Abstract
Please cite this paper as: Ahn et al. (2011) Role of procalcitonin and C‐reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza and Other Respiratory Viruses 5(6), 398–403.
Background Mixed bacterial infection is an important contributor to morbidity and mortality during influenza pandemics. We evaluated procalcitonin (PCT) and C‐reactive protein (CRP) in differentiating pneumonia caused by mixed bacterial and 2009 H1N1 influenza infection from 2009 H1N1 influenza infection alone.
Methods Data were collected retrospectively over a 7‐month period during the 2009 H1N1 influenza pandemic. Patients visiting emergency department and diagnosed as community‐acquired pneumonia caused by 2009 H1N1 infection were included (n = 60).
Results Mixed bacterial and viral infection pneumonia (n = 16) had significantly higher PCT and CRP levels than pneumonia caused by 2009 H1N1 influenza alone (n = 44, P = 0·019, 0·022 respectively). The sensitivity and specificity for detection of mixed bacterial infection pneumonia was 56% and 84% for PCT > 1·5 ng/ml, and 69% and 63% for CRP > 10 mg/dl. Using PCT and CRP in combination, the sensitivity and specificity were 50% and 93%, respectively.
Conclusion Procalcitonin and CRP alone and their combination had a moderate ability to detect pneumonia of mixed bacterial infection during the 2009 H1N1 pandemic. Considering high specificity, combination of low CRP and PCT result may suggest that pneumonia is unlikely to be caused by mixed bacterial infection.
Keywords: C‐reactive protein, H1N1 subtype, influenza, pneumonia, procalcitonin
Introduction
Since the first report of 2009 H1N1 influenza infection in Mexico, 1 the 2009 H1N1 infection rapidly spread around the world, leading to a pandemic. According to the May 2010 World Health Organization report, H1N1 infections occurred in more than 214 countries which brought about more than 18 000 deaths. 2 Pneumonia, mixed bacterial infection, and aggravation of underlying conditions such as heart failure are well‐known complications of influenza. 3 , 4 , 5 , 6 Among these complications, mixed bacterial infection is especially known to increase the mortality and morbidity of influenza. 7 , 8 As pandemic influenza strains generally cause self‐limiting illnesses, it is crucial to accurately diagnose concurrent mixed bacterial infections.
Ideal inflammatory biomarkers require accurate discriminatory effects between infectious and non‐infectious disease, ability to aid in early detection, and easy application. Classic inflammatory mediators, including tumor necrosis factor (TNF)‐α, interleukin (IL)‐6, and C‐reactive protein (CRP), and more recently developed markers such as procalcitonin (PCT), are widely used in the diagnosis of infectious and inflammatory diseases. PCT levels are known to be higher in bacterial, fungal, and parasitic infections than in viral infections, 9 and this has led to PCT being used as a guide to antibiotic treatment in community‐acquired pneumonia and acute exacerbations of chronic obstructive pulmonary disease. 10 , 11
Several studies regarding the use of inflammatory markers to predict mixed bacterial infection of 2009 H1N1 pneumonia exist, 12 , 13 , 14 but the results are not consistent, especially for CRP, and studies in emergency departments (EDs) are lacking. The aim of the present study was to evaluate the role of two serum inflammatory markers, alone and in combination, in discriminating pneumonia caused by mixed bacterial and 2009 H1N1 influenza infection from pneumonia caused by the 2009 H1N1 influenza infection alone.
Materials and methods
Study design
This was a retrospective study of adult patients, 18 years and older, who visited the emergency department of a tertiary‐care hospital in Seoul, Korea, during the 2009 H1N1 pandemic. Patients were eligible if they were diagnosed as having community‐acquired pneumonia caused by 2009 H1N1 influenza infection between August 2009 and February 2010. Patients transferred from other institutions with prior antibiotic administration were excluded from this study. Upon arrival to the ED, laboratory exams including serum PCT and CRP, blood and sputum cultures, and Gram stains and chest X‐rays were performed. The 2009 H1N1 influenza infection was confirmed by real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) on nasopharyngeal swabs. 15 Pneumonia severity index (PSI) was calculated for risk stratification. Serum PCT was measured by the VIDAS BRAHMS enzyme‐linked fluorescence assay (measurement range 0·05–200 ng/ml; bioMerieux, Lyon, France), and CRP was measured with an automated multichannel analyzer (model TBA‐30FR; Toshiba, Saitama, Japan). Treatment outcomes were classified into patients whose conditions improved to discharge and those who died following admission.
