary publications of RCTs of any medical treatment that were published in 6 high impact, general medical journals in 2014: Annals of Internal Medicine, British Medical Journal (BMJ), Journal of the American Medical Association (JAMA), JAMA Internal Medicine, Lancet, and New England Journal of Medicine. Articles were identified using a PubMed search conducted on January 26, 2015 (see Appendix A for strategy). The articles that were included in the review were reports of RCTs that compared at least 2 treatments, 1 of which could be a placebo, active comparator, or a wait-list control (i.e., the control group consisted of patients assessed over the same period of time that the intervention group received treatment, after which these participants received the study intervention)).
2.2 Study Selection
Two authors (RAK and JSG) independently screened all identified articles to determine whether they met the inclusion criteria.
Data Extraction and Quality Assessment
A coding manual was developed to evaluate the reporting of the use of DMC/DSMBs and their responsibilities and membership. For example, to identify reporting of a DMC/DSMB we searched for the following terms using the “control F” function: DSMB, DMC, DSMC, IMC, monitor, independent, board, committee. Coders were asked to identify the DMC/DSMB’s responsibilities, if any were described, whether the names and affiliations of the members were listed, and in what section the names were listed. The coding manual also included questions pertaining to trial characteristics (e.g., type of intervention, number of participants randomized, blinding status, and whether vulnerable subjects (e.g., children, cognitively impaired patients, or pregnant women) were included) (see Appendix B for a full list and wording of the questions). A trial was considered to have been stopped early if the authors stated that it was terminated earlier than planned or if the number of participants randomized was lower than 95% of the planned sample size. The duration of follow-up was coded as the longest duration participants were followed during the randomized phase of the study. A study was considered to be multi-site if the authors directly stated that participants were recruited from more than one site or if they indicated that IRB approval was obtained at multiple institutions. The coding manual was pre-tested and modified for clarity and content by JSG and RAK in 3 rounds, (i.e., 2 rounds of 3 articles each and a third round of 2 articles). The pre-test rounds used articles published between 2009 and 2013 that otherwise met the inclusion criteria. Coders (MRH, JP, and JL) were then trained on the coding manual using the same articles that were used during the pre-test rounds. The final list of included articles (see Appendix C) was organized into 2 randomized lists, and each article was coded by 2 independent coders. One author (MRH) coded all of the articles and 3 other coders (RAK, JP, and JL) each coded approximately one-third. After the articles were independently coded, 2 authors reviewed discrepancies; those due to obvious oversight were corrected by RAK, and those due to alternative interpretations were adjudicated by JSG.
For trials in articles that did not report a whether a DMC/DSMB was used, we checked registries (i.e., clinicaltrials.gov, isrctn.com, or anzctr.org) and published protocols (cited in the articles’ reference lists and supplementary materials and registries) for information regarding DSMB use. We then contacted the corresponding authors of the articles for which the information regarding DSMBs was not available in a registry or published protocol. We sent an initial email and then a follow-up email one week later if we did not receive a response to our initial email. We attempted to contact the authors by phone that we did not reach via email when we were able to find phone numbers via an internet search.
2.3 Data Synthesis and Analyses
Descriptive statistics were used to analyze trial characteristics for the total sample. Descriptive statistics were also used to identify the percentage of articles with a particular characteristic that reported a DMC/DSMB in (1) the manuscript or (2) via any method we used to identify DMC/DSMBs (i.e., reported in the manuscript, trial registries, published protocols, or personal communication). We also summarized the responsibilities and memberships of DMC/DSMBs that were reported in the articles.
2.4 Patient involvement
Patients were not involved in the design of this study.
3. Results
3.1 Coder Discrepancies
The total number of items coded for the project was 4681, and there were 696 (15%) discrepancies. Of the 696 discrepancies, 629 (90%) were obvious oversights by one of the coders, and the remaining 67 (10%) were due to differences in interpretation between coders. Of the 4,681 total items coded, 3,252 were trial characteristics and the other 1,429 were DSMB-related. Among the 1,429 DMC/DSMB data items, there were a total of 152 (11%) discrepancies. Of those, 121 (80%) were obvious oversights, and the remaining 31 (21%) were due to differences in interpretation. The most common discrepancy occurred when one of the coders interpreted an Institutional Review Board or on-site data monitor as a DMC/DSMB.
