Abstract
Background
Older age at initiation of combination antiretroviral therapy (cART) has been associated with poorer clinical outcomes. Our objectives were to compare outcomes between older and younger patients in our clinical cohort in Jos, Nigeria.
Methods
This retrospective cohort study evaluated patients enrolled on cART at the Jos University Teaching Hospital, Nigeria between 2004 and 2012. We compared baseline and treatment differences between older (≥50 years) and younger (15–49 years) patients. Kaplan-Meier analysis and Cox proportional hazard models estimated survival and loss to follow-up (LTFU) and determined factors associated with these outcomes at 24 months.
Results
Of 8352 patients, 643 (7.7%) were aged ≥50 years. The median change in CD4 count from baseline was 151 vs 132 (P = .0005) at 12 months and 185 vs 151 cells/mm3 (P = .03) at 24 months for younger and older patients, respectively. A total of 68.9% vs 71.6% (P = .13) and 69.6% vs 74.8% (P = .005) of younger and older patients achieved viral suppression at 12 and 24 months, with similar incidence of mortality and LTFU. In adjusted hazard models, factors associated with increased risk of mortality were male sex, World Health Organization (WHO) stage III/IV, and having a gap in care, whereas being fully suppressed was protective. The risk of being LTFU was lower for older patients, those fully suppressed virologically and with adherence rates >95%. Male sex, lack of education, WHO stage III/IV, body mass index <18.5 kg/m2, and having a gap in care independently predicted LTFU.
Conclusions
Older patients achieved better viral suppression, and older age was not associated with increased mortality or LTFU in this study.
Keywords: ART, HIV, LTFU, mortality, older
As more patients are placed on effective combination antiretroviral therapy (cART), there are a growing number of people aged 50 years and older living with human immunodeficiency virus (HIV) infection. For the first time since the start of the epidemic, 10% of the adult population living with HIV in low- and middle-income countries (LMIC) were aged 50 years or older at the end of 2012 [1]. Seventy-four percent of the 100000 new infections occurring among people aged 50 years and above in LMIC annually are acquired in sub-Saharan Africa (SSA). The aging of the HIV epidemic can be attributed to improved survival with cART and decrease in incident cases among young adults and youth [2, 3].
There are special challenges for delivery and monitoring of older patients on cART. Data from 12 LMIC where more than 1 in 10 people initiating ART are ≥50 years old found that mortality was higher in older compared with younger persons, with late presentation serving as an important cofactor [4, 5]. Older persons living with HIV are also known to experience higher burden of comorbidities [6–8] and are often diagnosed at a worse stage of HIV infection compared with their younger counterparts [9, 10]. Data from resource-rich settings reporting on cART outcomes among older patients indicate that despite experiencing higher rates of virologic suppression, older patients tend to have poorer rates of immune reconstitution and higher mortality compared with younger patients [8, 11–13], with others reporting mixed outcomes [14–16].
Studies from SSA have also reported similar outcomes to cART with respect to viral suppression, immunologic response, and survival [17–20]. In an outcomes study reporting on 7 African countries, mortality rates were higher and loss to follow-up (LTFU) rates lower among older patients [21]. However, this study did not present data on virologic or immunologic response. As more people in SSA initiate cART and live longer, the population of patients aged 50 years and above will increase, with older people remaining at increased risk of HIV acquisition [3]. In light of the above data, there is a need to better understand the response to cART in indigenous older patients in SSA and its related outcomes. Nigeria has the second highest burden of HIV globally [2] and is halfway into the second decade of her national antiretroviral therapy (ART) program. In this analysis, our objectives were to characterize the baseline features of older adults initiating cART and to report on outcomes to treatment in the first 2 years postinitiation in a comprehensive HIV treatment, care, and support program in Jos, north central Nigeria.
MATERIALS AND METHODS
This observational retrospective analysis was conducted using data collected routinely at the HIV clinic at the Jos University Teaching Hospital (JUTH), north central Nigeria. (1) The Government of Nigeria and (2) the US President’s Emergency Plan for AIDS Relief (PEPFAR) through a grant awarded to the Harvard T.H. Chan School of Public Health and the AIDS Prevention Initiative Nigeria, Ltd/Gte (APIN) jointly funded the program. For this evaluation, we accessed routine clinical data collected for patients enrolled for care between June 2004 and February 2012.