Definition
Community‐acquired pneumonia was diagnosed when the patient had respiratory symptoms with lung infiltration on chest X‐ray and rales on auscultation. 10 Patients with pneumonia and positive for 2009 H1N1 influenza PCR were diagnosed as having 2009 H1N1 pneumonia. Patients with pneumonia who were both positive for 2009 H1N1 influenza PCR and had detectable bacterial pathogens were defined as having pneumonia caused by mixed infection. The presence of bacterial pathogens was confirmed by positive Gram staining in respiratory samples, a pathogen concentration >105 colony‐forming units/ml in tracheobronchial aspirates or a blood culture revealing a bacterial pathogen in the absence of an extrapulmonary focus. 16 Exams for atypical pathogens were performed by serum enzyme immunoassay for mycoplasma antibodies, cold agglutinin test, and legionella urine antigen test. Organisms detected in sputum were considered pathogenic when sputum was qualified with <10 epithelial cells and more than 25 leukocytes per low power field. 10
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and as absolute or relative frequencies for categorical variables. Univariate analyses using contingency tables and basic descriptive statistics were performed. Statistical analysis was carried out using the chi‐squared test for nominal data, Mann–Whitney test for the medians of non‐parametric data, and Student’s t‐test for parametric data. To demarcate the PCT and CRP cutoff values in distinguishing mixed infection pneumonia from 2009 H1N1 viral pneumonia, receiver‐operating characteristic (ROC) curve analysis was carried out. All statistical analyses were performed with spss for Windows 11.0.1 (SPSS Inc, Chicago, IL, USA). All reported P‐values are two‐tailed, and P‐values <0·05 were considered statistically significant.
Results
Baseline characteristics of total patients
During the study period, a total of 96 patients were diagnosed as having community‐acquired pneumonia, and 60 of them were positive for the 2009 H1N1 influenza virus. Of these 60 patients, 44 had pneumonia caused by 2009 H1N1 infection alone and 16 had pneumonia caused by mixed bacterial infection. The most common bacterial organisms in mixed bacterial infection pneumonia were Streptococcus pneumoniae, followed by Staphylococcus aureus and Pseudomonas aeruginosa (Table 1). The numbers of specimen revealing causative organisms were 10 in blood cultures, five in sputum cultures, and four in urine samples of pneumococcal antigens. The mean age of the 60 patients was 49·4 ± 18·9 years, and 33 (55·0%) were men. Ten (16·7%) patients died following admission. The mean value of initial PCT concentration was 4·3 ± 11·6 ng/ml, and the CRP was 10·6 ± 9·9 mg/dl. Initial serum white blood cell (WBC) count was 9·2 ± 5·1 (×103/mm3).
Table 1.
Bacterial pathogens of mixed infection pneumonia
| Pathogen | Number |
|---|---|
| Streptococcus pneumoniae | 6 |
| Staphylococcus aureus | 4 |
| Pseudomonas aeruginosa | 3 |
| Haemophilus influenzae | 2 |
| Klebsiella pneumoniae | 1 |
Comparison between 2009 H1N1 pneumonia and mixed infection
Baseline characteristics, laboratory findings, and clinical presentations of 2009 H1N1 pneumonia and pneumonia with mixed bacterial infection were compared. Patients with 2009 H1N1 pneumonia were younger but this was not statistically significant. No difference in gender was found between the two groups. Vital signs and radiologic findings were not significantly different. The median value for PCT was statistically higher in patients with pneumonia caused by mixed bacterial infection than in patients with 2009 H1N1 pneumonia (3·45 versus 0·15 ng/ml, P = 0·019). The median value for CRP was also statistically higher in the mixed infection group than in the H1N1 infection group (14·8 versus 4·6 mg/dl, P = 0·022) (Table 2). Other laboratory findings were similar in the two groups. A significantly higher proportion of patients in the 2009 H1N1 pneumonia group (77·3%) exhibited cough compared to the mixed infection group (43·8%, P = 0·014), but other clinical presentations did not differ significantly. The mortality rate was higher in the mixed infection group than the H1N1 influenza group (25·0% versus 13·6%), but this difference was not statistically significant.