3.2 Trial Characteristics
The search yielded 379 records, of which 47 were excluded by scanning the title or abstract. We excluded 38 of the remaining 332 articles after scanning the full text. The most common reason for exclusion was that the article solely reported secondary analyses of clinical trial data (Figure 1). Of the 294 articles meeting the inclusion criteria, slightly fewer than half (42%) were published in the New England Journal of Medicine. The remaining articles were primarily split between JAMA (24%) and the Lancet (23%) (Table 1). Over half (57%) of the studies were sponsored at least in part by industry. Non-invasive pharmacologic treatments (i.e., treatments that do not cause a break in the skin, including transdermal and oral mucosal treatments) were studied most frequently (35%), followed by invasive pharmacologic treatments (i.e., any treatment that breaks the skin, including implanted pumps and intravenous delivery) (17%). Approximately half of the trials enrolled more than 500 participants. A majority of trials had multiple study sites (90%) and most did not include a vulnerable study population (77%). Fewer than one-half (43%) had a double-blind study design.
Figure 1.
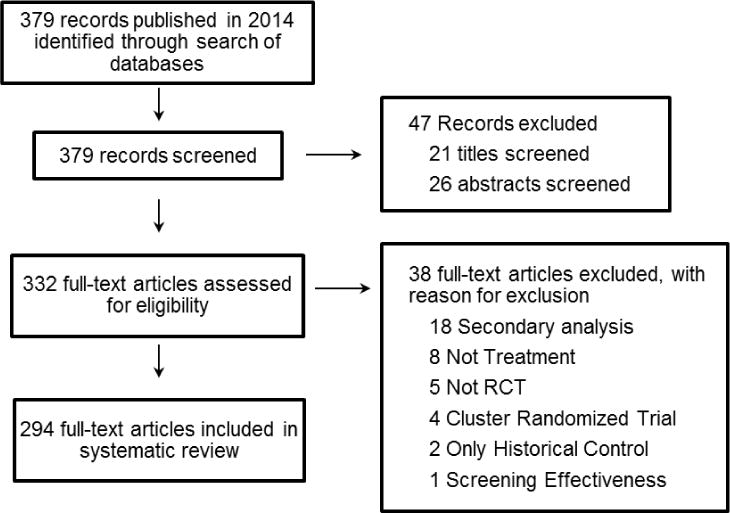
PRISMA diagram
Table 1.
Trial Characteristics
| Characteristic | N (% of total) | N (% of total with the characteristic) | |
|---|---|---|---|
|
| |||
| Total (N = 294) | Reported a DSMB in the manuscript | DSMB identified via any method* | |
|
| |||
| Journal | |||
| Annals of Internal Medicine | 8 (3) | 1/8 (13) | 4/8 (50) |
| BMJ | 13 (4) | 7/13 (54) | 8/13 (62) |
| JAMA | 71 (24) | 49/71 (69) | 62/71 (87) |
| JAMA Internal Medicine | 10 (3) | 0/10 (0) | 6/10 (60) |
| Lancet | 69 (23) | 41/69 (59) | 50/69 (72) |
| New England Journal of Medicine | 123 (42) | 77/123 (63) | 107/123 (87) |
|
| |||
| Sponsor | |||
| Industry | 169 (57) | 98/169 (58) | 136/169 (80) |
| Other | 125 (43) | 77/125 (62) | 101/125 (81) |
|
| |||
| Intervention type | |||
| Non-invasive pharmacologic | 103 (35) | 65/103 (63) | 84/103 (82) |
| Invasive pharmacologic | 50 (17) | 33/50 (66) | 42/50 (84) |
| Surgical | 11 (4) | 8/11 (73) | 10/11 (91) |
| Invasive device | 12 (4) | 5/12 (42) | 10/12 (83) |
