Abstract
Please cite this paper as: van Gageldonk‐Lafeber et al. (2011) The relative clinical impact of 2009 pandemic influenza A (H1N1) in the community compared to seasonal influenza in the Netherlands was most marked among 5–14 year olds. Influenza and Other Respiratory Viruses 5(6), e513–e520.
Background So far, most pandemic influenza reports were based on case studies focusing on severe disease. For public health policy, it is essential to consider the overall impact of the pandemic, including mild diseases.
Objectives The aim of our study is to gain insight into the epidemiology of 2009 pandemic influenza in the community and to estimate the relative impact of pandemic compared to seasonal influenza.
Methods The relative impact of pandemic influenza in the general population was assessed as the influenza‐like illness (ILI) incidence during the pandemic season compared with that during regular seasons. Influenza‐like illness incidences and virus diagnostics were derived from continuous sentinel surveillance systems. The incidence of hospital admissions, based on the mandatory notification of pandemic influenza, was used to relate the impact of severe disease to that in the community.
Results The overall incidence of general practitioners‐attended ILI was 96 consultations per 10 000 persons. Highest incidences were reported in children and lowest in persons aged ≥65 years. For 5–14 year olds, the incidence during the pandemic was higher than during all preceding seasons. Samples originating from 5 to 19 year olds were statistically significant more often positive for pandemic influenza A (H1N1) 2009 virus as compared with samples from 0 to 4 year olds. Moreover, the incidence of hospital admission owing to pandemic influenza was highest in the youngest children.
Conclusions Our study showed that while the absolute incidences of 2009 pandemic influenza were highest in children aged 0–4 years, the relative clinical impact in the community compared to seasonal influenza in previous years was most noticeable in healthy children 5–14 years of age.
Keywords: Epidemiology, hospitalization, primary health care, public health, seasonal influenza, swine origin influenza A H1N1 virus
Introduction
In 2009, an emerging influenza A (H1N1) virus, initially identified in April 2009, caused the first official pandemic since 1968. 1 Early case reports suggested serious morbidity and significant mortality. 2 , 3 , 4 Because of the rapid spread, it became urgent to gain insight into the impact of this new virus on human populations. In the Netherlands as elsewhere, first rapid assessments of this impact have been based on notified patients. 5 , 6 , 7 Initially, most (mandatory) notification systems addressed all possible, probable, and confirmed cases of pandemic influenza A (H1N1), but as the epidemic was extending globally WHO and ECDC recommended to focus in particular on severe acute respiratory illness caused by this new influenza virus. 8 , 9 Concurrently, it became clear that the clinical spectrum included asymptomatic infection, self‐limiting illness, severe illness requiring mechanical ventilation, and death. 10 , 11 , 12 , 13 , 14 , 15 , 16
Initial case studies, mainly based on severe influenza cases, provided vital information to guide management and control; 17 , 18 however, selection bias limits the generalizability of such studies. 19 Therefore, a more comprehensive analysis is needed to assess not only the absolute disease burden of severe cases but to relate this to the burden in the community and to estimate the relative clinical impact of pandemic influenza in the general population (community dwellers) compared to regular influenza in previous years. Continuous longitudinal surveillance systems enabled the assessment of this impact over time by providing a stable basis of observations. The Dutch sentinel influenza surveillance systems continued during the pandemic, thereby providing vital information about influenza‐like illness (ILI) and influenza A (H1N1) 2009 virus infections in primary care.
For over 40 years, the general practitioners (GPs) from the Continuous Morbidity Registration Sentinel Network of NIVEL, the Netherlands institute of health services research, register all patients who consult them for ILI. For about 25 years, these GPs take weekly nose and throat swabs from a sample of patients with acute respiratory tract infections (ARTI), including ILI. 20 , 21 , 22 Since in the Netherlands, medical care for elderly living in nursing homes is not provided by GPs but by elderly care physicians, a dedicated nursing home network was initiated in 2008. In this national sentinel surveillance network for infectious diseases in nursing homes (SNIV), the weekly numbers of ILI patients in participating nursing homes are reported and viral diagnostics is performed in a subset of patients, similar to the design of the GP sentinel surveillance. 23
The aim of our study is to gain insight into the epidemiology of 2009 pandemic influenza in the community, using continuous sentinel surveillance systems and the mandatory notification of pandemic influenza virus A (H1N1) hospitalization and to estimate the relative clinical impact of pandemic influenza compared to seasonal influenza in previous years, using longitudinal population‐based sentinel data for seasonal influenza.