Table 2.
Comparison of characteristics and parameters between the 2009 H1N1 pneumonia and mixed bacterial and 2009 H1N1 influenza infection
| 2009 H1N1 (n = 44) | Mixed infection (n = 16) | P‐value | |
|---|---|---|---|
| Age (years) (mean ± SD) | 47·2 ± 20·0 | 55·5 ± 14·5 | 0·070 |
| Male, n (%) | 24 (54·5) | 9 (56·3) | 0·907 |
| Underlying disease, n (%) | 22 (64·7) | 48 (77·4) | 0·180 |
| Hypertension | 9 (20·5) | 3 (18·8) | 0·884 |
| Diabetes | 13 (29·5) | 8 (50·0) | 0·142 |
| Chronic lung disease | 20 (45·5) | 10 (62·5) | 0·243 |
| Malignancy | 16 (36·4) | 9 (56·3) | 0·167 |
| Clinical presentations, n (%) | |||
| Sore throat | 13 (29·5) | 7 (43·8) | 0·302 |
| Rhinorrhea | 24 (54·5) | 9 (56·3) | 0·907 |
| Headache | 20 (45·5) | 7 (43·8) | 0·907 |
| Cough | 34 (77·3) | 7 (43·8) | 0·014* |
| Myalgia | 17 (38·6) | 9 (56·3) | 0·223 |
| Nausea/vomiting | 17 (38·6) | 7 (43·8) | 0·721 |
| Diarrhea | 14 (31·8) | 9 (56·3) | 0·085 |
| Vital signs (mean ± SD) | |||
| SBP (mmHg) | 121·4 ± 18·1 | 120·5 ± 23·2 | 0·785 |
| DBP (mmHg) | 71·8 ± 14·8 | 71·6 ± 14·5 | 0·621 |
| RR (/min) | 23·8 ± 6·2 | 22·8 ± 7·2 | 0·776 |
| PR (/min) | 114·2 ± 24·0 | 105·8 ± 22·5 | 0·092 |
| BT (°C) | 38·0 ± 1·0 | 38·2 ± 1·1 | 0·321 |
| SpO2 (%) | 90·7 ± 13·8 | 91·3 ± 9·1 | 0·935 |
| Initial laboratory findings (Median, range) | |||
| WBC (×103/mm3) | 8·9 (0·8–22·0) | 8·6 (0·9–19·2) | 0·616 |
| ANC (cells/mm3) | 6160 (0–18410) | 6295 (558–17310) | 0·786 |
| Lymphocyte (%) | 13·5 (1·2–97·6) | 10·9 (2·2–46·8) | 0·927 |
| Platelet (×103/mm3) | 184·0 (11–402) | 162·0 (8–285) | 0·256 |
| Procalcitonin (ng/ml) | 0·15 (0·05–44·4) | 3·45 (0·05–65·1) | 0·019* |
| C‐reactive protein (mg/dl) | 4·6 (0·13–43·0) | 14·8 (1·21–34·6) | 0·022* |
| PaO2 (mmHg) | 69·5 (56·8–80·0) | 63·5 (51·5–85·0) | 0·703 |
| pH | 7·5 (7·4–7·5) | 7·4 (7·4–7·5) | 0·304 |
| Radiologic findings | |||
| Bilateral infiltration, n (%) | 18 (40·9) | 9 (56·3) | 0·132 |
| Pleural effusion, n (%) | 9 (20·5) | 2 (12·5) | 0·382 |
| PSI (Median, range) | 59·0 (8–155) | 59·0 (13–192) | 0·423 |
| Death following admission, n (%) | 6 (13·6) | 4 (25·0) | 0·296 |
SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; PR, pulse rate; BT, body temperature; SpO2, oxygen saturation; WBC, white blood cell; ANC, absolute neutrophil count; PSI, pneumonia severity index.