| Non-invasive device | 6 (2) | 3/6 (50) | 3/6 (50) |
| Radiologic | 4 (1) | 3/4 (75) | 4/4 (100) |
| Biologic | 19 (6) | 11/19 (58) | 16/19 (84) |
| Psychological/Behavioral | 12 (4) | 6/12 (50) | 10/12 (83) |
| Physical therapy | 5 (2) | 3/5 (60) | 3/5 (60) |
| Dietary | 6 (2) | 4/6 (67) | 5/6 (83) |
| Other | 66 (22) | 34/66 (52) | 50/66 (76) |
|
| |||
| Number of sites | |||
| Multi-site | 263 (90) | 165/263 (63) | 219/263 (83) |
| Single-site | 27 (9) | 9/27 (33) | 16/27 (59) |
| Unclear | 4 (1) | 1/4 (25) | 2/4 (50) |
|
| |||
| Longest follow-up duration | |||
| < 3 months | 47 (16) | 27/47 (57) | 31/47 (66) |
| 3 months – 1 year | 127 (43) | 68/127 (54) | 96/127 (76) |
| >1 year – 5 years | 98 (33) | 68/98 (69) | 92/98 (94) |
| >5 years | 22 (7) | 12/22 (55) | 18/22 (82) |
|
| |||
| Number randomized | |||
| <100 | 12 (4) | 8/12 (67) | 9/12 (75) |
| 100–499 | 134 (46) | 72/134 (54) | 102/134 (76) |
| 500–999 | 70 (24) | 41/70 (59) | 59/70 (84) |
| >=1000 | 78 (27) | 54/78 (69) | 67/78 (86) |
|
| |||
| Blinding | |||
| Double-blind | 129 (44) | 78/129 (60) | 105/129 (81) |
| Patient and/or assessor blinded only | 99 (34) | 56/99 (57) | 77/99 (78) |
| None reported | 66 (22) | 41/66 (62) | 55/66 (83) |
|
| |||
| Vulnerable participant population | |||
| Yes | 68 (23) | 48/68 (71) | 55/68 (81) |
| Children | 43 (15) | 28/43 (65) | 32/43 (74) |
| Pregnant women | 9 (3) | 7/9 (78) | 8/9 (89) |
| Cognitively impaired | 15 (5) | 13/15 (87) | 14/15 (93) |
| Pregnant women and children | 1 (0.3) | — | 1/1 (100) |
| No | 226 (77) | 175/226 (77) | 182/226 (81) |
|
| |||
| Trial stopped earlier than planned | |||
| Yes | 58 (20) | 50/58 (86) | 54/58 (93) |
| No | 236 (80) | 125/236 (53) | 183/236 (78) |
Sponsor “other”: Studies that received no funding or donation of study treatment from industry; other sources included government agency, academic institution/departmental funds, or professional foundation
Intervention type “other”: multiple intervention types, induced hypothermia, different care protocols, care coordination
DMC/DSMB searched for on registries (e.g., clinicaltrials.gov), published protocols, and personal correspondence; 7 articles remained with no data pertaining to whether a DSMB was present.