Methods
The GP network of the Continuous Morbidity Registration constitutes a group of GPs in about 40 practices throughout the Netherlands. This network covers about 0·8% of the Dutch population and is nationally representative by age, gender, regional distribution, and population density. 20 On a weekly basis, the GPs register the number of consultations for ILI by age‐group according to the following definition: illness with an acute onset of symptoms (prodromal stage ≤4 days), fever (defined as a rise in rectal temperature to at least 38°C), and at least one of the following symptoms: cough, rhinitis, sore throat, frontal headache, retrosternal pain, or myalgia. As part of the virological surveillance, the GPs take nose swabs and throat swabs from two ILI patients per week. When sampling of ILI patients is not feasible, swabs are taken from patients with other ARTIs. These swabs are accompanied by a form on which the GP registers patient data including sex, date of birth, diagnosis, and underlying diseases. Samples are sent to the National Institute of Public Health and the Environment (Bilthoven, the Netherlands).
During the study period, the SNIV nursing homes network included 25 nursing homes throughout the Netherlands. They register the weekly number of ILI patients according to the definition: illness with acute start of symptoms and at least one of the following systemic symptoms: fever of febrile feeling, malaise, headache, myalgia, and at least one of the following three respiratory symptoms: cough, sore throat, shortness of breath. Sampling for the virological surveillance is similar to that for the GP surveillance, except for the requirement to sample patients aged 10 years or younger.
Influenza viruses in clinical specimens for both the GP and the SNIV nursing homes network were detected and further subtyped using a combination of virus isolation and characterization by HI assays (hemagglutination inhibition) and (real‐time) reverse transcriptase (RT)‐PCR (polymerase chain reaction). For rapid diagnostics of the pandemic influenza A (H1N1) 2009 virus, real‐time RT‐PCR for the general detection of influenza virus type A and B was combined with specific detection of the pandemic influenza A (H1N1) 2009 virus. 24
Data with respect to hospital admission for pandemic influenza were obtained from the national mandatory notification system as described by Van ‘t Klooster et al. 7 No comparable longitudinal hospital data are available to assess trends over time.
Statistical analyses
Both for seasonal and for pandemic influenza, the influenza season is defined as the period with ILI activity at GP level above the baseline threshold of a weekly ILI incidence of 5·1 per 10 000 persons. 20
For the GP network, incidences of ILI were calculated per 10 000 persons as the number of ILI consultations divided by the patient population × 10 000 both per week and per influenza season in total in the period between 1999/2000 and 2009/2010. This was calculated for the total population and stratified by age‐groups (0–4, 5–9, 10–14, 15–19, 20–44, 45–64, and ≥65 years of age). For the SNIV nursing home network, ILI incidences were calculated per 10 000 residents as the number of new ILI patients divided by the number of nursing home residents × 10 000, both per week and for the influenza seasons 2008/2009 and 2009/2010. As the ILI surveillance in SNIV nursing home network was operational from January 2009 onwards, only data from week 2 to 8 2009 of the season 2008/2009 were available. As proxy for the number of nursing home residents, we used the bed capacity in the registrating nursing homes. The bed occupancy in Dutch nursing homes is always close to 100%.
Incidence of hospital admissions owing to the pandemic influenza A (H1N1) 2009 virus in the pandemic season 2009/2010 was calculated per 10 000 persons as the number of hospitalizations divided by the Dutch population (determined in July 2009) × 10 000. This was calculated for the total population and stratified by age‐groups (0–4, 5–9, 10–14, 15–19, 20–44, 45–64, and ≥65 years of age).
The relative impact of the pandemic influenza virus in the general population was assessed as the ILI incidences during the pandemic season in different age‐groups compared with those during earlier regular influenza seasons, for both the GP network and the SNIV nursing home network. The incidence of hospital admissions owing to the pandemic influenza A (H1N1) 2009 virus in the pandemic season was used to relate the impact of severe disease, requiring hospital admission, to that of mild diseases in the general population.
