To the Editor:
The 2009 influenza season in temperate countries in the southern hemisphere has been well documented as moderately severe in terms of impact on healthcare systems. 1 , 2 , 3 However, experience with previous pandemics has demonstrated that in some cases, the second season of transmission can be worse than the initial one. 4 There has recently been evidence of this occurring in the UK where a large number of severe cases have been reported with the start of the 2011 influenza season. 5 As the second season has already come and gone in the temperate countries of the southern hemisphere, there is a unique opportunity to look back for evidence of changes in behavior or severity of the pandemic in two completed transmission seasons. Using data from FluNet 6 and ministries of health in Argentina, Chile, South Africa, Australia, and New Zealand, we compare influenza virus circulation, the time course, and geographic distribution of the peaks in activity; and the impact of influenza on healthcare systems during the 2010 winter compared to the 2009 pandemic season.
Virus circulation, time course, and geographic distribution
The H1N1 (2009) pandemic virus began to circulate in most temperate countries of the Southern Hemisphere near the start of their usual seasons of influenza virus transmission in 2009. 1 The H1N1pdm virus quickly became the predominant strain detected, even where other seasonal influenza viruses had already been detected that season. South Africa was the only temperate southern country where typical circulation of a seasonal virus was observed before the subsequent occurrence of an H1N1pdm wave in July–September (Figure 1). In the winter of 2010, a different and more diverse pattern emerged. In New Zealand and Australia, H1N1pdm was still predominant in 2010 representing approximately 67–84% (Figure 1A,B) of the viruses detected from epidemiologic week (EW) 1–39/40. 7 , 8 In contrast, very few H1N1 (2009) viruses were detected in Chile and Argentina in 2010. Seasonal H3N2 and influenza B (Chile) and influenza B strains (Argentina) were the most commonly detected influenza virus types identified in those two countries. 6 , 9 South Africa had three smaller waves of influenza B, seasonal H3N2, and H1N1pdm cocirculating simultaneously. 10 All of the H1N1pdm viruses tested in the five countries in 2010 are antigenically similar to the A/California/7/2009 strain. None of the previously circulating seasonal H1N1 viruses have been detected in any of the five countries since January of 2010. None of the >600 samples analyzed in the five countries during EW 1–33 2010 were resistant to oseltamivir, and only two isolates from Australia had the H274Y mutation. 9 , 10 , 11 , 12
Figure 1.
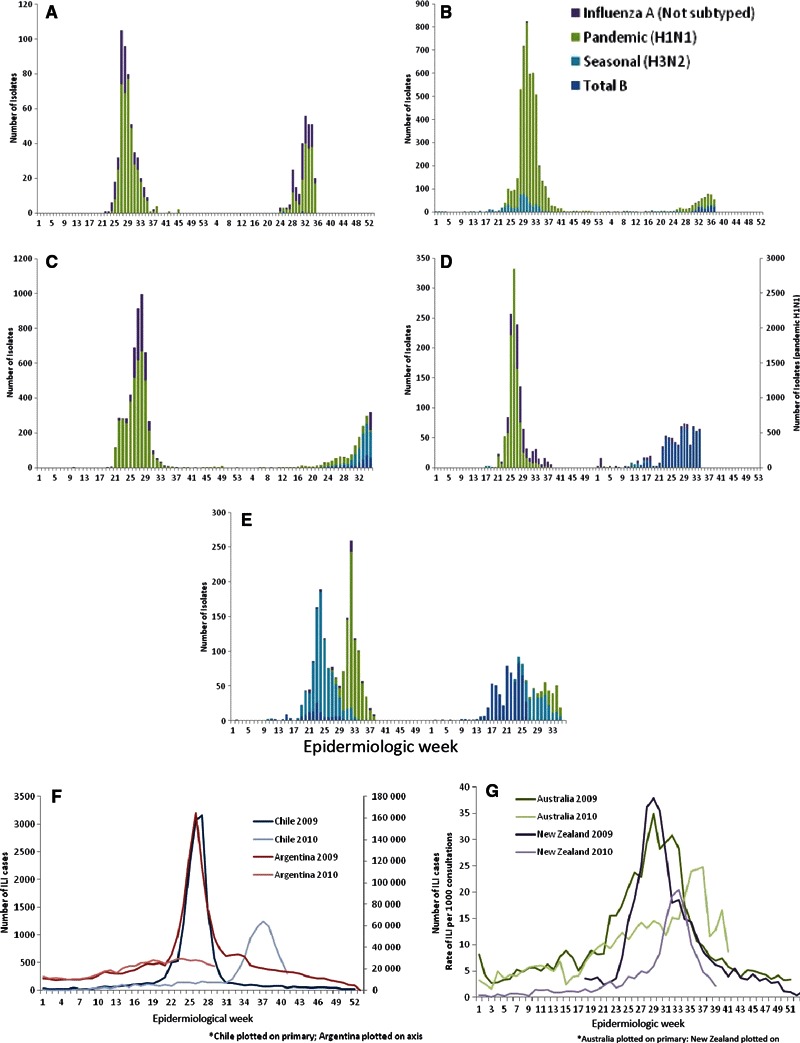
Influenza circulation during 2009 and 2010 in (A) New Zealand, (B) Australia, (C) Chile, (D) Argentina, (E) South Africa; and (F) number of reported ILI cases in Chile and Argentina and (G) ILI rates in Australia and New Zealand by epidemiologic week in 2009 and 2010.