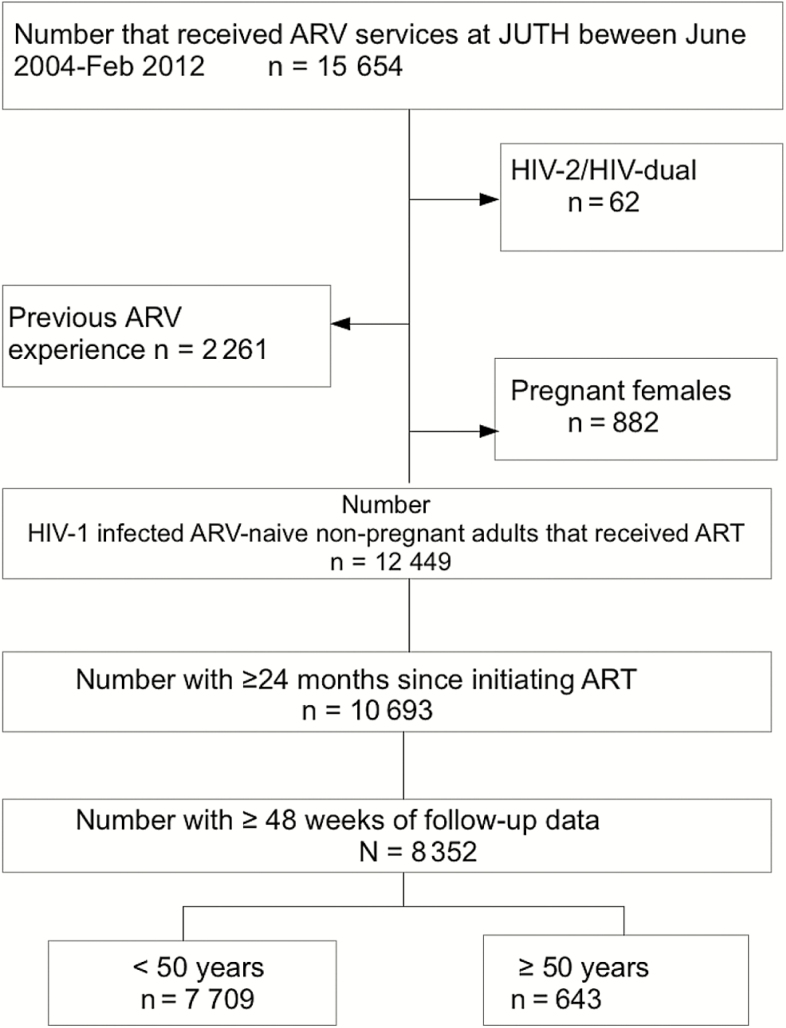
Inclusion criteria for this evaluation were age ≥15 years, ART-naive at enrollment, not pregnant at the time of enrollment, and not having HIV-2 or dual HIV-1/HIV-2 infection. Patients were excluded if they received cART for less than 48 weeks and/or had less than 24 months of follow-up (Figure 1). Patients were seen regularly after enrollment and cART initiation. Hepatitis B virus (HBV) surface antigen (Monolisa HBsAg Ultra3; Bio-Rad, France), and hepatitis C virus (HCV) antibody (DIA.PRO Diagnostic, Bioprobes srl, Milan, Italy) assessments were conducted at baseline. Immunonologic monitoring with CD4+ cell counts using flow cytometry (Partec, Munster, Germany) and HIV viral load (VL) assays were also performed routinely (Cobas Amplicor Monitor Assay, version 1.5; Roche Diagnostics, Mannheim, Germany). Patients were initiated on cART according to the National guidelines existing at the time of program entry (CD4+ cell count <200 cells/mm3, clinical WHO stage III/IV or clinical WHO stage III/IV with CD4+ cell count of <350 cells/mm3) with 1 nonnucleoside reverse-transcriptase inhibitor (NNRTI) and 2 nucleoside reverse-transcriptase inhibitors (NRTIs). During the period covered by this study, standard first-line ART regimens included stavudine or zidovudine (AZT) with abacavir (ABC) and tenofovir (TDF) as alternative options for NRTI and nevirapine or efavirenz as NNRTI options. At each visit, patients received adherence and nutrition counseling and ART refills. Monitoring laboratory tests, including hemoglobin (Hb), serum creatinine, and alanine aminotransferase (ALT), in addition to CD4+ cell counts and VLs were performed at 3 and 6 months, then 6-monthly thereafter. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood ≥90 mm Hg or the use of antihypertensive medications. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL, or use of hypoglycemic drugs).
Figure 1.
Flowchart of patients evaluated and included in this study. ART, antiretroviral therapy; ARV, antiretroviral; HIV, human immunodeficiency virus; JUTH, Jos University Teaching Hospital.
Data Collection
Patient demographic, clinical, laboratory, and therapeutic data were collected on standard forms at enrollment and at each follow-up visit. Data were entered into a secure computerized database designed solely for the purpose of patient care management [22]. The database was updated daily by data entry clerks, and the data management team ensured data accuracy by performing weekly quality assurance checks of the databases. Data collected for this study included baseline demographics, weight, height, World Health Organization (WHO) clinical stage, ART regimen at initiation, enrollment and visit dates, and date of discontinuation (either by death, LTFU, transfer, or self-withdrawal). Baseline and follow-up laboratory monitoring and drug use data were also collected.
Ethical Consideration
All patients in the Harvard/APIN program provided written consent for care. Only those who consented to the use of their information for research were included in this analysis. All patient data were delinked and deidentified. Institutional review boards at the Harvard T. H. Chan School of Public Health and the Jos University Teaching Hospital approved the use of data for this study.
Outcomes of Interest
The primary treatment outcomes were immunologic and virologic responses to cART at 6, 12, 18, and 24 months postinitiation with the main clinical outcomes being all-cause mortality (irrespective of time of cART initiation) and LTFU. A patient was considered LTFU if he/she had not attended the facility for a medication refill, a laboratory visit, or a clinicians visit in the 180 days preceding data abstraction. Mortality ascertainment occurred largely through passive reporting to the health facility by family or friends and, to a lesser extent, through site-specific tracing activities to locate patients LTFU. If the date of death was unknown, but a patient was known to have died, the date of the last encounter with the patient was used as the date of death. Other outcomes of interest included the incidence of select laboratory abnormalities post-cART initiation (Hb ≤8 g/dL, ALT ≥120 IU, and serum creatinine ≥260), rates of drug substitution, and calculated average medication adherence rates. Immunologic outcomes observed were the median change in CD4+ cell count over time from baseline. We also assessed the absolute cell increase from baseline to each time point. Virologic response was assessed using the proportion of patients with HIV VL <400 copies/mL of blood at each designated time point, and we assessed the proportion of patients achieving viral suppression from 6 through 24 months of therapy (fully suppressed-FS). Discontinuation was defined as death, LTFU, or withdrawal from the program. Time to discontinuation was calculated using the last recorded visit date. Percent adherence values were carried backward because adherence is calculated at the end of a prescription refill cycle. The average adherence rate was calculated as the proportion of time the patient had pills available (number of days supplied/number of days between refills) and was capped at 100%. A prespecified adherence cutoff of ≥95% was used for categorical assessment of optimal adherence. A gap in treatment was defined as any period ≥60 days when a patient was without pills. We assessed the number of gaps by age group for the first year of therapy as well as the second year of therapy. Given that there was no significant difference between the age groups in the numbers experiencing 1 or 2 gaps per treatment year, we dichotomized this outcome as “any gap” in treatment versus no gap in the multivariate analysis.