Uni‐ and multivariate logistic regression analyses were used to assess which patient characteristics were independently related to detection of influenza virus, both pandemic and seasonal, in nose swabs and throat swabs from ILI patients. These analyses were restricted to acute samples obtained within 4 days after the first day of illness, taken in the influenza seasons in the period between 2003/2004 and 2009/2010.
Statistical analyses were performed using sas software version 9·1·3 (SAS Institute, Cary, NC, USA).
Results
The pandemic season started in week 41, 2009 (October), and lasted for 10 weeks. This period included the so‐called autumn school holiday: week 43 for the northern and central part of the Netherlands and week 44 for the southern part. There was no spring or summer wave of pandemic influenza in the Netherlands. The duration of the influenza seasons between 1999/2000 and 2008/2009 varied from 4 to 15 weeks. The start of these seasons was between weeks 46 and week 8 and the end between weeks 3 and 13 (Table 1).
Table 1.
Period with heightened ILI activity for the influenza seasons 1999/2000–2009/2010
| Influenza season | Start season | End season | Duration (weeks) | ||
|---|---|---|---|---|---|
| Year | Week | Week | Year | ||
| 1999/2000 | 1999 | 51 | 5 | 2000 | 7 |
| 2000/2001 | 2001 | 3 | 6 | 2001 | 4 |
| 2001/2002 | 2002 | 4 | 12 | 2002 | 9 |
| 2002/2003 | 2003 | 9 | 12 | 2003 | 4 |
| 2003/2004 | 2003 | 50 | 3 | 2004 | 6 |
| 2004/2005 | 2005 | 4 | 12 | 2005 | 9 |
| 2005/2006 | 2006 | 1 | 13 | 2006 | 13 |
| 2006/2007 | 2007 | 8 | 11 | 2007 | 4 |
| 2007/2008 | 2008 | 5 | 9 | 2008 | 5 |
| 2008/2009 | 2008 | 46 | 8 | 2009 | 15 |
| 2009/2010 | 2009 | 41 | 50 | 2009 | 10 |
Pandemic influenza virus in primary care
General practice
The overall incidence of GP‐attended ILI in the period between week 41 and week 50, 2009, was 96 consultations per 10 000 persons. The highest incidences were reported in children aged <15 years, respectively, 285, 195, and 162 consultations per 10 000 persons in the 0–4, 5–9, and 10–14 year olds, and the lowest incidence in persons aged ≥65 years, 52 consultations per 10 000 persons (Figure 1).
Figure 1.
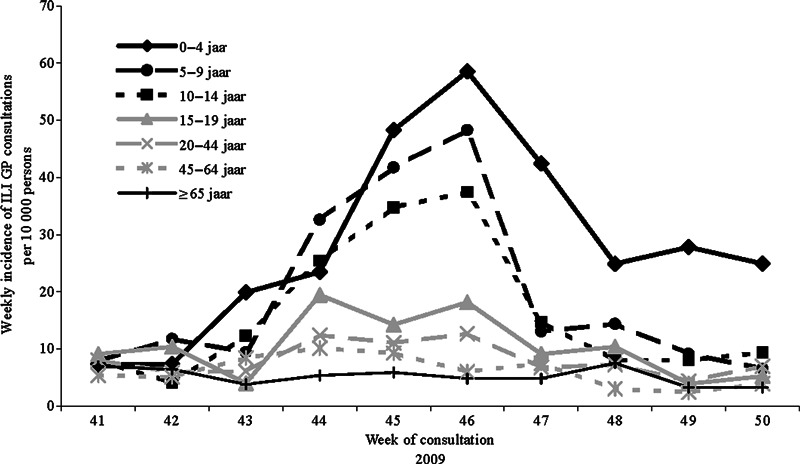
Weekly incidence of GP‐attended influenza‐like illness (ILI) per 10 000 persons for different age‐groups in the period from week 41 to 50 in 2009.