*P < 0·05.
In ROC curve analysis of PCT, the area under the curve was 0·698 [95% confidence interval (CI) 0·523–0·873]. A PCT cutoff of >1·5 ng/ml best identified patients with mixed infection pneumonia (sensitivity 56%, specificity 84%, positive predictive value 56% and negative predictive value 84%) (Figure 1). For CRP, the area under the curve was 0·696 (95% CI 0·539–0·852) and a cutoff of >10 ng/ml best identified patients with mixed infection pneumonia (sensitivity 69%, specificity 63%, positive predictive value 41% and negative predictive value 54%) (Figure 1). The distribution of PCT and CRP between pneumonia of 2009 H1N1 infection alone and pneumonia caused by mixed infection are also depicted (Figure 2). When PCT and CRP concentrations were considered together, the accuracy of diagnostic criteria for the detection of mixed infection pneumonia was as follows: sensitivity, 50%; specificity, 93%; positive predictive value, 73%; and negative predictive value, 84% (Table 3).
Figure 1.
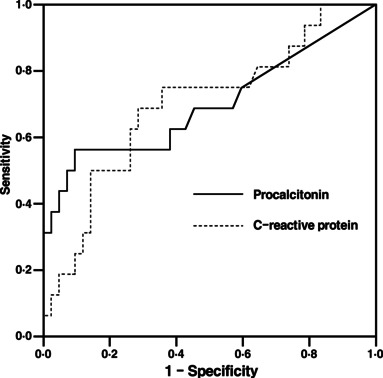
Receiver‐operating characteristics curve for discriminating between 2009 H1N1 pneumonia and mixed infection pneumonia for procalcitonin and C‐reactive protein (CRP) on initial emergency department visit [Area under curve 0·698 (95% confidence interval 0·523–0·873) for procalcitonin, 0·696 (95% confidence interval 0·539–0·852) for CRP].
Figure 2.
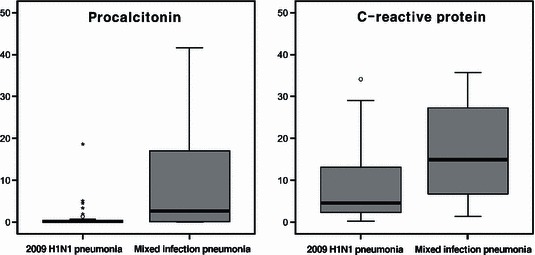
Box plot of procalcitonin and C‐reactive protein levels on initial emergency department visit between 2009 H1N1 pneumonia and mixed infection pneumonia. ○: minor outliers; *: extreme outliers.
Table 3.
Accuracy of diagnostic parameters
| CRP >10 mg/dl | PCT >1·5 ng/ml | CRP >10 mg/dl & PCT >1·5 ng/ml | |
|---|---|---|---|
| Sensitivity | 69% | 56% | 50% |
| Specificity | 63% | 84% | 93% |
| PPV | 41% | 56% | 73% |
| NPV | 84% | 84% | 84% |
| Accuracy | 64% | 77% | 82% |
| LR+ | 1·86 | 3·5 | 7·14 |
| LR− | 0·49 | 0·52 | 0·54 |
CRP, C‐reactive protein; PCT, procalcitonin; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
Discussion
This study showed that serum PCT concentration was significantly higher in patients with mixed infection pneumonia compared to those with 2009 H1N1 infection alone, indicating that this marker could be useful in discriminating between these conditions. Although statistically less powered, CRP could also aid in discriminating mixed bacterial infection from viral pneumonia. When both criteria are considered together, owing to a high specificity and high negative predictive value, they may further improve the accuracy of discrimination between pneumonia caused by mixed infection and pneumonia caused by 2009 H1N1 influenza infection alone.