Statistical Methods
Proportions, means (±standard deviation [SD]), and medians (interquartile ratios [IQR]) were calculated for baseline characteristics at the time of cART initiation. The primary predictor was age at cART initiation. This was categorized as 15–49 years (younger) or ≥50 years (older). Immunologic and virologic responses at months 6, 12, 18, and 24 between the 2 groups were also assessed. We also determined the proportions of patients fully suppressed (FS) for the 2 groups and determined factors associated with being FS in a logistic regression model. We determined the incidence of laboratory abnormalities in the first 24 months of cART for serum creatinine, Hb, and ALT. Abnormal cutoffs for each were defined as at least grade 2 according to severity grading of adult adverse events [23]. Finally, we determined rates of death and LTFU by age group. Patients were followed up until they achieved the outcomes of interest or the earliest among the following outcomes: death, LTFU, or the date December 2012 (the end of the follow-up period). To describe the probability of death and LTFU, we used Kaplan-Meier survival analysis.
To estimate the association between age group at treatment initiation, death, and LTFU, Cox proportional hazards models were used to estimate unadjusted and adjusted hazard ratios (aHRs) for each outcome separately with relevant covariates. The final Cox proportional hazards regression models included variables that were statistically significant as well as clinically relevant covariates (significant or not) in unadjusted analyses. Because a significant number of deaths are missed due to loss to follow-up in program settings in LMIC [24, 25], we constructed Cox proportional hazard models and estimated mortality rates after imputing ranked proportions (10%, 25%, and 50%) of patients documented to be LTFU as dead and compared these with the complete case model. The criterion for significance for analyses was 2-tailed P value <.05. Statistical analyses were performed with the statistical software packages Epi 7 (Centers for Disease Control and Prevention, Atlanta, GA) and STATA version 13.1 (StataCorp, College Station, TX).
RESULTS
During the period of June 2004 to February 2012, 15654 patients were enrolled for cART services at JUTH. Our final cohort had 8352 patients with 643 (7.7%) being 50 years or older at enrollment (Figure 1). The baseline characteristics of patients are summarized in Table 1. A greater proportion of older patients compared with younger patients were men (P < .001). We found significant differences in marital status, where a higher percentage of older patients were married or widowed compared with younger patients (P < .001), and educational level, where older patients were less likely to have higher-level education than the younger patients (P < .001). Older patients were more likely than their younger counterparts to have hepatitis C coinfection (P = .001), hypertension (P < .001), diabetes mellitus (P < .001), higher Hb concentration (P = .008), and higher serum creatinine (P < .001).
Table 1.
Baseline Characteristics of Study Population (n = 8 352)
| Characteristics | Total (n = 8352) | Young (15–49 Years) (n = 7709) | Older (≥50 Years) (n = 643) | P Value |
|---|---|---|---|---|
| Mean age, years (SD) | 35 ± 8 | 33 ± 7 | 55 ± 5 | <.001 |
| Sex, n (%) | <.001 | |||
| Male | 2856 (34.2) | 2486 (32.3) | 370 (57.5) | |
| Female | 5492 (65.8) | 5219 (67.7) | 273 (42.5) | |
| Missing | 4 (0.0) | 4 (0.0) | 0 (0.0) | |
| Marital Status | <.001 | |||
| Single | 1665 (19.9) | 1660 (21.5) | 5 (0.8) | |
| Married | 4471 (53.5) | 4079 (53.0) | 392 (60.9) | |
| Widowed | 1499 (18.0) | 1296 (16.8) | 203 (31.6) | |
| Divorced/Separated | 707 (8.5) | 664 (8.6) | 43 (6.7) | |
| Missing | 10 (0.1) | 10 (0.1) | 0 (0.0) | |
| Educational Level Attained | <.001 | |||
| No Formal Education | 1400 (16.8) | 1167 (15.1) | 233 (36.2) | |
| Primary | 1689 (20.2) | 1547 (20.0) | 142 (22.1) | |
| Secondary | 2475 (29.6) | 2415 (31.3) | 60 (9.3) | |
| Tertiary | 2692 (32.2) | 2509 (32.6) | 183 (28.5) | |