In the period between week 41 and 50, 2009, a total of 555 ILI patients were sampled as part of the virological surveillance, of which 43% were positive for the new pandemic influenza virus. The percentage of samples positive for pandemic influenza was highest in the age‐groups 5–9 and 10–14 years, respectively, 67% and 68%, and lowest in the ≥65 years of age, 27%. Although univariate analyses showed that samples originating from patients with a respiratory allergy were statistically remarkably more often positive for the pandemic influenza A (H1N1) 2009 virus, this difference was not found in the multivariate analyses. Both univariate and multivariate analyses showed that samples originating from children aged between 5 and 19 years were statistically remarkably more often positive for the pandemic influenza A (H1N1) 2009 virus as compared with samples from ILI patients younger than 5 years of age (Table 2). This was most pronounced for 5–9 and 10–14 year olds, with, respectively, OR = 5·1 and OR = 5·0.
Table 2.
Univariate and multivariate logistic regression analyses of the detection of new influenza A (H1N1) virus in nose swabs and throat swabs from patients with influenza‐like illness sampled in the period from week 41 to 50 in 2009
| No. samples and percentage positive for new influenza A (H1N1) virus No. (%) | Univariate logistic regression OR [95% CI] | Multivariate logistic regression OR [95% CI] | |
|---|---|---|---|
| Gender* | |||
| Female | 293 (43) | Ref | Ref |
| Male | 258 (45) | 1·1 [0·8–1·5] | 0·9 [0·7–1·4] |
| Age group (years) | |||
| 0–4 | 103 (29) | Ref | Ref |
| 5–9 | 73 (67) | 5·0 [2·6–9·5] | 5·1 [2·6–9·9] |
| 10–14 | 73 (68) | 5·3 [2·8–10·1] | 5·0 [2·6–9·7] |
| 15–19 | 42 (57) | 3·2 [1·5–6·8] | 3·1 [1·5–6·7] |
| 20–44 | 161 (32) | 1·2 [0·7–2·0] | 1·1 [0·6–1·9] |
| 45–64 | 88 (36) | 1·4 [0·8–2·6] | 1·2 [0·7–2·3] |
| ≥65 | 15 (27) | 0·9 [0·3–3·0] | 0·7 [0·2–2·7] |
| Respiratory allergy** | |||
| No | 512 (42) | Ref | Ref |
| Yes | 36 (61) | 2·2 [1·1–4·3] | 1·9 [0·9–4·2] |
| Chronic disease | |||
| No | 480 (43) | Ref | Ref |
| Yes | 67 (43) | 1·0 [0·6–1·7] | 1·0 [0·6–1·9] |
*Gender was missing for 4 of the sampled patients.
**Complaints of the respiratory tract caused by IgE‐mediated allergy, including asthma.
Bold text indicates statistically significant differences.
Nursing homes
The overall incidence of ILI in nursing home residents in the period between week 41 and week 50, 2009, was 123 per 10 000 persons. No effect of the pandemic in the incidence of ILI was visible in this population (Figure 2), and none of the 21 obtained nose and throat swabs were positive for the pandemic influenza A (H1N1) virus.
Figure 2.
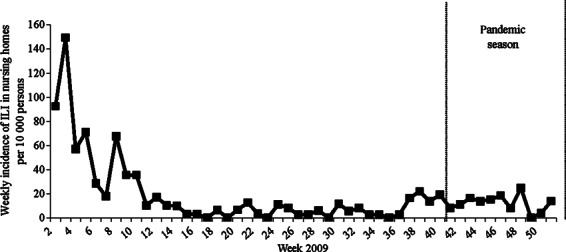
Weekly incidence of influenza‐like illness (ILI) per 10 000 nursing home residents in 2009.
Pandemic influenza virus in hospitals
The overall incidence of hospital admissions because of laboratory‐confirmed 2009 pandemic influenza A (H1N1) in the period from week 41 to 50 was 1·1 admissions per 10 000 persons. The incidence was highest in children aged 0–4 years (5·7 admissions per 10 000 persons) and lowest in persons aged ≥65 years (0·5 admissions per 10 000 persons; Figure 3).
Figure 3.
