Abstract
Despite successful treatment of the clonal plasma cell implicated in its pathogenesis, patients with AL amyloidosis (AL) experience significant morbidity related to underlying amyloid mediated organ dysfunction. While normalization of the serum free light chain measurements (normal ratio of involved and uninvolved free light chains (nFLCr)) is the goal of therapy and centerpiece of hematologic response criteria, achieving (or not achieving) meaningful organ response (OR) is clinically significant for its implications on long-term symptomatology as well as overall survival, and remains the ultimate goal of treatment. Expectations for organ recovery following successful therapy leading to nFLCr in AL remain poorly described. We evaluated the timeframe and predictive factors for OR, and long-term outcome, in 313 AL patients who achieved nFLCr following therapy initiation. OR was seen in 80% of surviving AL patients within one-year of nFLCr. Patients achieving early OR within 1 year of nFLCr had superior overall survival (OS) compared to those who despite obtaining nFLCr did not achieve early OR. We further evaluated factors predicting OR and OS among patients achieving nFLCr. Higher values of dFLC (involved-uninvolved) at diagnosis predict organ response, and early OR predicts improved overall survival following successful hematologic therapy in AL.
Keywords: primary systemic (AL) amyloidosis, organ involvement, response to treatment, free light chains
INTRODUCTION
Multi-organ dysfunction mediated by the deposition of misfolded free light chains in light chain amyloidosis (AL) presents a unique challenge for the treating physician, given the variable impact on disease morbidity, long term survival, and quality of life.1 Underlying organ damage can complicate initial treatment due to unanticipated toxicity, but is also an important consideration in the longitudinal management beyond the initial therapy.2 Treatment for AL targets the clonal plasma cell responsible for production of amyloidogenic FLC. Treatment efficacy is measured both in terms of hematologic response, relating to the reduction of the plasma cell clone and involved FLC (iFLC) burden, as well as organ response (heart, kidney, liver, nerve), both of which correlate with improved overall survival (OS) and quality of life.3,4 Patients who obtain a hematologic complete response (hCR), defined as a normal FLC ratio (nFLCr) and negative serum and urine immunofixation5, are more likely to obtain a clinical organ response, and likewise have improved overall survival as compared to patients who do not.6,7,8,9
The precise mechanisms of improvement in organ function following hCR are not completely understood. It has been observed that despite continued presence of organ amyloid deposition following hCR, reduction in surrogate biomarkers of organ function such as the amino terminal fragment of pro-brain natriuretic peptide (NT-proBNP), measure improvement in cardiac function and correlate with increased overall survival.10,11,12 These clinical observations lend credence to supporting a mechanism of ongoing FLC mediated direct toxicity in addition to damage by the fibrillar deposition,13 and support therapeutic strategies that achieve normalization of involved FLC and FLC ratio rapidly following their initiation.6,14 While achieving nFLCr is the initial goal of therapy, less is known about what predicts organ response following successful achievement of nFLCr, and the clinical implications of the kinetics of organ response on long term patient outcomes in AL. This is an important clinical question in the management of AL. That is, for the patient who has obtained nFLCr but has yet to have an organ response, are there factors predictive of an eventual organ response, and how should this potential organ response be interpreted in the context of their overall prognosis? This study was designed to evaluate the timeframe in which patients obtain documented organ response following successful nFLCr in AL and the clinical implications of the kinetics of organ response.