| Missing | 96 (1.2) | 71 (1.0) | 25 (3.9) | |
| WHO Stage | .09 | |||
| I | 2757 (33.0) | 2565 (33.27) | 192 (29.8) | |
| II | 2698 (32.3) | 2491 (32.31) | 207 (32.2) | |
| III | 2174 (26.0) | 1993 (25.85) | 181 (28.2) | |
| IV | 465 (5.6) | 418 (5.42) | 47 (7.3) | |
| Missing | 258 (3.1) | 242 (3.14) | 16 (2.5) | |
| WHO Stage III/IV | 2639 (31.6) | 2411 (31.2) | 228 (35.4) | .02 |
| Body mass index (kg/m2) | ||||
| Median (IQR) | 21.5 (19.3–24.0) | 21.5 (19.3–23.9) | 22.0 (19.7–24.9) | .0007 |
| BMIb Class | .002 | |||
| Underweight (<18.5) | 1128 (16.1) | 1046 (16.2) | 82 (15.0) | |
| Ideal weight (18.5–24.9) | 4590 (65.5) | 4260 (66.0) | 330 (60.3) | |
| Overweight (25.0–29.9) | 1035 (14.8) | 925 (14.3) | 110 (20.1) | |
| Obese ≥30 | 250 (3.6) | 225 (3.5) | 25 (4.6) | |
| Baseline CD4+ Cell Count | ||||
| Median (IQR) | 136 (71–201) | 136 (72–201) | 139 (69–200) | .75 |
| % With CD4 <200 | 5941 (71.1) | 5480 (71.1) | 461 (71.7) | .74 |
| Median baseline HIV RNA (cp/mL) (IQR) | 53 253 (12 743–174 358) |
52 527 (12 533–170 899) |
70 733 (18 025–212 684) |
.003 |
| Baseline Hemoglobin | ||||
| Median, g/dL (IQR) | 11.0 (10–13) | 11.0 (10–13) | 12 (10–13) | .008 |
| <8 g/dL, n (%) | 459 (5.5) | 433 (5.6) | 26 (4.0) | .09 |
| Baseline ALT, median (IQR)-IU/L | 24 (16–39) | 25 (16–39) | 24 (16–36) | .22 |
| Baseline serum creatinine, median (IQR)-µmol/L | 81.0 (65.2–101.3) |
80.2 (64.9–100.7) |
91.3 (70.5–113.1) |
<.001 |
| Median eGFRa, mLs/min per1.73 m2 (IQR) | 96.4 (74.9–116.6) | 97.9 (76.2–118.0) | 80.9 (63.9–101.0) | <.001 |
| HBsAg at baseline, n (%) | <.001 | |||
| Positive | 1232 (14.7) | 1168 (15.1) | 64 (9.9) | |
| Negative | 4975 (59.6) | 4554 (59.1) | 421 (65.5) | |
| Missing | 2145 (25.7) | 1987 (25.8) | 158 (24.6) | |
| Anti-HCVab at baseline, n (%) | .001 | |||
| Positive | 911 (10.9) | 811 (10.5) | 100 (15.6) | |
| Negative | 5294 (63.4) | 4908 (63.7) | 386 (60.0) | |
| Missing | 2142 (25.7) | 1990 (25.8) | 157 (24.4) | |
| Hypertension at baseline, n (%) | 136 (1.6) | 85 (1.1) | 51 (7.9) | <.001 |
| Type 2 diabetes at baseline, n (%) | 34 (0.3) | 21 (0.3) | 13 (2.0) | <.001 |
| Year of cART initiation | .28 | |||
| 2004 | 163 (2.0) | 155 (2.0) | 8 (1.2) | |
| 2005 | 1922 (23.0) | 1775 (23.0) | 147 (22.9) | |
| 2005 | 2200 (26.4) | 2037 (26.4) | 163 (25.3) | |
| 2007 | 1682 (20.1) | 1544 (20.0) | 138 (21.5) | |
| 2008 | 1450 (17.4) | 1339 (17.4) | 111 (17.3) | |
| 2009 | 846 (10.1) | 772 (10.0) | 74 (11.5) | |
| 2010 | 85 (1.0) | 83 (1.1) | 2 (0.3) | |
| NRTI Backbone in cART | <.001 | |||
| Zidovudine (AZT) | 4130 (49.4) | 3847 (49.9) | 283 (44.0) | |
| Tenofovir-disoproxil fumarate (TDF) | 1816 (21.8) | 1626 (21.0) | 190 (29.5) | |
| Stavudine (d4T) | 951 (11.4) | 888 (11.5) | 63 (9.8) | |
| Abacavir (ABC) | 238 (2.8) | 222 (2.9) | 16 (2.5) | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; cART, combination antiretroviral therapy; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; HBsAg, hepatitis B surface antigen; HCVab, hepatitis C virus antibody; HIV, human immunodeficiency virus; IQR, interquartile range; NRTI, nucleoside reverse-transcriptase inhibitor; RNA, ribonucleic acid; SD, standard deviation; WHO, World Health Organization.
aEstimated glomerular filtration rate using the CKD-EPI formula.
bBody mass index.
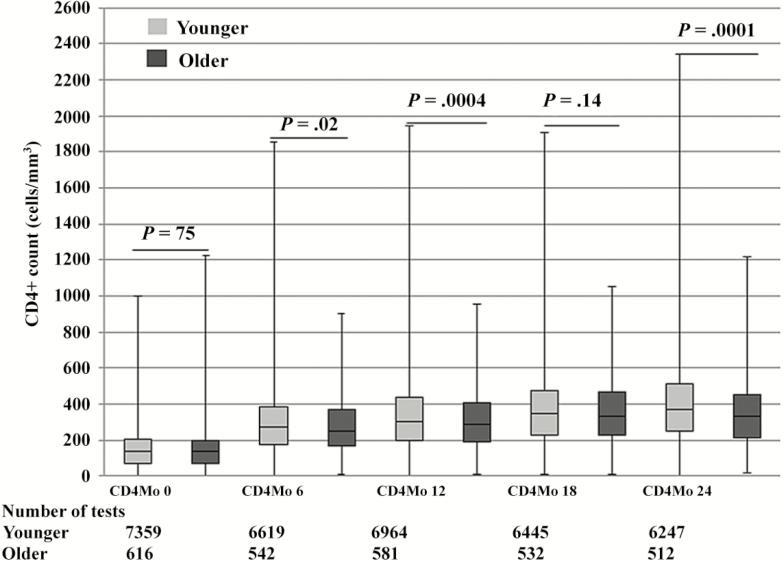
Median CD4+ cell counts post-cART initiation through 24 months increased significantly for both groups (Figure 2). Median CD4+ counts (IQR) were higher at months 6 (272, IQR 177–386 vs 251, IQR 166–367; P = .02), 12 (303, IQR 200–433 vs 283, IQR 186–406; P = .004), and 24 (370, IQR 250–511 vs 331, IQR 215–453; P = .0001) for younger patients compared with older patients. Among patients with CD4+ count <200 cells/mm3 at baseline, 57.9% of older patients compared with 64.5% of younger patients (P = .004) had achieved CD4 ≥200 at the end of follow up. The median change in CD4+ count from baseline was also higher for younger patients at 6 (112 vs 94, P = .0002), 12 (151 vs 132, P = .0005), and 24 (181 vs 151, P < .001) months (Table 2).