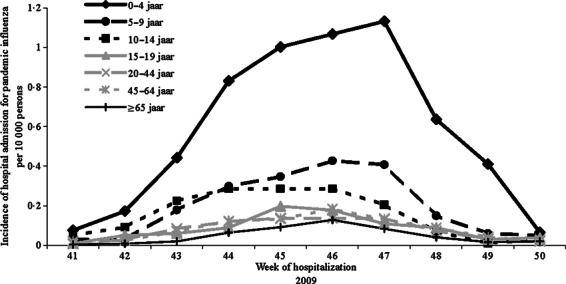
Weekly incidence of hospital admissions because of 2009 pandemic influenza A (H1N1) by age‐group per 10 000 persons between week 41 and 50 in 2009.
Pandemic versus seasonal influenza virus in primary care
General practice
Both for pandemic influenza in 2009/2010 and for seasonal influenza in 1999/2000–2008/2009, the highest incidence of GP‐attended ILI was reported for children aged <5 years (Figure 4). During the pandemic, this incidence was lower than the seasonal influenza incidence in this age‐group in 2008–2009, but higher than that in the other seasons in the preceding decade. For the 5–14 year olds, the incidence of GP‐attended ILI for pandemic influenza was less than the incidence in 0–4 year olds, but remarkably higher than the seasonal influenza incidence in 5–14 year olds in all preceding years. While the highest incidence for 0–4 year olds was reported in the season 2008/2009, for 15–19 year olds, this was in the season 2005/2006 and for persons aged 20 years and older in 1999/2000.
Figure 4.
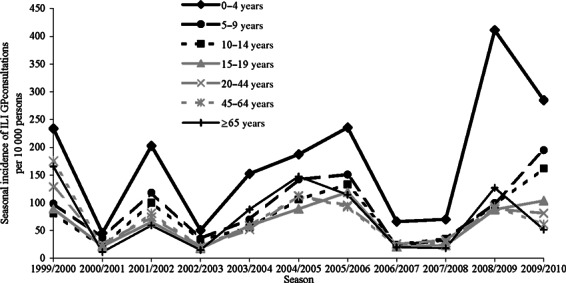
Seasonal incidence of general practitioner (GP)‐attended influenza‐like illness (ILI) by age‐group per 10 000 persons in the seasons 1999/2000–2009/2010.
The number of sampled ILI patients in the seasons 2003/2004 to 2008/2009 varied between 44 in 2003/2004 and 301 in 2008/2009, and the percentage of samples positive for influenza virus varied between 38% in 2006/2007 and 53% in 2004/2005. In contrast to season 2009/2010, multivariate logistic regression analyses showed that there were no differences in the proportions of influenza‐positive nose and throat swabs between the different age‐groups during regular influenza seasons (data not shown). Because of the limited number of sampled patient, these analyses were restricted to the seasons 2005/2006, 2007/2008, and 2008/2009.
Nursing homes
During the pandemic, the incidence of ILI in nursing home residents was considerably lower compared to the incidence of seasonal influenza in 2008/2009 (respectively, 123 and 484 per 10 000 persons –Figure 2), even though the 2008/2009 incidence was only based on data from week 2 to 8 in 2009.
Discussion
Our study relates the epidemiology of pandemic influenza in primary care ILI patients and in hospitalized patients with confirmed pandemic influenza to the epidemiology of seasonal influenza, using a long‐term validated sentinel GP surveillance system as well as a recently started nursing home network, both covering ILI activity and virological surveillance. While absolute incidences of pandemic influenza were highest in children below 5 years of age, the largest relative impact of pandemic compared to seasonal influenza in previous years was observed among children 5–14 years of age. No noticeable impact was seen in persons aged ≥65 years.
The strength of our study is the inclusion of long‐term longitudinal population‐based data derived from continuous sentinel surveillance systems next to data from the mandatory notification system. Studies focusing on notified and/or hospitalized patients only showed that pandemic influenza A (H1N1) caused severe illness, particularly in young persons and those with underlying medical conditions. 12 , 25 In addition to this, our study showed that the relative clinical impact in the general population of the 2009 pandemic influenza compared to seasonal influenza in previous years was most felt in healthy children aged 5–14 years of age. This is in agreement with preliminary data on European all‐cause mortality during the pandemic reported by EuroMOMO, 26 using longitudinal data series on crude mortality by age‐group, indicating a cumulative excess mortality in 5–14 year olds. Furthermore, Lemaitre and Carrat 27 showed that young age (<20 years) was a principal mortality risk factor of the 2009 pandemic.