PATIENTS AND METHODS
Patients
Three hundred thirteen patients with pathologically confirmed AL, treated and followed at Mayo Clinic Rochester between 1998 and 2013, who obtained a response to therapy defined as two consecutive normal values of the serum FLC ratio (nFLCr), were retrospectively studied for improvement in organ function and response. All demographic and clinical information including age, gender, hematologic and organ related laboratory tests were obtained from medical records. The study was performed with IRB approval and in accordance with the principles of the Helsinki declaration. Organ involvement for heart, kidney, liver, and nerve was defined per consensus standards.4 Organ response criteria specifically included cardiac response defined as NT-proBNP reduction of >30% and >300 pg/mL with a baseline NT-proBNP of ≥650 pg/mL, kidney response defined as a 50% decrease and at least 0.5 grams/day in 24-hour urine protein with baseline proteinuria ≥0.5 g/day and creatinine clearance not worsening by >25% over baseline, liver response defined as a 50% decrease in abnormal alkaline phosphatase value, and nerve response defined by improvement in electromyogram conduction velocity (requiring both an abnormal baseline and subsequent study showing improvement).4,5
Statistical Analysis
Continuous data were described with median and range or interquartile range. To detect differences in continuous variables between groups the Kruskal-Wallis test was used. Fisher’s exact test was used to test differences in nominal variables where applicable. Survival curves were constructed per Kaplan-Meier estimates and comparisons were made using the log-rank test. Landmark analysis to compare groups of organ responders and non-responders at the landmark point was performed at one-year from initiation of therapy as well as one-year from achievement of nFLCr. Patients were excluded from the landmark survival analysis if they had died or did not have follow up until the landmark point. Any organ response prior to the landmark point led the patient to be included in the organ responder group, whereas patients with no organ response at the landmark point were included in the non-responder group even if they were known to have obtained organ response beyond the landmark point. Laboratory markers of organ function and baseline amyloid data at diagnosis such as quantitative serum FLC were analyzed as dichotomized variables with specific cut-offs. Cut-offs were either determined from the previously reported literature, from the upper limit of normal of the laboratory reference, or from the median value of our patient cohort. Univariate and multivariate Cox-proportional hazard analysis was performed to assess variables influencing both time to organ response and overall survival on both a landmarked population and where indicated the entire study population. Effect likelihood ratio tests, hazard rates and 95% confidence intervals were calculated to determine significance. Statistical analysis was performed using JMP v.10 statistical software (SAS, Cary, NC).
RESULTS
The final study population consisted of 313 patients with AL who obtained nFLCr after therapy and had adequate follow up for assessment of organ function. Median age at diagnosis was 60 years, and 62% of patients were male. The median estimated follow up for the entire cohort from diagnosis was 46.1 months (95% CI; 42, 52); 241 (77%) patients were alive at last follow up. Renal, heart, liver and nervous system involvement were seen in 75%, 54%, 14%, and 12% of patients at onset of therapy. Half of the patients had single organ involvement, primarily kidney (33%), or cardiac (13%). Fifty-eight percent were Mayo 2004 Cardiac Stage 2 or higher at diagnosis.15 Baseline characteristics are detailed in Table 1.