Figure 2.
Box plot of median CD4+ counts from baseline (Mo) through 24 months by age group. The box plot used minimum value (lower error bar), the the 25th percentile (lower border of the box), the median value/50th percentile (the line in the middle of the box), the 75th percentile (the top border of the box), and the maximum value (the top error bar).
Table 2.
Follow-Up Data on Treatment From Baseline Through 24 Months of cART for Young and Older Patients Initiating Treatment
| Variable | Total | Young (15–49 Years) | Older (≥50 Years) | P Value |
|---|---|---|---|---|
| Days on HIV care, median (IQR) | 1541 (1051–2051) | 1547 (1056–2052) | 1468 (1020–2004) | .01 |
| Days on cART, median (IQR) | 1610 (1092–2112) | 1614 (1093–2114) | 1586 (1064–2081) | .10 |
| Median CD4+ Cell Increase From Baseline (IQR), Cells/mm3 | ||||
| Month 6 | 110 (25–200) | 112 (27–202) | 94 (2–179) | .0002 |
| Month 12 | 149 (55–254) | 151 (56–256) | 132 (43–232) | .0005 |
| Month 18 | 165 (39–284) | 116 (40–286) | 153 (30–265) | .03 |
| Month 24 | 182 (21–312) | 185 (24–318) | 151 (0–266) | <.001 |
| Viral Suppression (% <400 copies/mL) | ||||
| Month 6 | 4807 (70.5) | 4424 (70.2) | 383 (73.9) | .04 |
| Month 12 | 4992 (69.1) | 4596 (68.9) | 396 (71.6) | .13 |
| Month 18 | 4451 (68.8) | 4100 (68.7) | 351 (70.8) | .24 |
| Month 24 | 4200 (70.0) | 3867 (69.6) | 333 (74.8) | .005 |
| Number of Treatment Gaps | .47 | |||
| None | 7330 (87.8) | 6760 (87.7) | 570 (88.6) | |
| ≥1 gap | 1022 (12.2) | 949 (12.3) | 73 (11.4) | |
| ≥95% adherence | 4898 (58.6) | 4523 (58.7) | 375 (58.3) | .86 |
| Drug Switch at 6 Month, n (%) | .59 | |||
| Yes | 18 (0.2) | 17 (0.2) | 1 (0.2) | |
| No | 8334 (99.8) | 7692 (99.8) | 642 (99.8) | |
| Patient Status at Censoring, n (%) | ||||
| Alive (in site) | 5765 (69.0) | 5320 (69.0) | 445 (69.2) | .29 |
| LTFU | 1533 (18.4) | 1425 (18.4) | 108 (16.8) | |
| Transferred | 953 (11.4) | 873 (11.4) | 80 (12.4) | .31 |
| Died | 83 (1.0) | 73 (1.0) | 10 (1.6) | |
| Withdrew | 18 (0.2) | 18 (0.2) | 0 (0) | .14 |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; LTFU, loss to follow up.
Given the differences observed in the use of NRTI backbone at baseline, we further analyzed CD4 response by thymidine-analogue-based NRTI backbone (AZT and 3TC) versus others (TDF and ABC). Median CD4+ counts (IQR) were similar at months 6 (268 vs 276, P = .38), 12 (302 vs 303, P = .92), and 18 (344 vs 336, P = .36) but differed at 24 months (370 vs 356, 0.003) for thymidine-analogue NRTI backbone versus others.
The proportion of patients achieving virologic suppression was significantly higher for older patients at months 6 (73.9% vs 70.2%, P = .04) and 24 (74.8% vs 69.6%, P = .005) than for younger patients (Table 2). When we assessed the proportions of all patients who sustained viral suppression from month 6 of cART to month 24 (FS), there was no significant difference between older and younger patients (15.7 vs 17.0, P = .41). However, in an adjusted logistic regression model, female patients (odds ratio [OR] = 1.24; 95% confidence interval [CI], 1.09–1.40) and those with medication adherence >95% (OR = 1.38; 95% CI, 1.22–1.56) were more likely to be FS compared with their relevant counterparts. Patients with low baseline body mass index ([BMI] OR, 0.72; 95% CI, 0.60–0.87), WHO stages III/IV disease (OR, 0.74; 95% CI 0.65–0.84), or those lacking formal education (OR, 0.83; 95% CI, 0.60–0.87) were less likely than their respective comparators to be FS from 6 through 24 months.