By assessing both absolute incidences and relative increases in incidences compared to seasonal influenza incidences, we could show that during the pandemic in the Netherlands, the 5–14 year olds suffered the largest relative clinical impact compared to previous years. While absolute incidences of GP consultations and hospitalizations were lower than the incidences of the 0–4 year olds, the 5–14 year olds was the only age‐group where the pandemic incidence of GP consultations did not fall within the range of observed incidences over the past decade. Furthermore, we found a higher proportion of nose and throat swabs positive for the pandemic influenza A (H1N1) virus in this age‐group compared to that found in 0–4 year olds, while no differential positivity was observed in previous seasons. Therefore, in the general population, the relative clinical impact of pandemic influenza compared to seasonal influenza in children aged between 5 and 14 years is probably even higher than expected based only on the incidence of GP‐attended ILI.
The incidence of ILI in nursing home residents cannot be compared directly to that found in GP patients aged ≥65 years, because of differences in the general state of health of the both populations as well as differences in the organization of both sentinel surveillance systems. The elderly care physicians examine all nursing home residents with (mild) ILI, while GPs examine only those patients who consult them because of ILI. Nevertheless, both surveillance systems confirm the relatively minor impact of the pandemic seen in persons aged ≥65 years, as has been reported also for other countries. 10 , 11 , 25 , 28
The incidence of GP‐attended pandemic ILI was highest in the 0–4 year olds, as also seen in regular influenza seasons, 21 and also the pandemic hospitalization rate was highest in this age‐group. This is in line with earlier studies on pandemic influenza, where also high incidences in young persons both for hospitalization because of laboratory‐confirmed 2009 pandemic influenza A (H1N1) and for GP‐attended ILI were observed. 29 , 30 However, part of the high consultation rates in the youngest children might be associated with a high level of parental anxiety, 21 possibly even more heightened by the great media attention during the pandemic. Indeed, relatively few of the Dutch‐hospitalized children were admitted to an intensive care unit (ICU), indicating that part of them might have been admitted for precautionary reasons or for supervised oseltamivir therapy rather than primarily for the severity of the illness. 7
Because of the relatively short duration of the pandemic season and the timing of the vaccination campaign, it is plausible that the effect of the vaccination campaign for healthy children aged between 6 months and 4 years on the assessed impact has been negligible (A Steens, EG Wijnans, JP Dieleman, MCJM Sturkenboom, MAB van der Sande, W van der Hoek, Submitted). The first pandemic vaccinations for healthy children were administered in week 48 of 2009 and the second vaccinations about 3 weeks later, while the pandemic season peaked in week 46 and lasted until week 50.
Our study has some limitations. First, the observed incidences based on GP‐attended ILI will underestimate the real incidence of ILI in the general population, because patients with mild complaints will not always seek medical care. However, this also applies for regular influenza seasons and is unlikely to have affected the age‐specific comparison of the pandemic with the preceding influenza seasons owing to the use of longitudinal data. Another limitation is the limited robustness of the nursing home surveillance because combined clinical and virological ILI surveillance had only been operational for one regular season. Next to this, the robustness of the virological surveillance depends on the number of sampled ILI patients, which unfortunately was low for some of the studied influenza seasons. A third limitation is the probability that during the pandemic small children with more serious illness were directly admitted to hospital and therefore not captured by the GP system. However, there are no indications that this happened on a different scale than in other years. In general, the majority of hospital admissions in Netherlands occur following referral by a GP. During the pandemic, hospital admission owing to influenza A (H1N1) 2009 virus became a notifiable disease, enabling us to study the incidence by age‐group and allowing a rapid focus on the severity of pandemic influenza. A limitation, however, is the lack of reliable historical records of hospital admission related to laboratory‐confirmed influenza virus for the regular influenza seasons. Finally, ascertainment bias might have resulted in more frequent testing for influenza in hospitalized patients with respiratory complaints during the pandemic and might also have influenced the sampling in the GP network. However, there are no indications that selection for the swab collection in relation to the ILI reporting was different from that in previous years.