Influenza seasons in temperate Southern Hemisphere countries typically occur during the winter months of June–August (approximately EW 23–36) each year. The peak activity during the 2009 influenza season, as measured by influenza‐like‐illness (ILI) reported cases per week (Chile; Figure 1F), influenza cases per week (Argentina; Figure 1F), or weekly ILI consultation rates (Australia, New Zealand; Figure 1G), occurred within this period. However, the peak of activity in 2010 occurred slightly later than the recent seasonal peaks in Chile (EW 33 9 ) and Australia (EW 38 7 ), and there was no apparent peak of activity in Argentina (Figure 1F). New Zealand’s 2010 peak in influenza activity was similar in timing to the 2008 peak [mid‐August (EW 33)] but somewhat later than the 2009 peak (Figure 1G). 11 , 13 The duration of influenza activity was shorter in New Zealand in 2010 with activity above baseline levels for 17 weeks in 2009 versus only 7 weeks in 2010. 14 South Africa followed a similar pattern in 2010 of a first H3N2 peak in EW 33, followed by a peak of H1N1pdm in EW 34–38. This was slightly later than the 2009 peak, which occurred in EW 31 (Figure 1E). 10
The geographic distribution of symptomatic influenza cases in all five countries was similar during the 2009 and 2010 seasons with uneven geographic distribution reported throughout each country over the course of the influenza season. 7 , 12 , 15 , 16 , 17 There was some evidence from New Zealand and Chile of regions (e.g., Waikato, Lakes, and Bay of Plenty and Bio Bio, Los Lagos, Los Rios, respectively), which reported lower numbers of cases in 2009 compared with 2010. 9 , 11 Serologic results from Australia and New Zealand indicate that infection levels during the first wave of the pandemic across five of Australia’s six states ranged from 18% to 31% 18 and, similarly, ranged from 19% to 30% in seven geographic locations in New Zealand. 19
Impact of 2009 versus 2010 influenza seasons
As of 8 October, <10 000 laboratory confirmed cases were reported in all five countries combined, 7 , 8 , 9 , 15 , 20 which is up to 500 times lower than the reported number of cases reported in each country in 2009. Similarly, reported H1N1pdm deaths have been up to 600 times fewer across the five countries than reported in 2009. 7 , 8 , 9 , 12 , 21 Overall, the impact of influenza, especially H1N1pdm, in terms of severity of illness, mortality, and impact on healthcare systems as reported by numbers of cases and deaths of H1N1pdm, and ILI and severe acute respiratory illness (SARI) activity in all five countries, was notably lower in the 2010 season compared with the 2009 season.