Loss to follow-up rates were similar for the younger versus older age groups (0.59 vs 0.60 per 1000 person years [PY], P = .88). The probability of LTFU between the 2 groups were different, with younger patients having a higher likelihood of LTFU (log rank P = .04). In a Cox proportional hazard regression model adjusting for baseline demographic, clinical and treatment characteristics (Table 3), older patients (aHR = 0.74; 95% CI, 0.58–0.93), patients who were FS (aHR = 0.50; 95% CI, 0.41–0.59), and those with adherence >95% (aHR, 0.62; 95% CI, 0.52–0.64) had a lower hazard of LTFU than their respective comparator groups. The independent predictors of LTFU were male sex (aHR = 1.20; 95% CI, 1.06–1.36), lack of formal education (aHR = 1.94; 95% CI, 1.66–2.26), WHO stage III/IV at baseline (aHR = 1.43; 95% CI, 1.26–1.63), BMI <18.5 kg/m2 at baseline (aHR, 1.23; 95% CI, 1.07–1.42), and experiencing a gap in treatment (aHR = 1.90, 95% CI, 1.66–2.18).
Table 3.
Association of Age Group With Risk of LTFU and Death Among (Older vs Younger) HIV-Infected Nigerians Initiating First-Line ART
| LTFU | Died | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Characteristic | AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | AHR (95% CI) |
| Age group, | |||||
| years | Ref | Ref | Ref | Ref | Ref |
| Young (15–49) Older (≥50) |
0.74 (0.58–0.93)a | 1.38 (0.70–2.71) | 1.32 (0.70–2.46) | 0.74 (0.47–1.16) | 0.67 (0.48–0.95)a |
| Sex, Male | 1.20 (1.06–1.36)a | 1.92 (1.24–2.98)a | 1.12 (0.76–1.64) | 1.50 (1.19–1.90)b | 1.26 (1.06–1.49)a |
| No formal education | 1.94 (1.66–2.26)b | 1.07 (0.48–2.41) | 1.74 (1.06–2.85)a | 1.95 (1.44–2.64)b | 1.86 (1.50–2.32)b |
| WHO stage III/IV | 1.43 (1.26–1.63)b | 2.70 (1.67–4.36)b | 1.05 (0.68–1.61) | 1.36 (1.05–1.75)a | 1.33 (1.10–1.59)a |
| BMI <18.5 kg/m2 | 1.23 (1.07–1.42)a | 1.58 (0.94–2.65) | 1.82 (1.21–2.73)a | 1.13 (0.86–1.50)a | 1.01 (0.82–1.24)a |
| HBV coinfection | 1.11 (0.96–1.28) | 1.19 (0.68–2.10) | 1.57 (1.02–2.42)a | 1.56 (1.19–2.04) | 1.39 (1.14–1.70) |
| HCV coinfected | 1.04 (0.89–1.21) | - | - | - | - |
| Fully suppressed | 0.52 (0.43–0.64)b | 0.14 (0.03–0.61)a | 0.62 (0.33–1.13) | 0.54 (0.36–0.80)a | 0.77 (0.66–0.91)a |
| Adherence >95% | 0.68 (0.60–0.76)b | 1.00 (0.64–1.55) | 0.65 (0.44–0.94)a | 0.63 (0.50–0.80)b | 0.77 (0.66–0.91)a |
| Any gap in care | 1.90 (1.66–2.18)b | 3.69 (2.35–5.80)b | 2.12 (1.39–3.22)b | 1.92 (1.47–2.50)b | 1.83 (1.51–2.22)b |
Abbreviations: AHR, adjusted hazard ratio; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LTFU, loss to follow-up; Ref, reference; WHO, World Health Organization.
NOTES: Model 1, complete case analysis; Model 2, 10% LTFU imputed as dead; Model 3, 25% LTFU imputed as dead; Model 4, 50% LTFU imputed as dead.
a P < .05.
b P < .001.
Mortality rates were similar for older and younger patients. Of 83 documented deaths within the follow-up period, 73 were aged 15–49 years, whereas 10 were aged ≥50 years, with 7 (0.1%) dying early (within the 90 days of initiating cART). The mortality rate was 0.05 vs 0.03 per 1000PY for older and younger patients, respectively (P = .10). Survival rates among those aged ≥50 years were also similar to those aged 15–49 years (log rank P = .06; Supplementary Figure). After controlling for socio-demographic (sex, marital status, level of education), clinical (WHO stage, BMI), biologic (HBV and HCV coinfection status, full viral suppression), and behavioral (medication adherence and gaps in treatment) characteristics (Table 3), male sex (aHR, 1.92; 95% CI, 1.24–2.98), WHO stages III/IV at baseline (aHR, 2.70; 95% CI, 1.67–4.36), and having gaps in treatment (aHR, 3.69; 95% CI, 2.35–5.80) were the only significant predictors of mortality. However, being fully virologically suppressed reduced the risk of death, with an aHR of 0.14 (95% CI, 0.03–0.61).
After imputation of mortality data, 3 additional Cox proportional hazard models were generated to control for the effect of missing mortality data as shown in Table 3. Model 1, which is the complete case analysis, was unable to detect the predictive effect of additional factors such as the lack of formal education, hepatitis B coinfection, low BMI, and optimal medication adherence on mortality. Although the hazard estimates/function for mortality increased progressively from 1.5 (95% CI, 1.00–2.00) for younger and 2.3 (95% CI, 1.10–4.60) for older patients (P = .14) using Model 1 to 13.5 (95% CI, 12.4–14.7) for younger and 11.3 (95% CI, 8.30–15.2) for older patients using Model 4 (P = .52), the risk for mortality remains similar for both age groups (Supplementary Table).