3.3 Reporting of DMC/DSMB Use and Responsibilities
Of the 294 articles included, 175 (60%) reported the use of a DMC/DSMB. Only one article explicitly stated that a DMC/DSMB was not used. The article that explicitly stated that a DMC/DSMB was not used provided the rationale that minimal risk to participants did not necessitate the use of such a committee. The remaining 118 (40%) articles did not mention a DMC/DSMB. Fifty-eight (49%) of the 118 articles that did not mention a DMC/DSMB reported the intention to use a DMC/DSMB either in the registry or the published protocol. Thirty-seven (31%) of the 118 that did not mention a DMC/DSMB in the article reported that they did not plan to use a DMC/DSMB. For the 22 remaining articles, we could not find information regarding whether a DMC/DSMB had been used in a registry or in the published protocol. For these 22 articles, 15 of the corresponding authors responded to our email or phone inquiries and 4 of those trials included a DMC/DSMB. Thus, in total, 237 (81%) of the 294 trials included in the articles reviewed likely included a DMC/DSMB, and 62 (26%) of those 237 DMC/DSMBs were not reported in the publication. Several characteristics were associated with an increased percentage of trials using a DMC/DSMB, including (1) recruitment from multiple sites (vs. a single site), (2) randomization of more than 500 participants, (3) participant follow-up longer than 1 year, (4) inclusion of pregnant women or cognitively impaired patients, and (5) stoppage of the trial earlier than planned (Table 1).
Of the 175 articles that reported use of a DSMB, 126 (72%) described at least one responsibility of the DMC/DSMB. Of the responsibilities described, monitoring safety data was the most frequently reported (n=78/126, (62%)), followed by monitoring efficacy data for futility or efficacy (n= 45/126, (36%)). The remaining responsibilities included advising on other trial adaptations (n=21/126, (17%)), providing input on or approving the protocol, or both (n=10/126, (8%)), monitoring trial performance (n=5/126, (4%)), examining final efficacy analyses (n=3/126, (2%)), and providing comments on the manuscript (n=1/126, (1%)). Forty-five (26%) of the 175 articles that reported use of a DMC/DSMB reported all of the members’ names and another 25 (14%) reported their names and affiliations. Eighteen of the 175 (10%) specifically identified the DMC/DSMB chair and biostatistician and provided their names and affiliations with varying amounts of information about the other members. DMC/DSMB members were reported in the Acknowledgements sections in all of the articles that reported members.
Twenty-nine of the 175 articles that reported a DMC/DSMB directly addressed whether the data presented to the committee were blinded. The following terms were used to describe the data presented to the DMC/DSMB: unblinded (n=14), blinded (n=13), semi-blinded (n=1), and coded assignments (n=1). Twenty-eight articles reported a specific interval for which the DMC/DSMB reviewed the data (e.g., annually or after accumulation of a specified number of participants). Eight articles stated the frequency with which the data were reviewed using an imprecise term such as “regularly” or “periodically”. The remaining 139 did not provide any details regarding how frequently the DMC/DSMB reviewed the data.
4. Discussion
Sixty percent of articles reported a DMC/DSMB in our sample. This percentage is considerably higher than the 25% of articles that reported a DMC/DSMB in a previous review of general medical journals of similar impact published in 2000 [9] and the 7% in the NEJM published between 1989 and 1991 [17]. However, using trial registries, published protocols, and personal communications, we found that 26% of the DMC/DSMBs that were presumably used were not reported in the peer-reviewed publications. Of the publications that indicated use of a DMC/DSMB, 28% provided no details pertaining to the responsibilities of the DMC/DSMB suggesting room for improvement in reporting of DMC/DSMB details. Deficiencies in the reporting of DMC/DSMB responsibilities are not surprising, considering that neither the author guidelines of the included journals nor the CONSORT statement [19] specifically requires DMC/DSMB reporting. Although authors may have assumed that the term “Data Monitoring Committee” or “Data and Safety Monitoring Board” implied the responsibilities of the group, further specification of the major roles of the DMC/DSMB would clarify the scope of work. This is especially important for trials that include interim analyses for efficacy or futility, in which case readers need to be able to evaluate the validity of the final statistical analyses that were performed (i.e., whether they properly accounted for the interim looks at the data).
Reporting whether or not a DMC/DSMB was involved in a trial and which aspects of a trial it monitored provides important information to readers about study procedures. The use of a DMC/DSMB that monitors safety data is especially important when the intervention is potentially of high risk. When the trial involves interim efficacy analyses, having an independent, unbiased group of experts monitoring the trial is important in maintaining integrity. An independent DMC/DSMB instills confidence that risk or toxicity has not been neglected and that the quality of the data and analyses have been critically monitored and evaluated [20].