Table 1.
Baseline variables of 313 patients obtaining normalization of sFLC ratio
| Variable | (%) | Median (range) |
|---|---|---|
| Age at diagnosis | --- | 60 (34–89) |
| Male gender | 62 | --- |
| Duration: diagnosis to nFLCr, months | --- | 7.6 (5.3–10.5)* |
| Duration: treatment to nFLCr, months | --- | 4.8 (3.3–8.7)* |
| Number of organs involved | --- | 1 (1–3) |
| 1 organ involvement | 50 | --- |
| 2 organ involvement | 37 | --- |
| 3 organ involvement | 10 | --- |
| Heart | 54 | --- |
| Ejection fraction % | --- | 62 (55–67)* |
| cTnT ng/mL | --- | 0.04 (<0.01–0.07)* |
| NT-proBNP pg/mL | --- | 3358 (1649–6637)* |
| Kidney | 75 | --- |
| 24 hour proteinuria g/dL | --- | 5.5 (2.6–8.8)* |
| Liver | 14 | --- |
| Nerve | 12 | --- |
| Mayo Clinic stage I–II | 69 | --- |
| Mayo Clinic stage III | 31 | --- |
| Heavy chain present | 51 | --- |
| Serum FLC difference mg/dL | --- | 9.48 (3.76–22.6)* |
| Serum FLC difference > 18.0 mg/dL | 29 | --- |
| Bone marrow plasma cell % | --- | 5 (4–10)* |
| Plasma cell FISH | ||
| t(11;14) IGH/CCND1 fusion | 25 | --- |
| −13 | 18 | --- |
| Hyperdiploidy | 13 | --- |
| Kappa light chain/lambda light chain% | 16/84 | --- |
--- indicates not applicable,
indicates 25%–75% quartile range
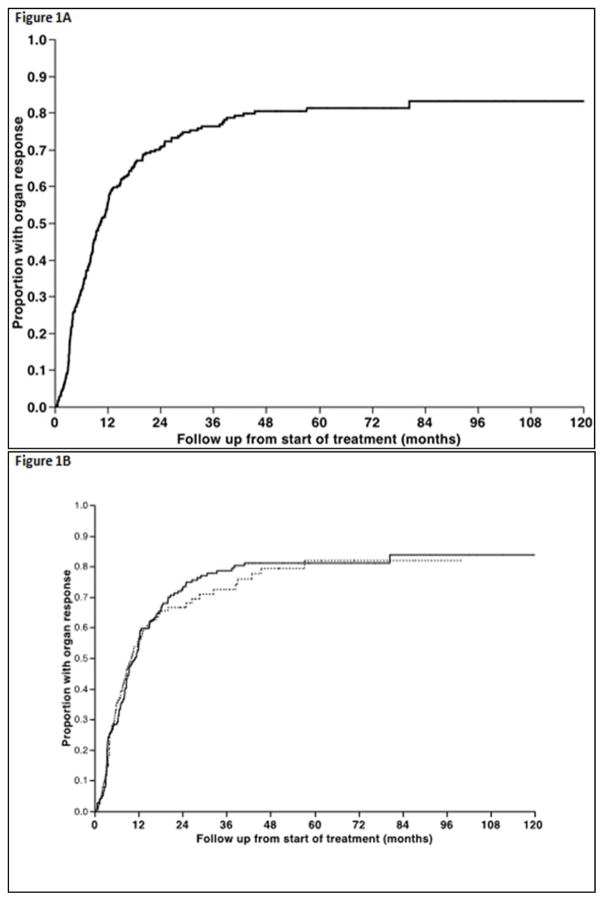
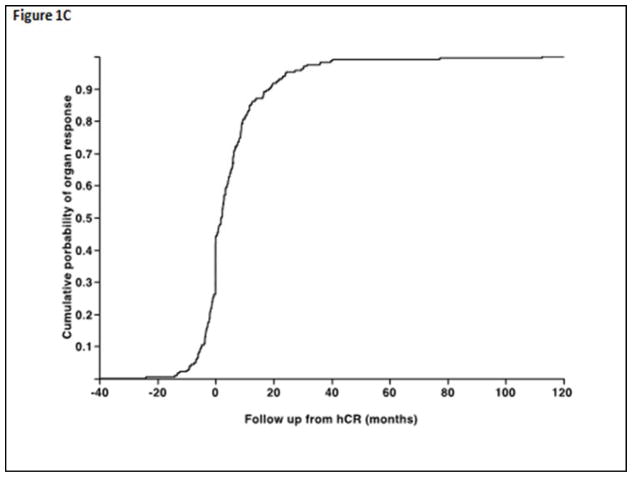
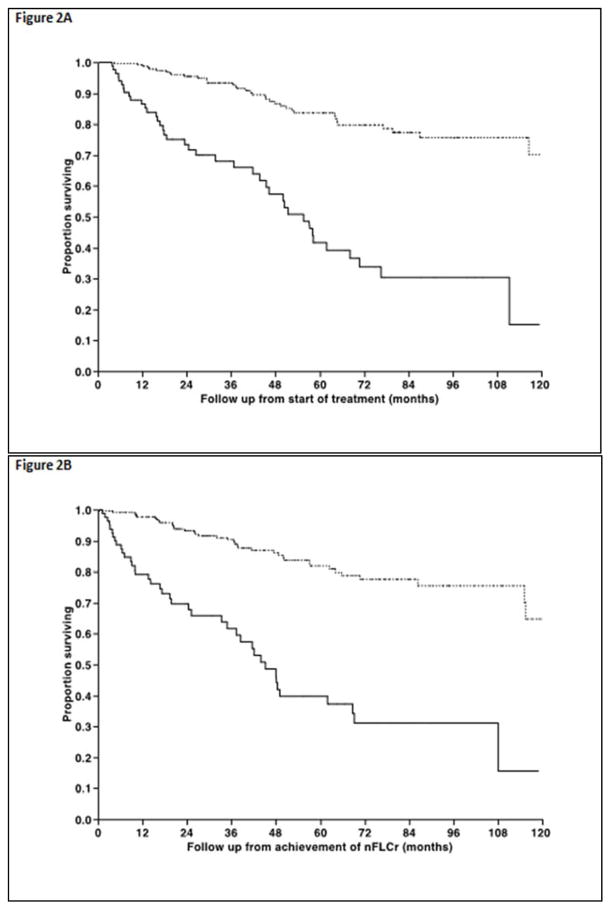
In terms of primary therapy, 54% underwent autologous stem cell transplantation (ASCT) as primary treatment, whereas 28% initially received melphalan and dexamethasone (Mel-Dex) while 13% received a proteasome inhibitor (PI) based initial therapy. The median time to nFLCr from diagnosis was 7.6 months (range, 1.6–45) and the median time to nFLCr from start of initial therapy was 3.8 months (range 0.5–45). The median time to nFLCr was shorter for ASCT compared with other treatment approaches, 3.4 months versus 5 months (P < 0.01). The median estimated time to first OR from start of treatment was 10.4 months (95% CI; 8.7, 12.1) for the entire cohort (Figure 1A) and was not different for the different types of therapy, including ASCT versus non-ASCT types of therapy (p=NS, Figure 1B). Overall, 231 (74%) patients among the entire study population achieved a documented OR in at least one of the affected organs. Among the 231 patients, the median time to the first organ response was 2.1 months (25–75% interquartile range −0.9 to +8.6) from documented nFLCr (Figure 1C). An OR preceded achievement of documented nFLCr in 63 (20%) patients. The median duration from nFLCr to cardiac response among responders was 0.0 months (25–75% interquartile range [−0.7]–[+7.1]), whereas for kidney responders was 2.5 months (25–75% interquartile range [−1.9]–[+9.0]), for liver responders 2.3 months (25–75% interquartile range [−0.2]–[+8.2]), and for nerve responders 0.0 months (25–75% interquartile range [−1.0]–[+15.5]). The overall response rates in individual organs and the timeframe of observed response among those who achieve organ specific response are detailed in Table 2. Fifty-percent of all patients achieved a documented organ response within six-months of nFLCr (62% within 12 months), while among patients with an eventual organ response, 70% achieved this within six-months of nFLCr (86% within 12 months). Among the entire study population, 40 (13%) patients died without obtaining OR; with the median survival following nFLCr being 18.3 months (95% CI; 9.1, 37.4).