DISCUSSION
In this large retrospective evaluation of HIV-infected patients in a high burden resource-limited treatment site, we compared immunologic, virologic, and clinical outcomes within the first 2 years of cART among different age groups. Older age was associated with reduced CD4+ cell recovery and improved virologic suppression. Differences in LTFU rates were seen when comparing young and older patients. Older age was not shown to impact the risk of death compared with younger age in our study, and this finding differs from previous reports from urban and rural African settings [17, 19, 21, 26, 27]. However, we note the low number of deaths documented in our cohort.
Data on the effect of age on immune response to cART in our study and from other SSA countries [17, 19, 20] is comparable to studies from high-income countries [11–13, 15, 28]. Immune recovery after cART tends to be slower in older patients, with some studies reporting a loss of this advantage with longer periods of follow up after an initial slower response [8]. Aging has been associated with decreased production of interleukin (IL)-2 and its receptors, where the lower responsiveness to IL-2 has been implicated as a mechanism for compromised T-cell function, leading to a shift in T-cell phenotype [29, 30]. It has also been reported that a reduced CD4+ response in elderly patients may be related to involution of the thymus gland, with decreased productivity. Increased age is further associated with diminished T-cell functionality, reduced memory T-cell populations, and fewer numbers of properly functioning CD8+ cytotoxic T cells [31, 32].
The goal of cART is sustained suppression of HIV replication. Our study, as well as other studies reporting results of virologic response, shows that older patients have higher rates of viral suppression in spite of higher initial viral burden compared with younger patients [8, 14, 15, 17, 33]. Older patients also tend to have shorter time to viral suppression, although our study did not address this outcome [8]. Better medication adherence rates have been reported to play a key role in older patients achieving higher viral suppression rates compared with younger patients, yet our analyses did not indicate a difference in overall adherence in older patients compared with younger adults [34]. Our study also showed that female patient’s and those with medication adherence rates >95% were more likely to be FS, with low rates of sustained virologic suppression occurring in both groups. Barriers to optimal adherence among younger patients in sub-Saharan Africa have included nondisclosure to loved ones, fear of stigmatization, substance abuse, complexity of drug regimens, and cost of healthcare services in countries that do not subsidize treatment. Some of these factors are dependent on the treatment setting, but they tend to overlap where they exist [35, 36]. Efforts to improve medication adherence with consequent increase in rates of viral suppression among younger patients (and subsequent community-level viral suppression) should be explored.
Overall, LTFU rates in this study were lower than those reported in other studies from Africa, Asia, and various resource-rich settings [37]. We found that the hazard of LTFU was lower for those aged ≥50 years compared with patients aged 15–49 years. Our findings about LTFU in young compared with older adults corroborate findings from a study conducted using data from 7 African countries [21] and a recent multisite study in Nigeria [38]. The higher risk for LTFU among younger, compared with older, patients increases their risk for death and other adverse outcomes, including their likelihood of transmitting HIV to seronegative sex partners. Effective interventions to reduce LTFU for youth could help reduce losses and lower HIV incidence in this age group.
Amongst documented deaths, mortality was low for our cohort and older age was not found to be a risk factor for mortality. This finding is consistent with reports from Uganda [39] and Malawi [18] as well as in the United States [14]. Several other studies have reported contrary findings regarding age and mortality in settings similar to ours [17, 19, 21] as well as in resource-rich settings [11, 16]. Studies reporting higher rates of mortality among older patients have attributed this association to delayed presentation, more rapid disease progression, as well as higher incidence of cART-related adverse reactions. We must note that a major limitation in our mortality analyses is that mortality data are collected in a passive nature and, therefore, mortality is underreported.
This study has some notable strengths. We used routine program data from a large urban HIV clinic that provided care to a wide variety of patients from different localities. Cohort studies have the advantage of providing longer follow-up periods for a large number of patients in real-life scenario, with greater generalizability to target populations and are, therefore, appropriate for studying long-term responses of treatment to chronic conditions such as HIV. Our results are likely generalizable in settings similar to ours. The large sample size and sociodemographic characteristics at baseline reflect the profile of patients who enroll for care in most sites in sub-Saharan Africa. We also note some limitations in the fact that we were somewhat disadvantaged by our inability to fully ascertain the true reasons for those lost to follow-up, and so it is possible that we overestimated LTFU and underestimated mortality. We used imputation [39] to mitigate this limitation. Our diagnosis of hepatitis B and C status was based on presence of surface antigen and anti-HCV antibodies only, because the program did not have routine access to confirmatory tests. We were also not able to assess the impact of other significant variables such as substance abuse, smoking, and alcohol use on our outcomes of interest due to lack of data. Finally, because this study was a retrospective analysis, we could not attribute causality to our outcomes.
CONCLUSIONS
In conclusion, clinical management of older persons with HIV is an increasingly important issue because of the large impact of cART on improved survival. Approaches to improve the outcomes of older HIV patients in SSA could include interventions to optimize CD4+ cell gains on cART. Additional investigation in resource-constrained settings is needed to optimize the care of this emerging group in light of the presence of other comorbidities. With the increased availability and use of cART in resource-constrained areas, the number of older persons with HIV who are on cART will continue to increase. Increased knowledge of the clinical issues that affect older persons with HIV is urgently needed in developing countries.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We are grateful to the patients whose data were used in this study. We are also grateful to the clinical and support staff of the Harvard/AIDS Prevention Initiative in Nigeria (APIN) President’s Emergency Plan for AIDS Relief (PEPFAR) program at the Jos University Teaching Hospital.