In conclusion, so far most pandemic influenza reports were based on case studies focusing on severe disease and thereby ignore the bulk of the public health impact of milder disease as observed in the community and reflected in GP consultations. By analyzing hospitalization, nursing home and GP surveillance data concurrently and using longitudinal trend data to assess the relative impact of pandemic influenza on the population compared to seasonal influenza, we could show that while the absolute incidences of 2009 pandemic influenza were highest among children 0–4 years of age, the relative clinical impact in the community compared to seasonal influenza in previous years was most noticeable in healthy children aged between 5 and 14 years of age.
Funding
Authors received no financial support.
Conflicts of interest
Authors have no conflicts of interest.
Acknowledgements
We thank all participating GPs and elderly care physicians, their patients, and the Netherlands institute of health services research for their cooperation in the data collection; all municipal health services, hospitals, and laboratories in the Netherlands for providing data; and the technicians of the Diagnostic Laboratory for Infectious Diseases and Perinatal Screening of the National Institute of Public Health and the Environment for performing the virological diagnostic tests.
Members of the Dutch Novel Influenza A (H1N1) Investigation Team are gratefully acknowledged for their for their input in establishing and modifying the notification system (M. van Ballegooijen, D. Beaujean, T. Beersma, C. Boucher, M. van Boven, P. Brandsema, E. de Bruin, N. Brunner, R. Coutinho, M. van Dam, C. Deuning, F. Dijkstra, S. Dittrich, T. Donker, A. van Eijk, R. Fouchier, S. Hahné, P. ten Ham, J. van der Have, A. van den Hoek, L. Isken, A. Jacobi, P. Jacobs, M. Jonges, H. van den Kerkhof, R. van Kessel, M. Koopmans, A. Kroneman, T. Leenstra, J. Monen, A. Osterhaus, P. Overduin, M. Petrignani, H. Ruijs, R. ter Schegget, F. Schellevis, M. Schutten, M. Siebbeles, J. van Steenbergen, A. Steens, C. Swaan, A. Timen, H. Vennema, L. Verhoef, R. Vriend, T. Waegemaekers, J. Wallinga, B. Wilbrink, J. IJzermans).
We thank the NIVEL Scientific Panel for critical reading of the manuscript.
References
- 1. Centers for Disease Control and Prevention (CDC) . Swine influenza A (H1N1) infection in two children – Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:400–402. [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) . Outbreak of swine‐origin influenza A (H1N1) virus infection – Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:467–470. [PubMed] [Google Scholar]
- 3. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]
- 4. Chowell G, Bertozzi SM, Colchero MA et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679. [DOI] [PubMed] [Google Scholar]
- 5. Hahné S, Donker T, Meijer A et al. Epidemiology and control of influenza A(H1N1)v in the Netherlands: the first 115 cases. Euro Surveill 2009; 14: pii=19267. [DOI] [PubMed] [Google Scholar]
- 6. Vriend HJ, Hahné AJM, Donker T et al. The new influenza A(H1N1) epidemic in the Netherlands. Statistics from 30 April–14 August 2009. Ned Tijdschr Geneeskd 2009; 153:A969. [Google Scholar]
- 7. van‘t Klooster TM, Wielders CC, Donker T et al. Surveillance of hospitalisations for 2009 pandemic influenza A(H1N1) in the Netherlands, 5 June–31 December 2009. Euro Surveill 2010; 15: pii=19461. [DOI] [PubMed] [Google Scholar]
- 8. Cauchemez S, Ferguson NM, Wachtel C et al. Closure of schools during an influenza pandemic. Lancet Infect Dis 2009; 9:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ECDC Meeting Report Surveillance and studies in a pandemic: Fourth meeting of the SSiaP working group, Stockholm. [Web Page]. 14 July 2009–15 July 2009; Available at http://ecdc.europa.eu/en/publications/Publications/0908_MER_Surveillance_and_Studies_in_a_Pandemic_Meeting_Report.pdf (Accessed 29 June 2010).