Figure 1.
Figure 1A. Time from initial treatment to first documented response in any involved organ. 1B. Time from initial treatment to first documented organ response comparing ASCT (solid line) versus non-initial ASCT therapies (dashed line) showing no significant difference in the time to obtain a first documented organ response. 1C. Among the subpopulation of those who obtained an organ response following therapy, the timeframe of first documented organ response is shown in relationship to documented nFLCr.
Table 2.
Organ specific response rates among 313 patients with AL achieving nFLCr.
| Percent of eventual organ responders achieving OR within
|
|||||
|---|---|---|---|---|---|
| Organ | Overall Response Rate % | 6 months of therapy | 12 months of therapy | 6 months from nFLCr | 12 months from nFLCr |
| Heart | 64 | 40 | 75 | 75 | 87 |
|
|
|||||
| Kidney | 66 | 46 | 70 | 64 | 84 |
|
|
|||||
| Liver | 70 | 58 | 74 | 71 | 87 |
|
|
|||||
| Nerve | 18 | 13 | 50 | 63 | 75 |
|
|
|||||
We then analyzed the outcomes based on a one-year landmark from start of therapy to allow for adequate duration of therapy as well as from the time of achievement of nFLCr. Among the group of patients surviving at least one year from the start of therapy (n=291), 78% had at least one organ response. Specifically looking at the group of patients surviving at least 1 year from the achievement of nFLCr, 80% had at least one OR. Among the patients with multi-organ involvement, a response in every organ (Complete OR) was seen in 58 (38%) patients.
We then examined the factors predicting for an organ response by utilizing a Cox-proportional hazard model utilizing time from start of therapy to first organ response for the entire study population. Univariate analysis as well as a multivariable model were evaluated and are summarized in Table 3. On univariate analysis, having dFLC (defined as the involved minus uninvolved free light chain) greater than 18.0 was predictive of having an organ response following nFLCr. Other significant factors predicting organ response include the presence of monosomy 13 on plasma cell FISH panel, (but not the presence of t(11;14) IGH/CCND1 fusion), having more than one organ involved at diagnosis, and being Mayo Stage II or III at diagnosis. Interestingly, the type of free light chain (kappa versus lambda) did not have an effect for predicting organ response when all organs were included. We then repeated this analysis for cohorts with specific cardiac and renal organ involvement and evaluated variables associated with organ specific response (Supplement Tables 1 and 2). Of note, initial therapy with melphalan and dexamethasone was significantly associated with not obtaining a cardiac organ response on univariate analysis (HR (95% CI) 0.65 (0.43–0.97) p = 0.034) while initial therapy with a proteasome inhibitor was associated with cardiac organ response, although not to a level of statistical significance (HR (95% CI) 1.61 (0.93–2.64) p = 0.09). Particular to patients with renal amyloid involvement was the significant negative association of having an involved lambda FLC, in contrast to kappa, as this was highly predictive failing to obtain renal OR despite nFLCr (HR (95% CI) 0.61 (0.44–0.86) p = 0.005). Also, patients with proteinuria at diagnosis of less than 5.5 g/dL were more likely to obtain a renal response than those with higher levels of baseline proteinuria. High dFLC (>18.0) at diagnosis did not predict for obtaining renal response, but it did for response of other organs including cardiac (HR (95% CI) 1.56 (1.07–2.27) p = 0.021).