Author contributions. P. A. A., P. J. K., and S. T. M. conceptualized the study. P. J. K., J. A. I., and H. M. S. reviewed initial draft for content. P. A. A., O. O. A., and H. M. S. collected data. P. A. A., P. O., S. T. M., and H. M. S. finalized data analysis and concluded the complete draft. All authors reviewed, edited, and approved the final version of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not represent the official views of the funders. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Financial support. Patient care was funded in part by the US Department of Health and Human Services, Health Resources and Services Administration (U51HA02522) and the Centers for Disease Control and Prevention through a cooperative agreement with APIN Lte (PS 001058).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. HIV and aging: a special supplement to the UNAIDS report on the global AIDS epidemic. Geneva: Joint UN Programme on HIV/AIDS Available at: http://www.unaids.org/sites/default/files/media_asset/20131101_JC2563_hiv-and-aging_en_0.pdf Accessed 20 April 2015.
- 2. UNAIDS report on the global AIDS epidemic Available at: http://www.unaids.org/en/resources/campaigns/globalreport2013 Accessed 20 April 2015.
- 3. Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS 2014; 28(Suppl 4):S453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Brien D, Spelman T, Greig J, et al. Risk factors for mortality during antiretroviral therapy in older populations in resource-limited settings. J Int AIDS Soc 2016; 19:20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013; 26:17–25. [DOI] [PubMed] [Google Scholar]
- 6. Balderson BH, Grothaus L, Harrison RG, et al. Chronic illness burden and quality of life in an aging HIV population. AIDS Care 2013; 25:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Negin J, Martiniuk A, Cumming RG, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS 2012; 26(Suppl 1):S55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med 2007; 167:684–91. [DOI] [PubMed] [Google Scholar]
- 9. Gebo KA. Epidemiology of HIV and response to antiretroviral therapy in the middle aged and elderly. Aging Health 2008; 4:615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abel T, Werner M. HIV risk behaviour of older persons. Eur J Public Health 2003; 13:350–2. [DOI] [PubMed] [Google Scholar]
- 11. Nogueras M, Navarro G, Antón E, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis 2006; 6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tumbarello M, Rabagliati R, de Gaetano Donati K, et al. Older age does not influence CD4 cell recovery in HIV-1 infected patients receiving highly active antiretroviral therapy. BMC Infect Dis 2004; 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grabar S, Kousignian I, Sobel A, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS 2004; 18:2029–38. [DOI] [PubMed] [Google Scholar]
- 14. Perez JL, Moore RD. Greater effect of highly active antiretroviral therapy on survival in people aged > or =50 years compared with younger people in an urban observational cohort. Clin Infect Dis 2003; 36:212–8. [DOI] [PubMed] [Google Scholar]
- 15. Ryan R, Dayaram YK, Schaible D, et al. Outcomes in older versus younger patients over 96 weeks in HIV-1-infected patients treated with rilpivirine or efavirenz in ECHO and THRIVE. Curr HIV Res 2013; 11:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenbaum AH, Wilson LE, Keruly JC, et al. Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. AIDS 2008; 22:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One 2011; 6:e21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Negin J, van Lettow M, Semba M, et al. Anti-retroviral treatment outcomes among older adults in Zomba district, Malawi. PLoS One 2011; 6:e26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinikoor MJ, Joseph J, Mwale J, et al. Age at antiretroviral therapy initiation predicts immune recovery, death, and loss to follow-up among HIV-infected adults in urban Zambia. AIDS Res Hum Retroviruses 2014; 30:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balestre E, Eholié SP, Lokossue A, et al. Effect of age on immunological response in the first year of antiretroviral therapy in HIV-1-infected adults in West Africa. AIDS 2012; 26:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004-2013. MMWR Morb Mortal Wkly Rep 2014; 63:1097–103. [PMC free article] [PubMed] [Google Scholar]
- 22. Chaplin B, Meloni S, Eisen G, et al. Scale-up of networked HIV treatment in Nigeria: creation of an integrated electronic medical records system. Int J Med Inform 2015; 84:58–68. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Departmemt of Health and Human Services, National Institutes of Health, National Institutes of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS Table for grading the severity of adult and pediatric adverse events, version 2.0. Available at: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed August 19, 2016 [Google Scholar]
- 24. Yiannoutsos CT, An MW, Frangakis CE, et al. Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in Western Kenya. PLoS One 2008; 3:e3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006; 367:817–24. [DOI] [PubMed] [Google Scholar]
- 26. Walker AS, Prendergast AJ, Mugyenyi P, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis 2012; 55:1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bakanda C, Birungi J, Mwesigwa R, et al. Association of aging and survival in a large HIV-infected cohort on antiretroviral therapy. AIDS 2011; 25:701–5. [DOI] [PubMed] [Google Scholar]
- 28. Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS 2002; 16:1859–66. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging 2008; 3:453–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wellons MF, Sanders L, Edwards LJ, et al. HIV infection: treatment outcomes in older and younger adults. J Am Geriatr Soc 2002; 50:603–7. [DOI] [PubMed] [Google Scholar]
- 31. Orlando G, Meraviglia P, Cordier L, et al. Antiretroviral treatment and age-related comorbidities in a cohort of older HIV-infected patients. HIV Med 2006; 7:549–57. [DOI] [PubMed] [Google Scholar]
- 32. Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr 2009; 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med 2006; 3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr 2003; 34:281–8. [DOI] [PubMed] [Google Scholar]
- 35. Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ 2008; 86:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mocroft A, Kirk O, Aldins P, et al. Loss to follow-up in an international, multicentre observational study. HIV Med 2008; 9:261–9. [DOI] [PubMed] [Google Scholar]
- 38. Meloni ST, Chang C, Chaplin B, et al. Time-dependent predictors of loss to follow-up in a large HIV treatment cohort in Nigeria. Open Forum Infect Dis 2014; 1:ofu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bakanda C, Birungi J, Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: findings from a nationally representative cohort in Uganda. PLoS One 2011; 6:e19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.