Serving on a DMC/DSMB involves significant responsibility and time commitment [21,22]. With one-quarter of the trials that likely used a DMC/DSMB not reporting its use in publications in 6 high-impact journals, academics may not receive sufficient recognition for their efforts in their promotion documents or grant submissions. If more authors acknowledged DMC/DSMB members and their responsibilities, it might help establish that serving on a DMC/DSMB should be a valued consideration in academic promotion, as has been suggested by Armstrong and Califf [23]. Furthermore, considering the amount of time and effort that is required when serving on a DMC/DSMB and the importance of the DMC/DSMB’s role in a trial, acknowledgement of the scope of the members’ work is certainly appropriate. Finally, because the DMC/DSMB can influence the outcome of a clinical trial by overseeing trial execution and making recommendations regarding protocol changes and early stopping, the DMC/DSMB chair and statistician ideally should have appreciable experience with clinical trials and DMC/DSMBs [3,4,24]. If readers can identify the DMC/DSMB member, they may better evaluate the members’ expertise in relation to the trial subject matter. The combination of these points is the basis for our recommendation to acknowledge the DSMB members in publications. This recommendation is contingent on the DMC/DSMB members agreeing to be acknowledged, as there are circumstances in which they may prefer to remain anonymous.
Some limitations of this study should be considered when interpreting the results. Although we consulted clinical trial registries, searched for published protocols associated with the articles included, and contacted corresponding authors for articles in which it was unknown whether a DMC/DSMB was included, we were unable to determine whether 7 trials that did not report a DMC/DSMB intended to or did use one. We did not contact investigators of all of the articles that reported a DMC/DSMB to assess the independence of the DMC/DSMBs (i.e., DMC/DSMB members were free of conflicts of interest and investigators and sponsors were blinded to confidential DSMB activities (e.g., unblinded interim analyses)) [4,20,25]. We investigated RCTs for any treatment of any condition in only 6 major medical journals. The results presented here may not apply to RCTs published in other journals, and may not reflect reporting for specific conditions or treatments. Although, none of the journals have unique reporting guidelines related to DMC/DSMBs. Finally, although the quality of the reports to the DMC/DSMB is crucial to its function, we did not attempt to assess the quality of the DMC/DSMB reports as this was outside the scope of our review and such information would be very challenging, if not impossible, to obtain.
This review highlights a frequent lack of clarity in primary publications regarding whether or not a DMC/DSMB was used and the details of the composition and role of reported DMC/DSMBs. Furthermore, these data demonstrate that almost one quarter of the articles reviewed failed to report a DMC/DSMB that was reported in a registry, published protocol, or via personal communication In accordance with and building on recommendations by other authors [9,12], we suggest that authors of publications of RCTs follow the recommendations pertaining to DMC/DSMB reporting presented in Table 2 and that if necessary, editors consider revising journal policies to facilitate adherence to providing the recommended information. We suggest that authors use similar language to that presented in the table in order to standardize reporting of DMC/DSMB responsibilities. We also suggest that the final version of the DMC/DSMB charter with amendments should be included as an online supplement and referenced in the main manuscript. If a DMC/DSMB was not used, the article should specifically state this fact; otherwise, when a DMC/DSMB is not mentioned, readers do not know whether a DMC/DSMB was used. We hope that highlighting the important role of DSMBs in clinical trials will increase the number of motivated and qualified individuals interested in serving on DSMBs.
Table 2.