Rates of hospitalization and intensive care unit (ICU) admissions because of H1N1pdm, as well as ILI and SARI rates in all five countries, were >2 times higher in 2009 compared with 2010. For example, hospitalization rates in New Zealand in 2010 have been about half those in 2009 (13·8/100 000 11 versus 26·1/100 000, 1 respectively), whereas ICU admissions during 2010 were approximately 75% of that in 2009. In Chile, there have been 1·5/100 000 confirmed cases of H1N1pdm SARI reported up to EW 36 2010 in Chile 9 as compared with 9·5/100 000 in 2009. 16 The proportion of confirmed H1N1pdm cases admitted to hospital was lower in Australia and higher in New Zealand in 2010 compared with 2009, 1 , 14 , 22 whereas the death to hospitalization ratio was higher in Australia (0·134 versus 0·045) and lower in New Zealand (0·02 versus 0·042) in 2010 compared with 2009. 1 , 14 , 22 , 23
Risk groups for H1N1pdm
Age and underlying chronic conditions continue to be significant risk factors for severe H1N1pdm infection. The highest rates of H1N1pdm cases in New Zealand in 2010 were seen among <10‐ and 20‐ to 29‐year‐olds, and for hospitalized patients, among <5 and 20–29 year olds, whereas most deaths occurred in the 50–59 year old age group. 11 In South Africa, the median age of confirmed and fatal cases in 2009 was 15·5 and 33·5 years old, respectively. 17 In 2010, the highest proportion of confirmed H1N1pdm cases from ILI surveillance was in the 10–19 years old age group, followed by 20–29, and the highest proportion of SARI H1N1pdm cases in 2010 was reported in the ≥45 year old age group. 10 There have been no deaths reported in South Africa in 2010. In Chile in 2009, the highest rates of severe illness seen in children <1 and 1–4 years old (76·1/100 000, 20·5/100 000 respectively) followed by those 50–59 and >60 years old (13·2/100 000, 12·4/100 000 respectively). 16 The highest rates of SARI because of H1N1pdm in Chile in 2010 have been reported in <1 (15·9/100 000 and 1–4 years old (3·0/100 000). 9 The number of cases because of H1N1 (2009) in Argentina in 2010 is too small to make meaningful comparisons.
Among H1N1pdm confirmed cases, the proportions who were hospitalized, admitted to ICU, or died may have increased in 2010; however, data are not available for comparison from all five countries. In Australia, for example, 67%, 79%, and 100% of all hospitalized, ICU‐admitted, and fatal H1N1pdm cases in 2010 had at least one comorbidity (specific conditions nor provided), 12 compared with 49%, 70%, 64%, respectively in 2009. 24 In Chile, approximately 71% (of 150 deaths with information available) reported in 2009 had an underlying chronic medical condition, 16 compared with 87·5% of 12 deaths occurring thru EW 36 in 2010. 9 Australian and New Zealand Intensive Care Society (ANZICS) has reported 85 ICU admissions among H1N1pdm confirmed patients in 2010, 80·0% of which had known comorbidities. 12 In South Africa in 2009, 21% of deaths for which data were available (76/91 deaths) had no comorbidities identified. 17 No deaths have been reported in South Africa in 2010.
Ethnic minority groups in New Zealand were again noted to have high rates of severe illness during the 2010 season, as in 2009 season, with the rates of cumulative H1N1pdm cases hospitalized in Maori and Pacific populations more than double that of European populations. 11 However, indigenous people in Australia made up only 4% and 2% of hospitalized and ICU‐admitted H1N1pdm patients, respectively, between 1 March and 17 September 2010, 14 whereas 21% and 20% of hospitalized and ICU‐admitted H1N1pdm patients, respectively, in 2009 were among indigenous people. 24
Conclusions
The temperate countries of the Southern Hemisphere offered a unique opportunity to observe and measure the transmission and impact of the H1N1 (2009) pandemic virus in an area where it was first introduced at the start of the usual winter period of transmission. While observations comparing seasons and data between countries were complicated by the reliance on routinely collected surveillance data that are often influenced by public concern, changing methods of monitoring in response to the situation, and the non‐standardization of data collection between countries, some conclusions can, nonetheless, be drawn from such comparisons. Virologically, the 2009 season was characterized by a predominance of one strain of virus, the H1N1pdm virus, which quickly spread nationally and appeared to displace other influenza viruses that were starting to appear around the same time. The previously circulating seasonal influenza A (H1N1) viruses appear to have subsequently completely disappeared from circulation. In August 2010, the Director General of the World Health Organization declared an end to the pandemic, implying that the pandemic virus was now displaying behavior characteristic of a seasonal virus. Indeed, observations during the 2010 Southern Hemisphere winter have shown that H1N1pdm was no longer completely dominant, and cocirculation of multiple virus types [notably seasonal A (H3N2) and influenza B] was observed in different areas of the Southern Hemisphere.