Mixed bacterial infection is an important contributor to morbidity and mortality during influenza pandemics 7 , 8 and also during periods of seasonal influenza. 17 This has been explained by an increased pathogenicity and virulence of bacterial organisms in such co‐infection settings. 18 Therefore, it is crucial to differentiate mixed bacterial infections from influenza viral infections. In our study, the cutoff values that best differentiated mixed bacterial infection pneumonia from viral pneumonia were 1·5 ng/ml for PCT and 10 mg/dl for CRP. The cutoff value for PCT in discriminating bacterial infections from viral infections varies among previous studies: 0·4 ng/ml in a study by Chirouze et al. 19 1·0 ng/ml in a study of severe acute respiratory syndrome by Chua et al. 20 and 0·8 ng/ml in a study by Ingram et al. 13 The variety in presented cutoff values may be attributed to the presence of a large overlap in PCT concentrations between viral and bacterial infections. 21 The utility of PCT and CRP as biomarkers has been discussed in various studies. 22 , 23 , 24 And the role of PCT and CRP in discriminating bacterial/mixed infection from 2009 H1N1 pneumonia has been reported in a recent study of 38 patients by Guervilly et al. 12 and their results showed that only PCT values were statistically higher in patients with mixed bacterial infections. They measured PCT values in the 24 hours after admission. With the cutoff of 0·5 ng/ml for PCT, the sensitivity, specificity, negative predictive value, and positive predictive values were 100%, 52·5%, 100%, and 42%, respectively. Our study, in comparison, examined the PCT and CRP values on initial ED visit. Our data showed that both PCT and CRP were useful in discriminating viral infection from mixed bacterial infection, with the cutoff was 1·5 ng/ml for PCT, and 10 mg/dl for CRP. This distinction in the cutoffs and performance of markers may be attributed to the difference in the timing of measurement of inflammatory markers, and total patient numbers between the two studies. Our study included 62 patients, all of whom had PCT and CRP measured on arrival to ED.
As mixed bacterial infection is an important contributor to poor outcome in influenza, pertinent antibiotic administration is crucial. However, indiscriminant antibiotic usage may lead to bacterial resistance and complications of the drug itself. Therefore, the decision when to stop empirical antibiotics during the course of influenza undoubtedly possesses problems. Wright et al. 25 described diffuse infiltration on chest X‐ray and leukopenia in favoring infections caused by influenza alone, and lobar infiltration and leukocytosis in favoring mixed bacterial infections. However, discrimination of mixed bacterial infection from influenza infection by this method would not be possible in most cases during a pandemic. In our study, no significant differences in radiographic findings were found. Furthermore, WBC, absolute neutrophil count, and lymphocyte counts were not significantly different between the two groups. Therefore, clinicians cannot rely on radiographic findings or blood cell counts. Inflammatory markers including serum PCT and CRP concentrations are required to aid in the diagnosis and discrimination of the different types of pneumonia. According to the management protocols of H1N1 influenza at our institute, all patients were initially administered oseltamivir and prophylactic antibiotics, and in this setting, combination of a low PCT and low CRP may be useful to reduce the duration of antibiotic administration.
This study has several limitations. The sample size was small and by nature retrospective studies have innate limitations. Recent studies have shown that when serum PCT concentration is used to make decisions concerning antibiotics administration and duration, the trend in PCT values over time may be more important than the initial PCT level itself. 26 , 27 However, our study only evaluated the initial PCT values at the ED visit. PCT determination is not covered by medical insurance in Korea; therefore, cost was a prohibitive factor in the routine use of this biomarker during follow‐up. We also acknowledge that, by limiting mixed infection group to those in whom microbiologically confirmed bacterial diagnosis was made, we may have created a bias. In fact, causative bacterial organisms cannot be confirmed solely with respiratory samples, blood cultures, and immunoassay. Lack of demonstration of bacterial etiology could not rule out its role.
Conclusion
Our study demonstrated that the biological markers PCT and CRP, alone and in combination, had a moderate ability to detect pneumonia caused by mixed bacterial infection during the 2009 H1N1 pandemic. Because of its high specificity, using PCT and CRP in combination may be able to discriminate pneumonia caused by mixed infection from pneumonia caused by viral infection. This may then aid clinicians in more accurately identifying those patients in which administration of empirical antibiotics could be stopped. Further prospective studies to validate this result are required.