Recommendations for reporting of DSMBs in publications of RCTs
| Minimum reporting recommendations |
|---|
| Report whether or not a DMC/DSMB was used in peer-reviewed publications and on ClinicalTrials.gov (or a comparable well-established clinical trial registration website) |
| If a DMC/DSMB was used: |
| • Report the following in the text of the article: |
| • Brief description of major responsibilities, including monitoring participant safety, benefit:risk assessment, recruitment, data quality, and study integrity, as well as any involvement in interim analyses • Any DMC/DSMB recommendations that significantly modified the protocol after study initiation or an indication that no such recommendations occurred • Names and affiliations of DMC/DSMB members • Frequency of DMC/DSMB meetings • Whether the data presented to the DSMB were unblinded, separated by treatment group but blinded to treatment assignments (i.e., “blinded assignments”), or not separated by treatment group (i.e., aggregate sample data only) |
| • Report the following in supplemental material (if not included in a publicly-available charter): |
| • Whether or not investigators and sponsors were blinded to DMC/DSMB deliberations involving unblinded data and any other DMC/DSMB activities that should generally not be revealed to investigators or sponsors • Whether or not the DMC/DSMB members were evaluated for conflicts of interest • Who was responsible for preparing the reports for the DMC/DSMB (e.g., independent statistical group, unblinded statistician from the organization conducting the trial, or study sponsor personnel) • DMC/DSMB charter with any amendments indicated |
Possible sections in which this information can be included in the article text include: (1) the Methods section, (2) Acknowledgements, (3) separate section before the References, (4) list of contributors, or (5) in a footnote. This placement will likely depend on journal policy.
Supplementary Material
What is new?
DMC/DSMBs are still under-reported in primary reports of RCTs published in 6 major medical journals in 2014
This is the first review to compare the reporting of DMC/DSMBs in the peer-reviewed literature to what is reported on trial registries and in published protocols or communicated by the corresponding authors of the publications
Acknowledgments
This article was reviewed and approved by the Executive Committee of the ACTTION public-private partnership with the United States Food and Drug Administration. The views expressed in this article are those of the authors and no official endorsement by the Food and Drug Administration (FDA) or the pharmaceutical and device companies that provided unrestricted grants to support the activities of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership should be inferred.
Funding
This work was supported by the ACTTION public-private partnership which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, and other sources. Those parties contributing funding had no influence over this research.
Declaration of Conflicting Interests
All authors declare no support from any organization for the submitted work, with the exception that ACTTION provided salary support during the production of this manuscript for JS Gewandter, R.A. Kitt, M.R. Hunsinger, MP McDermott, DC Turk, and RH Dworkin.
References
- 1.Heart Special Project Committee to the National Advisory Heart Council. Organization, review, and administration of cooperative studies (Greenberg Report): A report from the Heart Special Project Committee to the National Advisory Heart Council, May 1967. Control Clin Trials. 1988;9:137–48. doi: 10.1016/0197-2456(88)90034-7. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Harrell FE, Buyse M, Emberson JR, Altman D. Ensuring trial validity by data quality assurance and diversification of monitoring methods. Clin Trials. 2008;5:49–55. doi: 10.1177/1740774507087554. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Trials Transformation Initiative. CTTI recommendations: Data Monitoring Committees. doi: 10.1177/1740774517707743. Accessed 10-4-2016 [ https://www.ctti-clinicaltrials.org/files/recommendations/dmc-recommendations.pdf] [DOI] [PMC free article] [PubMed]
- 4.Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, Elbourne DR, McLeer SK, Parmar MKB, Pocock SJ, Spiegelhalter DJ, Sydes MR, Walker AE, Wallace SA, DAMOCLES study group Issues in data monitoring and interim analysis of trials. Health Technology Assessment. 2005;9:iii–237. doi: 10.3310/hta9070. [Accessed 10-5-2016 https://researchonline.lshtm.ac.uk/13859/1/FullReport-hta9070.pdf] [DOI] [PubMed] [Google Scholar]
- 5.O’Neill RT. Regulatory perspectives on data monitoring. Stat Med. 2002;21:2831–42. doi: 10.1002/sim.1287. [DOI] [PubMed] [Google Scholar]
- 6.Sartor O, Halabi S. Independent data monitoring committees: an update and overview. Urol Oncol. 2015;33:143–8. doi: 10.1016/j.urolonc.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellenberg SS. Protecting clinical trial participants and protecting data integrity: are we meeting the challenges? PLoS Med. 2012;9:e1001234. doi: 10.1371/journal.pmed.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellenberg SS, Fleming TR, DeMets DL. Data Monitoring Committees in Clincal Trials: A Practical Perspective. John Wiley & Sons; 2003. p. 174. [Google Scholar]
- 9.Sydes MR, Altman DG, Babiker AB, Parmar MKB, Spiegelhalter DJ, DAMOCLES group Reported use of data monitoring committees in the main published reports of randomized controlled trials: a cross-sectional study. Clin Trials. 2004;1:48–59. doi: 10.1191/1740774504cn003oa. [DOI] [PubMed] [Google Scholar]
- 10.Dixon DO, Weiss S, Cahill K, et al. Data and safety monitoring policy for National Institute of Allergy and Infectious Diseases clinical trials. Clin Trials. 2011;8:727–35. doi: 10.1177/1740774511425181. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency, Committee for medical products for human use. Guidelines on data monitoring committees. 2005 (Accessed November 11, 2015 at http://osp.od.nih.gov/sites/default/files/resources/WC500003635.pdf.