Table 3.
Variables predicting first organ response among AL patients achieving nFLCr
| Variable | HR (95% CI) | P |
|---|---|---|
| Univariable analysis | ||
| Male gender | 0.97 (0.75–1.27) | 0.85 |
| dFLC > 18.0 mg/dL at diagnosis | 1.38 (1.03–1.83) | 0.029* |
| Age at diagnosis > 60 years | 1.01 (0.78–1.31) | 0.92 |
| Non IgG heavy chain | 0.75 (0.50–1.07) | 0.12 |
| t(11;14) IGH/CCND1 fusion FISH | 1.28 (0.91–1.81) | 0.16 |
| −13 chromosome FISH | 1.50 (1.04–2.14) | 0.026* |
| Hyperdiploidy FISH | 0.89 (0.56–1.36) | 0.60 |
| Lambda iFLC | 0.78 (0.59–1.06) | 0.11 |
| Primary treatment | ||
| autologous HCT | 1.04 (0.80–1.35) | 0.80 |
| melphalan and dexamethasone | 0.87 (0.64–1.16) | 0.34 |
| proteasome inhibitor inclusive | 0.99 (0.63–1.49) | 0.97 |
| >1 organ involved | 1.48 (1.14–1.93) | 0.003* |
| Time from treatment to nFLCr < 3.8 months¶ | 1.12 (0.87–1.45) | 0.38 |
| Time from diagnosis to nFLCr < 7.6 months¶; | 1.18 (0.91–1.53) | 0.21 |
| Mayo stage 2 or higher | 1.30 (1.00–1.70) | 0.050* |
| Multivariable analysis | ||
| dFLC > 18.0 at diagnosis | 1.18 (0.43–1.28) | 0.43 |
| −13 chromosome FISH | 1.55 (1.07–2.23) | 0.021* |
| > 1 organ involved | 1.52 (1.02–2.26) | 0.037* |
| Mayo stage 2 or higher | 1.20 (0.78–1.85) | 0.41 |
indicates p<0.05,
Reflects median for this cohort
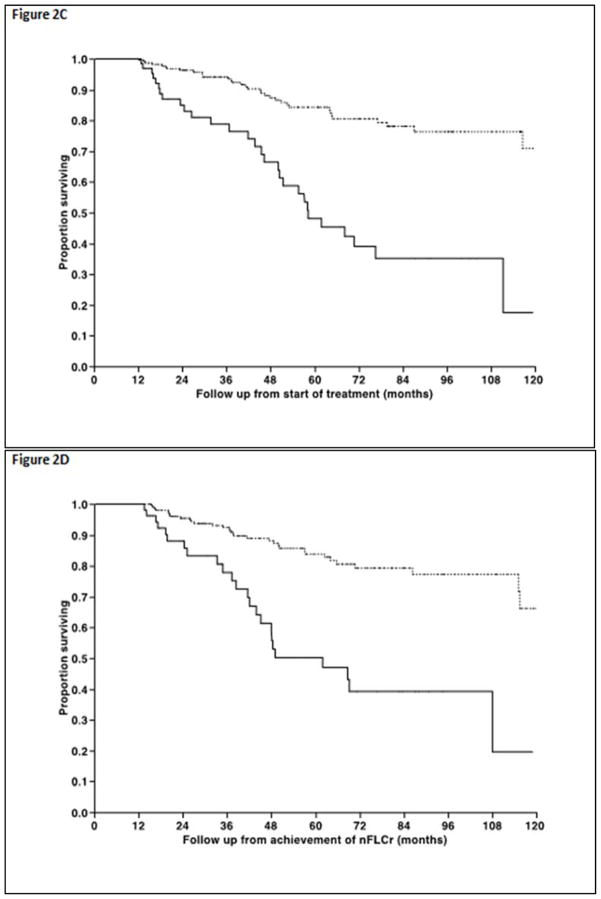
Median overall survival for the entire cohort was not reached, while estimated 5 year survival was 73%. The median OS from initiation of therapy for patients achieving an organ response was not reached compared with 55.7 months (95% CI; 44, 68) for those not achieving an organ response despite achieving the nFLCr (Figure 2A, P < 0.001). Similarly, the median OS from achieving nFLCr was not reached for the organ responders compared with 45.2 months (95% CI; 35, 69) for the rest (Figure 2B, P < 0.001). Landmark analysis of patients surviving at least 12 months from the start of treatment, as well as 12 months from attainment of nFLCr, shows superior overall survival in organ responders (log-rank test p<0.001 for both) Figure 2C, D. Patients who died prior to or did not have follow up post landmark point were excluded, and this amounted to 22 patients when the landmark point was 12 months following start of treatment, and 48 patients when the landmark point was 12 months following obtaining nFLCr. Cox-proportional hazard analysis was performed to identify factors affecting survival following achievement of nFLCr, results are as shown in Table 4. Univariate analysis showed that high Mayo Cardiac Stage at diagnosis, increasing age at diagnosis, and initial treatment with melphalan and dexamethasone were associated with increased rates of all-cause mortality, while lower NT-proBNP at diagnosis and initial treatment with autologous stem cell transplantation were associated with decreased hazard of death (all p-values less than 0.05). Multivariate survival analysis showed that lower initial NT-proBNP values were protective (p=0.0151) and initial treatment with autologous HCT was associated with improved survival (p=0.0006).