The pandemic of 2009 in the Southern Hemisphere, as in the rest of the world, was also characterized by a high attack rate, especially in young people. Individuals with chronic illnesses, pregnancy, at the extremes of age, or belonging to certain disadvantaged populations were particularly susceptible to developing severe complications, a pattern that continued into the second winter season. In contrast to 2009, however, the overall attack rate and impact in terms of severe illness was considerably less in 2010. This is not surprising given the high rates of infection in 2009, relatively high vaccination coverage in many of the areas under observation (indeed, immunity is expected to have developed in most individuals undergoing vaccination), and the fact that the virus has not perceptibly changed antigenically. It has been noted by some observers that in New Zealand, a number of areas with lower attack rates in 2009 were more severely affected in 2010 than they had been in the previous year (M. Jacobs, personal communication).
It is important to note that high‐risk individuals continue to be at increased risk of severe disease and death with all types of influenza virus, including H1N1 (2009), even with the low rates of transmission. WHO continues to recommend vaccination for high‐risk individuals where vaccination is available and notes that the lack of antigenic drift in the virus over the last year implies that the match between the H1N1 strain in the current seasonal influenza vaccine and the circulating strain is a good one. 25
Our findings demonstrate the characteristic unpredictability of flu, notably the wide variations in experience around the globe. WHO also advises countries to continue to develop effective surveillance, in compliance with International Health Regulations 2005, and further encourages the open and transparent international sharing of surveillance data to improve global understanding of influenza and other respiratory disease transmission.
Acknowledgements
The authors would like to recognize the hard work of all the individuals, including healthcare workers, Municipal Health Centres, hospitals, virology laboratories and reference laboratories, and the Ministries of Health, who are responsible for providing data to the ministries of health so that they may generate informative web‐based influenza weekly reports. The authors would also like to thank Angus Nicoll for reviewing and commenting on the manuscript and the Medical Research Council UK and Bill and Melinda Gates Foundation for funding (MVK).
© 2011 Blackwell Publishing Ltd. The World Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication.
References
- 1. Van Kerkhove MD, Mounts AW, Mall S et al. Epidemiologic and virologic assessment of the 2009 influenza A (H1N1) pandemic on selected temperate countries in the southern hemisphere: Argentina, Australia, Chile, New Zealand and South Africa. 2010. (in process). [DOI] [PMC free article] [PubMed]
- 2. Baker M, Kelly H, Wilson N. Pandemic H1N1 influenza lessons from the southern hemisphere. Euro Surveill 2009; 14:pii: 19370. [DOI] [PubMed] [Google Scholar]
- 3. Falagas ME, Koletsi PK, Baskouta E, Rafailidis PI, Dimopoulos G, Karageorgopoulos DE. Pandemic A(H1N1) 2009 influenza: review of the Southern Hemisphere experience. Epidemiol Infect 2011; 139:27–40. [DOI] [PubMed] [Google Scholar]
- 4. Viboud C, Grais RF, Lafont BAP, Miller M, Simonsen L, for the Multinational Influenza Seasonal Mortality Study Group . Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248. [DOI] [PubMed] [Google Scholar]
- 5. UK Health Protection Agency . HPA National Influenza Report. Available at http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1287147913271 (Accessed 4 February 2011).
- 6. WHO . FLUNET the reporting tool of the Global Influenza Surveillance Network. Available at: http://gamapserver.who.int/GlobalAtlas/home.asp (Accessed 13 October 2010).
- 7. Australian Influenza Surveillance Summary Report: No. 40, 2010, Reporting Period: 2 October–8 October 2010. Available at http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/ozflu2010‐oct‐dec‐pdf‐cnt.htm/$File/ozflu‐no40‐2010.pdf (Accessed 18 October 2010).
- 8. Public Health Surveillance Information for New Zealand Public Health Action . Influenza Weekly Update 2010/39: 27 September–3 October 2010. Available at http://www.surv.esr.cri.nz/PDF_surveillance/Virology/FluWeekRpt/2010/FluWeekRpt201039.pdf (Accessed 13 October 2010).
- 9. Ministerio de Salud Chile . Influenza Pandemica (H1N1) 2010, Ministerio de Salud de Chile. Informe de Influenza Semana Epidemiológica 36 (5 al 11 de septiembre 2010). Available at http://www.minsal.cl/ (Accessed 1 October 2010).