- 10. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 11. Fielding J, Higgins N, Gregory J et al. Pandemic H1N1 influenza surveillance in Victoria, Australia, April–September, 2009. Euro Surveill 2009; 14: pii=19368. [DOI] [PubMed] [Google Scholar]
- 12. Halasa NB. Update on the 2009 pandemic influenza A H1N1 in children. Curr Opin Pediatr 2010; 22:83–87. [DOI] [PubMed] [Google Scholar]
- 13. Chang YS, van Hal SJ, Spencer PM, Gosbell IB, Collett PW. Comparison of adult patients hospitalised with pandemic (H1N1) 2009 influenza and seasonal influenza during the “PROTECT”phase of the pandemic response. Med J Aust 2010; 192:90–93. [DOI] [PubMed] [Google Scholar]
- 14. Koliou M, Soteriades ES, Toumasi MM, Demosthenous A, Hadjidemetriou A. Epidemiological and clinical characteristics of influenza A(H1N1)v infection in children: the first 45 cases in Cyprus, June–August 2009. Euro Surveill 2009; 14: pii=19312. [DOI] [PubMed] [Google Scholar]
- 15. Jamieson DJ, Honein MA, Rasmussen SA et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–458. [DOI] [PubMed] [Google Scholar]
- 16. Gordon CL, Johnson PD, Permezel M et al. Association between Severe Pandemic 2009 Influenza A (H1N1) Virus Infection and Immunoglobulin G(2) Subclass Deficiency. Clin Infect Dis 2010; 50:672–678. [DOI] [PubMed] [Google Scholar]
- 17. Pandemic (H1N1) 2009 [Web Page]. Available at http://www.ecdc.europa.eu/en/healthtopics/Documents/091109_Influenza_A(H1N1)_Weekly_Executive_Update.pdf (Accessed 26 April 2011).
- 18. World Health Organization (WHO) . Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec 2009; 84:481–484. [PubMed] [Google Scholar]
- 19. Lipsitch M, Hayden FG, Cowling BJ, Leung GM. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet 2009; 374:1209–1211. [DOI] [PubMed] [Google Scholar]
- 20. Donker GA. Continuous Morbidity Registration at Dutch Sentinel Stations, 2008. [Web Page]. Available at http://www.nivel.nl/pdf/Rapport‐CMR‐2008‐engels.pdf (Accessed 26 April 2011).
- 21. van Gageldonk‐Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case–control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 2005; 41:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dijkstra F, Donker GA, Wilbrink B, Van Gageldonk‐Lafeber AB, Van Der Sande MA. Long time trends in influenza‐like illness and associated determinants in The Netherlands. Epidemiol Infect 2009; 137:473–479. [DOI] [PubMed] [Google Scholar]
- 23. Brunner NA, Veldman‐Ariesen M‐J, Haenen A, van de Sande H, Benthem BH. Healthcare‐associated infection surveillance in nursing homes in the Netherlands. Clin Microbiol Infect 2010; 16:S409 (abstract). [Google Scholar]
- 24. Meijer A, Beerens A, Claas E et al. Preparing the outbreak assistance laboratory network in the Netherlands for the detection of the influenza virus A(H1N1) variant. J Clin Virol 2009; 45:179–184. [DOI] [PubMed] [Google Scholar]
- 25. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009; 361:1935–1944. [DOI] [PubMed] [Google Scholar]
- 26. Mazick A, Gergonne B, Wuillaume F et al. Higher all‐cause mortality in children during autumn 2009 compared with the three previous years: pooled results from eight European countries. Euro Surveill 2010; 15: pii=19480. [PubMed] [Google Scholar]
- 27. Lemaitre M, Carrat F. Comparative age distribution of influenza morbidity and mortality during seasonal influenza epidemics and the 2009 H1N1 pandemic. BMC Infect Dis 2010; 10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cullen G, Martin J, O’Donnell J et al. Surveillance of the first 205 confirmed hospitalised cases of pandemic H1N1 influenza in Ireland, 28 April–3 October 2009. Euro Surveill 2009; 14: pii 19389. [PubMed] [Google Scholar]
- 29. Turbelin C, Pelat C, Boelle PY et al. Early estimates of 2009 pandemic influenza A(H1N1) virus activity in general practice in France: incidence of influenza‐like illness and age distribution of reported cases. Euro Surveill 2009; 14:19341. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) . Patients hospitalized with 2009 pandemic influenza A (H1N1) – New York City, May 2009. MMWR Morb Mortal Wkly Rep 2010; 58:1436–1440. [PubMed] [Google Scholar]