Figure 2.
Figure 2A and 2B. Non-landmarked overall survival curves for organ responders (dashed line) and non-responders (solid line) from treatment start and nFLCr among the 313 pts in this study who obtained nFLCr. (log rank p = <0.001 for both)
Figure 2C and 2D. Landmarked overall survival curves for organ responders (dashed line) and non-responders (solid line) from 12 months beyond treatment start and nFLCr among the 313 pts in this study who obtained nFLCr. (log rank p = <0.001 for both)
Table 4.
Variables associated with inferior OS in AL patients achieving nFLCr (n=313)
| Variable | HR (95% CI) | P |
|---|---|---|
| Univariable analysis | ||
| Male gender | 1.40 (0.88–2.28) | 0.15 |
| Differential FLC > 18.0 at diagnosis | 0.89 (0.51–1.48) | 0.66 |
| Age at diagnosis > 60 | 1.66 (1.06–2.63) | 0.027* |
| Non IgG heavy chain | 1.09 (0.56–1.94) | 0.79 |
| t(11;14) IGH/CCND1 fusion FISH | 0.72 (0.36–1.38) | 0.32 |
| −13 chromosome FISH | 0.71 (0.32–1.44) | 0.35 |
| Hyperdiploidy FISH | 1.81 (0.85–3.57) | 0.12 |
| iFLC lambda | 1.10 (0.67–1.90) | 0.70 |
| Primary treatment | ||
| autologous HCT | 0.29 (0.17–0.46) | <.0001* |
| melphalan and dexamethasone | 3.08 (1.94–4.88) | <.0001* |
| proteasome inhibitor inclusive | 1.47 (0.56–3.17) | 0.40 |
| >1 organ involved | 1.53 (0.97–2.45) | 0.07 |
| Time from treatment to nFLCr < 3.8 months | 1.01 (0.64–1.58) | 0.97 |
| Time from diagnosis to nFLCr < 7.6 months | 1.38 (0.88–2.18) | 0.15 |
| Mayo stage 2 or higher | 3.05 (1.81–5.39) | <.0001* |
| 24 hour urine protein < 5.5 g/dL | 1.41 (0.88–2.31) | 0.15 |
| NTproBNP <3358 pg/mL | 0.34 (0.22–0.54) | <.0001* |
| Multivariable analysis | ||
| Primary treatment auto HCT | 0.39(0.23–0.65) | 0.0003* |
| NTproBNP <3358 pg/mL | 0.49 (0.30–0.80) | 0.0049* |
| Age at diagnosis > 60 | 1.37 (0.86–2.18) | 0.19 |
indicates p<0.05
DISCUSSION
Immunoglobulin free light chains drive amyloid fibril organ deposition, and superior clinical outcomes have been shown to closely correlate with their reduction and normalization (nFLCr).8,16 The current retrospective study of AL patients uses normalization of the free light chain ratio (nFLCr) as the time point surrogate for hematologic complete response. We justify the use of this surrogate for three reasons; firstly, given lack of expected individual FLC normalization while assessing quantitative FLC assays in patients with underlying renal dysfunction, we rely on the normalization of the FLC ratio on consecutive laboratory measurements to indicated normalization of the involved FLC. Secondly, the inclusion of requiring negative serum and urine immunofixation assays in addition to nFLCr would have introduced a new time to laboratory attainment bias, particularly those patients without baseline renal amyloid involvement, who did not have as frequent urine laboratory studies. Thirdly, our fundamental question was in regard to what should be expected in terms of organ response following successful treatment of AL that reduces to normal, the levels of the involved amyloidogenic free light chain. The use of nFLCr as a surrogate for hCR does not compromise the ability to answer that question, and we feel the nFLCr is therefore a useful surrogate and appropriate for use in this current study.