References
- 1. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]
- 2. WHO . Pandemic (H1N1) 2009 – update 87. Available at http://www.who.int/csr/don/2010_02_12/en/index.html (Accessed 18 February 2010).
- 3. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 4. Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 5. Choi WJ, Kim WY, Kim SH et al. Clinical characteristics of pneumonia in hospitalized patients with novel influenza A (H1N1) in Korea. Scand J Infect Dis 2010; 42:311–314. [DOI] [PubMed] [Google Scholar]
- 6. Choi SS, Kim WY, Kim SH et al. Associated factor related to major complications of patients with hospitalized for 2009 H1N1 influenza pneumonia. Tuberc Respir Dis 2010; 68:162–167. [Google Scholar]
- 7. Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christ‐Crain M, Jaccard‐Stolz D, Bingisser R et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004; 363:600–607. [DOI] [PubMed] [Google Scholar]
- 11. Stolz D, Christ‐Crain M, Bingisser R et al. Antibiotic treatment of exacerbations of COFD – a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest 2007; 131:9–19. [DOI] [PubMed] [Google Scholar]
- 12. Guervilly C, Coisel Y, Botelho‐Nevers E et al. Significance of high levels of procalcitonin in patients with influenza A (H1N1) pneumonia. J Infect 2010; 61:355–358. [DOI] [PubMed] [Google Scholar]
- 13. Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C‐reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piacentini E, Sanchez B, Arauzo V, Calbo E, Cuchi E, Nava JM. Procalcitonin levels are lower in intensive care unit patients with H1N1 influenza A virus pneumonia than in those with community‐acquired bacterial pneumonia. A pilot study. J Crit Care 2010; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Duncan C, Guthrie JL, Tijet N et al. Analytical and clinical validation of novel real‐time reverse transcriptase‐polymerase chain reaction assays for the clinical detection of swine‐origin H1N1 influenza viruses. Diagn Microbiol Infect Dis 2011; 69:167–171. [DOI] [PubMed] [Google Scholar]
- 16. Soler N, Torres A, Ewig S et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157:1498–1505. [DOI] [PubMed] [Google Scholar]
- 17. Murata Y, Walsh EE, Falsey AR. Pulmonary complications of interpandemic influenza A in hospitalized adults. J Infect Dis 2007; 195:1029–1037. [DOI] [PubMed] [Google Scholar]
- 18. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chirouze C, Schuhmacher H, Rabaud C et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis 2002; 35:156–161. [DOI] [PubMed] [Google Scholar]
- 20. Chua AP, Lee KH. Procalcitonin in severe acute respiratory syndrome (SARS). J Infect 2004; 48:303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toikka P, Irjala K, Juven T et al. Serum procalcitonin, C‐reactive protein and interleukin‐6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J 2000; 19:598–602. [DOI] [PubMed] [Google Scholar]
- 22. Lacour AG, Gervaix A, Zamora SA et al. Procalcitonin, IL‐6, IL‐8, IL‐1 receptor antagonist and C‐reactive protein as identificators of serious bacterial infections in children with fever without localising signs. Eur J Pediatr 2001; 160:95–100. [DOI] [PubMed] [Google Scholar]
- 23. Moulin F, Raymond J, Lorrot M et al. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child 2001; 84:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon L, Gauvin F, Amre DK, Saint‐Louis P, Lacroix J. Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis 2004; 39:206–217. [DOI] [PubMed] [Google Scholar]
- 25. Wright PF, Kirkland KB, Modlin JF. When to consider the use of antibiotics in the treatment of 2009 H1N1 influenza‐associated pneumonia. N Engl J Med 2009; 361:e112. [DOI] [PubMed] [Google Scholar]
- 26. Boussekey N, Leroy O, Alfandari S, Devos P, Georges H, Guery B. Procalcitonin kinetics in the prognosis of severe community‐acquired pneumonia. Intensive Care Med 2006; 32:469–472. [DOI] [PubMed] [Google Scholar]
- 27. Christ‐Crain M, Stolz D, Bingisser R et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia a randomized trial. Am J Respir Crit Care Med 2006; 174:84–93. [DOI] [PubMed] [Google Scholar]