- 10. NICD . The South African 2010 Influenza Season for AIVC. 2010.
- 11. Huang QS, Bandaranayake D. For the WHO Consultation on the Composition of Seasonal Influenza Vaccines for the Southern Hemisphere 2011 and on Vaccine Viruses for Pandemic Preparedness and Response & For the Australian Influenza Vaccine Committee Meeting on the Composition of Seasonal Influenza Vaccines for Australia New Zealand and South Africa for 2011. The New Zealand 2010 Influenza Data, 2010.
- 12. Australian Influenza Surveillance Summary Report: No. 37 2010, Reporting period: 11 September–17 September 2010. Available at http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/ozflu2010‐jul‐sep‐pdf‐cnt.htm/$File/ozflu‐no37‐2010.pdf (Accessed 1 October 2010).
- 13. Public Health Surveillance Information for New Zealand Public Health Action . Influenza Weekly Update 2010/37: 13–19 September 2010. Available at http://www.surv.esr.cri.nz/virology/influenza_weekly_update.php (Accessed 23 September 2010).
- 14. Australian Influenza Surveillance Report No. 36, 2010. Reporting Period: 4 September–10 September 2010. Available at http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/ozflu2010‐jul‐sep‐pdf‐cnt.htm/$File/ozflu‐no36‐2010.pdf (Accessed 23 September 2010).
- 15. NICD . Consolidated Influenza Surveillance Weekly Reports. Available at http://www.nicd.ac.za/ (Accessed 5 October 2010).
- 16. Ministerio de Salud Chile . 2009 Final report on pandemic influenza (H1N1) in January 2010, 13 January 2010. Available at http://www.pandemia.cl/templates/pandemia/documentos/Informe_13_enero.pdf (Accessed 7 October 2010).
- 17. Archer B, Cohen C, Naidoo D et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill 2009; 14:pii: 19369. [DOI] [PubMed] [Google Scholar]
- 18. McVernon J, Laurie K, Nolan T et al. Seroprevalence of 2009 pandemic influenza A(H1N1) virus in Australian blood donors, October–December 2009. Euro Surveill 2010; 15:19678. [DOI] [PubMed] [Google Scholar]
- 19. Bandaranayake D, Huang QS, Bissielo A, Wood T. Seroprevalence of the 2009 influenza A (H1N1) pandemic in New Zealand; in ESR (ed.): Available at http://www.moh.govt.nz/moh.nsf/indexmh/seroprevalence‐2009‐flu‐nz (Accessed 7 October 2010).
- 20. Ministerio de Salud Argentina . Informe Semanal De Vigilancia de Infecciones Respiratorias Agudas en Argentina, Fecha Infrome. Available at http://www.msal.gov.ar (Accessed 26 August 2010).
- 21. New Zealand Ministry of Health . Media Release: Pandemic Influenza H1N1 2009 (swine flu) – Update 210. Available at http://www.moh.govt.nz/moh.nsf/indexmh/influenza‐a‐h1n1‐update‐210‐300910 (Accessed 30 September 2010).
- 22. New Zealand Ministry of Health . Media Release: Pandemic Influenza H1N1 2009 (swine flu) – Update 211. Available at http://www.moh.govt.nz/moh.nsf/indexmh/influenza‐a‐h1n1‐update‐211‐071010 (Accessed 7 October 2010).
- 23. New Zealand Ministry of Health . Report for the Minister of Health from the Pandemic Influenza Mortality and Morbidity Review Group. Wellington. Available at http://www.pmmrc.health.govt.nz/moh.nsf/pagescm/7745/$File/report‐pandemix‐influenza‐mortality‐morbidity‐review‐group.pdf (Accessed 15 October 2010).
- 24. Australian Influenza Surveillance Summary Report: No. 33, 2009, Reporting Period: 19 December 2009–1 January 2010. Available at http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/18D06BAC4644C98DCA25763E00823442/$File/ozflu‐no33‐2009.pdf; http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/EB136394E79CA5E2CA2576A50010783A/File/ozflu‐no8‐2010.pdf (Accessed 7 October 2010).
- 25. World Health Organization . WHO informal consultation for improving influenza vaccine virus selection. Available at http://www.who.int/csr/disease/influenza/influenzanetwork/virusselectionmeeting2010/en/ (Accessed 13 October 2010).