The 313 AL patients in this study represent a favorable risk cohort compared to a general AL population, because they were included in our study by design only if they successfully achieved nFLCr following therapy. Therefore, we acknowledge that we should not extrapolate this data to a general AL population at the time of diagnosis or therapy initiation to predict organ response expectations. It has previously been shown in a general AL population that increased dFLC at diagnosis correlated with a higher risk of death.17 Interestingly, in this highly selected cohort, a high (>18.0) dFLC at diagnosis correlated with a higher likelihood of organ response, but did not have an effect on overall survival. This suggests that while high iFLC at diagnosis correlates with shorter overall survival, among a subset of patients with high iFLC at diagnosis who achieve nFLCr following therapy, the prognostic value of initial high iFLC is less predictive of poor clinical outcomes. It also raises the possibility that the amyloidogenic threshold, the inherent propensity of the iFLC to misfold, deposit in organs, and cause dysfunction, could be relatively greater, thus accounting for the higher rate of organ response seen. These observations suggest heterogeneity within the biology of AL, and raise important questions about the potential hematologic response duration in this high iFLC presenting group which were beyond the scope of our current study.
It has previously been shown that renal response correlates with improved overall survival, and that lambda light chain involvement predicts an increased likelihood of renal involvement in AL.18,19 We have shown that the presence of iFLC lambda predicts a reduced likelihood of having a renal response despite achieving nFLCr. However, the presence of iFLC lambda did not predict death following successful treatment and nFLCr in our cohort. The underlying mechanism as to why the presence of lambda free light chain has a preponderance for renal involvement in AL, and for why in spite of successful treatment and nFLCr there is less renal response observed in patients with iFLC lambda remains unclear.
Cardiac response in our cohort of patients achieving nFLCr was less likely in those patients treated with melphalan and dexamethasone. Bortezomib in combination with dexamethasone plus/minus cyclophosphamide has been reported as an effective therapy that obtains rapid reduction of iFLC in patients with AL.20,21 Therapy regimens in our patients inclusive of a proteasome inhibitor were most closely associated with cardiac response, although this association did not meet statistical significance. Also, in our cohort shorter time from initiation of therapy to nFLCr predicted overall survival. Bortezomib and proteasome inhibitors remain an attractive regimen for patients who do not meet criteria or do not choose to undergo high dose melphalan and autologous hematopoietic stem cell transplantation. While these observations do correlate nicely with mechanistic theorizing, we would again note with caution that our study population was highly selected and extrapolating a benefit for PI based regimens at the time of diagnosis or therapy initiation would be unfair based only on the retrospective data from our selected study population.
The significance of molecular abnormalities in AL are less well described as compared to other plasma cell disorders.22 Chromosomal gains, and the loss of chromosome 13 were observed in similar frequencies in our cohort. Loss of chromosome 13 predicted for an organ response among nFLCr patients, but did not have any effect on overall survival. While t(11;14) FISH abnormalities have previously been associated with improved overall survival in the myeloma population23, there was no association found predicting improved organ response or overall survival for AL patients with t(11;14) FISH abnormalities. The prognostic significance, if any, of various plasma cell FISH markers remains unclear.
Factors predicting overall survival in our cohort achieving nFLCr include younger age, treatment with stem cell transplantation, lower baseline levels of NT pro-BNP, and shorter time from initiation of treatment to recorded nFLCr. Eligibility itself for autologous stem cell transplantation has previously shown to be a prognostic factor for survival.24 While many agree that the goal of therapy in AL should be the rapid reduction of iFLC14 it is interesting that a shorter time from therapy initiation to achieving nFLCr was associated with improved overall survival, but not an increased likelihood of obtaining organ response in our data, suggesting the possibility of other baseline factors influencing organ response over and above duration of exposure to increased levels of amyloidogenic FLC in the serum. Organ response within 12 months of achieving nFLCr strongly predicts improved overall survival in AL. These results provide new and significant clinical insight for the prognostication and management of successfully treated AL. The results can also be of utility in targeting the design of early intervention trials among patients not achieving an organ response, especially with new classes of drugs aimed at enhancing dissolution of amyloid fibrils.
Supplementary Material
Patients with AL have superior OS when obtaining organ response within 1 year of obtaining hematologic response to therapy.
We identified several novel predictors of organ response including high dFLC, and non iFLC lambda in AL patients with renal involvement.