- 12.Fernandes RM, van der Lee JM, Offringa M. A systematic review of the reporting of Data Monitoring Committees’ roles, interim analysis and early termination in pediatric clinical trials. BMC Pediatr. 2009;9:77. doi: 10.1186/1471-2431-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIH. Further Guidance on a Data and Safety Monitoring for Phase I and Phase II Trials. 2000 (Accessed November 11, 2015, at https://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-038.html)
- 14.NIH. NIH Policy for Data and Safety Monitoring. 1998 (Accessed November 11, 2015, at https://grants.nih.gov/grants/guide/notice-files/not98-084.html)
- 15.U.S. Health and Human Services, Food and Drug Administration. Guidance for Clinical Trial Sponsors: Establishment and Operation of Clinical Trial Data Monitoring Committees. 2006 (Accessed November 11, 2015, at http://www.fda.gov/RegulatoryInformation/Guidances/ucm127069.htm)
- 16.Fernandes RM, van der Lee JH, Offringa M. Data monitoring committees, interim analysis and early termination in paediatric trials. Acta Paediatr. 2011;100:1386–92. doi: 10.1111/j.1651-2227.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 17.Wittes J. Behind closed doors: the data monitoring board in randomized clinical trials. Stats in Med. 1993;12:419–24. doi: 10.1002/sim.4780120504. [DOI] [PubMed] [Google Scholar]
- 18.Yuill K, Carandang C. Safety methodology in pediatric psychopharmacology trials. J Child Adolesc Psychopharmacol. 2013;23:148–62. doi: 10.1089/cap.2011.0142. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 20.DeMets DL, Furberg CD, Friedman LM. Data Monitoring in Clinical Trials: A Case Studies Approach. Springer; 2006. p. 370. [Google Scholar]
- 21.DeMets DL, Ellenberg SS. Data Monitoring committees- Expect the unexpected. N Engl J Med. 2016;375:1365–71. doi: 10.1056/NEJMra1510066. [DOI] [PubMed] [Google Scholar]
- 22.Hess CN, Roe MT, Gibson CM, et al. Independent data monitoring committees: preparing a path for the future. Am Heart J. 2014;168:135–41 e1. doi: 10.1016/j.ahj.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong PW, Califf RM. Data and safety monitoring boards: academic credit where credit is due? JAMA. 2013;310:1563–4. doi: 10.1001/jama.2013.280383. [DOI] [PubMed] [Google Scholar]
- 24.Fleming TR, Hennekens CH, Pfeffer MA, DeMets DL. Enhancing trial integrity by protecting the independence of data monitoring committees in clinical trials. J Biopharm Stat. 2014;24:968–75. doi: 10.1080/10543406.2014.925719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drazen JM, Wood AJJ. Don’t mess with the DSMB. N Engl J Med. 2010;363:477–8. doi: 10.1056/NEJMe1007445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