Acknowledgments
This work is supported in part by: Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA96028). Also supported in part by grants CA 107476, CA62242, CA100707 and CA 83724 from the National Cancer Institute, Rockville, MD,USA;
References
- 1.Kyle R, Gertz M. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 2.Gertz M. Immunoglobulin light chain amyloidosis: 2011 update on diagnosis, risk-stratification, and management. American Journal of Hematology. 2011;86:181–186. doi: 10.1002/ajh.21934. [DOI] [PubMed] [Google Scholar]
- 3.Seldin D, Anderson J, Sanchorawala V, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004;104(6):1888–1893. doi: 10.1182/blood-2004-01-0089. [DOI] [PubMed] [Google Scholar]
- 4.Gertz M, Comenzo R, Falk R, et al. Definition of Organ Involvement and Treatment Response in Immunoglobulin Light Chain Amyloidosis (AL): A Consensus Opinion From the 10th International Symposium on Amyloid and Amyloidosis. American Journal of Hematology. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G, Dispenzieri A, Gertz M, et al. New Criteria for Response to Treatment in Immunoglobulin Light Chain Amyloidosis Based on Free Light Chain Measurement and Cardiac Biomarkers: Impact on Survival Outcomes. J Clin Oncol. 2012;30(36):4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 6.Lebovic D, Hoffman J, Levine B, et al. Predictors of survival in patients with systemic light-chain amyloidosis and cardiac involvement initially ineligible for stem cell transplantation and treated with oral melphalan and dexamethasone. British Journal of Haematology. 2008;143:369–373. doi: 10.1111/j.1365-2141.2008.07327.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Dispenzieri A, Lacy M, et al. Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. American Journal of Hematology. 2011;86:251–255. doi: 10.1002/ajh.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gertz M, Kyle R, Greipp P. Response rates and survival in primary systemic amyloidosis. Blood. 1991;77(2):257–262. [PubMed] [Google Scholar]
- 9.Jaccard A, Moreau P, Leblond V, et al. High-Dose Melphalan versus Melphalan plus Dexamethasone for AL Amyloidosis. N Engl J Med. 2007;357:1083–1093. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 10.Palladini G, Barassi A, Klersy C, et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116(18):3426–3430. doi: 10.1182/blood-2010-05-286567. [DOI] [PubMed] [Google Scholar]
- 11.Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107(10):3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 12.Merlini G, Seldin D, Gertz M. Amyloidosis: Pathogenesis and New Therapeutic Options. J Clin Oncol. 2011;29(14):1924–1933. doi: 10.1200/JCO.2010.32.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, Guan J, Jiang B, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci. 2010;107:4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merlini G, Palladini G. Amyloidosis: is a cure possible? Annals of Oncol. 2008;19(supplement 4):iv63–iv66. doi: 10.1093/annonc/mdn200. [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Dispenzieri A, Lacy M, Katzmann J, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantion. Blood. 2006;107(8):3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachmann H, Gallimore R, Gillmore J, et al. Outcome in systemic AL amyloidosis in relation to change in concentration of circulating free immunoglobulin light chains following chemotherapy. British Journal of Haematology. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 18.Leung N, Dispenzieri A, Fervenza F, et al. Renal Response After High-Dose Melphalan and Stem Cell Transplantation is a Favorable Marker in Patients With Primary Systemic Amyloidosis. American Journal of Kidney Diseases. 2005;46(2):270–277. doi: 10.1053/j.ajkd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Gertz M, Leung N, Lacy M, et al. Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol Dial Transplant. 2009;24:3132–3137. doi: 10.1093/ndt/gfp201. [DOI] [PubMed] [Google Scholar]
- 20.Kastritis E, Anagnostopoulos A, Roussou M, et al. Treatmen of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92(10):1351–1358. doi: 10.3324/haematol.11325. [DOI] [PubMed] [Google Scholar]
- 21.Mikhael J, Schuster S, Jimenez-Zepeda V, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19):4391–4394. doi: 10.1182/blood-2011-11-390930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochtler T, Hegenbart U, Heiss C, et al. Hyperdiploidy is less frequent in AL amyloidosis compared with monoclonal gammopathy of undetermined significance and inversely associated with translocation t(11;14) Blood. 2011;117(14):3809–3815. doi: 10.1182/blood-2010-02-268987. [DOI] [PubMed] [Google Scholar]
- 23.Gertz M, Lacy M, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dispenzieri A, Lacy M, Kyle R, et al. Eligibility for Hematopoietic Stem-Cell Transplantation for Primary Systemic Amyloidosis Is a Favorable Prognostic Factor for Survival. Journal of Clinical Oncology. 2001;19(14):3350–3356. doi: 10.1200/JCO.2001.19.14.3350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.