Abstract
Please cite this paper as: Song et al. (2011). Clinical, laboratory and radiologic characteristics of 2009 pandemic influenza A/H1N1 pneumonia: primary influenza pneumonia versus concomitant/secondary bacterial pneumonia. Influenza and Other Respiratory Viruses 5(6), e535–e543.
Background Although influenza virus usually involves the upper respiratory tract, pneumonia was seen more frequently with the 2009 pandemic influenza A/H1N1 than with seasonal influenza.
Methods From September 1, 2009, to January 31, 2010, a specialized clinic for patients (aged ≥15 years) with ILI was operated in Korea University Guro Hospital. RT‐PCR assay was performed to diagnose 2009 pandemic influenza A/H1N1. A retrospective case–case–control study was performed to determine the predictive factors for influenza pneumonia and to discriminate concomitant/secondary bacterial pneumonia from primary influenza pneumonia during the 2009–2010 pandemic.
Results During the study period, the proportions of fatal cases and pneumonia development were 0·12% and 1·59%, respectively. Patients with pneumonic influenza were less likely to have nasal symptoms and extra‐pulmonary symptoms (myalgia, headache, and diarrhea) compared to patients with non‐pneumonic influenza. Crackle was audible in just about half of the patients with pneumonic influenza (38·5% of patients with primary influenza pneumonia and 53·3% of patients with concomitant/secondary bacterial pneumonia). Procalcitonin, C‐reactive protein (CRP), and lactate dehydrogenase were markedly increased in patients with influenza pneumonia. Furthermore, procalcitonin (cutoff value 0·35 ng/ml, sensitivity 81·8%, and specificity 66·7%) and CRP (cutoff value 86·5 mg/IU, sensitivity 81·8%, and specificity 59·3%) were discriminative between patients with concomitant/secondary bacterial pneumonia and patients with primary influenza pneumonia.
Conclusions Considering the subtle manifestations of 2009 pandemic influenza A/H1N1 pneumonia in the early stage, high clinical suspicion is required to detect this condition. Both procalcitonin and CRP would be helpful to differentiate primary influenza pneumonia from concomitant/secondary bacterial pneumonia.
Keywords: 2009 H1N1, C‐reactive protein, influenza, pneumonia, procalcitonin
Introduction
Since early April 2009, the 2009 pandemic A/H1N1 virus has spread and persisted noticeably over the seasonal baseline. According to the Korean Influenza Sentinel Surveillance (KISS) report in 2009, weekly influenza‐like illness (ILI) rates had already exceeded the seasonal outbreak criteria (2·6 per 1000 cases) in week 34 and were about 10‐fold higher than the recent seasonal average between October and December of 2008. 1
Of the large number of patients with influenza infection, many required hospitalization and some of these patients presented with pneumonia. The clinical features and significance of influenza pneumonia need to be further elucidated before the predicted second influenza outbreak in the upcoming 2010–2011 influenza season. In this study, we described the clinical, laboratory and radiologic characteristics of 2009 pandemic influenza A/H1N1 pneumonia and compared these between concomitant/secondary bacterial pneumonia and primary influenza pneumonia during the 2009–2010 pandemic.
Materials and methods
Study design
Korea University Guro Hospital (KUGH) is a 1000‐bed tertiary acute care hospital in southwestern Seoul, Korea. From September 1, 2009, to January 31, 2010, a specialized clinic for patients with ILI was operated in KUGH; patients aged ≥15 years were cared for at the Department of Internal Medicine. To confirm 2009 pandemic influenza A/H1N1, we performed real‐time, reverse transcriptase‐polymerase chain reaction (RT‐PCR) assays using respiratory specimens in accordance with the published guidelines of the US Centers for Disease Control and Prevention (CDC). In addition to the RT‐PCR assay, serum hemagglutinin inhibition (HI) assays were performed to diagnose influenza pneumonia in cases of suspicious influenza pneumonia with negative RT‐PCR results.
Specimens were collected either by a nasopharyngeal/throat swab or by nasopharyngeal aspiration. The proportion of pneumonia and fatal cases among patients (aged ≥15 years) with laboratory‐confirmed 2009 pandemic influenza A/H1N1 was calculated. We also collected retrospective data regarding patient demographics, co‐morbidities, clinical manifestations, laboratory and radiologic findings, duration of hospital stay, duration of intensive care unit (ICU) stay, duration of mechanical ventilation, treatment modalities, and clinical outcomes. The severity of illness was also assessed at presentation using a scoring system: the CURB‐65 scoring system was used for all patients with pneumonia and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system was used for patients admitted to the ICU. Using clinical data obtained from electronic medical records, we performed a case–case–control study to determine the predictive factors for 2009 pandemic influenza A/H1N1 pneumonia and to discriminate concomitant/secondary bacterial pneumonia from primary influenza pneumonia. Two kinds of influenza pneumonia groups were defined as cases: patients with primary influenza pneumonia (case 1 group) and patients with concomitant/secondary bacterial pneumonia (case 2 group). The controls were selected from hospitalized patients with non‐pneumonic laboratory‐confirmed influenza during the same calendar month that influenza pneumonia occurred (frequency‐matched controls). Chest X‐ray was taken in all study subjects, and chest computed tomography (CT) was taken in all subjects with unremarkable chest X‐ray finding to exclude pneumonia development. Patients with extra‐pulmonary bacterial infections were excluded. This study was approved by the ethics committee of KUGH and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Case definition
ILI was defined as sudden onset fever (≥38°C) with respiratory symptoms (cough, sore throat, or nasal stuffiness). The following criteria were used to diagnose pneumonia: a chest radiograph revealing a new infiltrate consistent with pneumonia and at least one of the following: fever, chills, hypothermia, cough, or abnormal white blood cell count (>11 × 109/l, or <3 × 109/l) with or without abnormal differential. In addition to the RT‐PCR assay, a serum HI assay was performed to detect 2009 pandemic influenza A/H1N1. Patients were regarded as positive for 2009 pandemic influenza A/H1N1 when the RT‐PCR was positive or the HI titers for the 2009 A/H1N1 met the following criteria: either convalescent (3 weeks later from ILI onset) titers ≥1:160 or seroconversion (a more than fourfold increase at the convalescent stage compared with the acute stage). 2 , 3 Concomitant/secondary bacterial pneumonia was defined according to current guidelines, and following laboratory tests were taken for all study subjects with pneumonia on admission; a sputum gram stain and culture, blood cultures, urinary antigen tests for Legionella pneumophila and Streptococcus pneumoniae, and paired (acute convalescent) serologic tests for atypical pathogens (Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella species). 4 Sputum specimens were regarded optimal if gram stain showed <10 squamous epithelial cells/low power field (10 times normal). Bronchiolitis was diagnosed clinically in the presence of respiratory symptoms combined with wheezing on physical examination after excluding asthma, chronic obstructive lung diseases, and pneumonia.
HI assays
The influenza virus used for the HI assay was A/Califonia/7/2009 NYMC X‐179A, which is distributed by the National Institute for Biological Standards and Control in the United Kingdom. Sera were treated with receptor‐destroying enzyme and absorbed with erythrocytes to remove non‐specific hemagglutination. Our HI assay was performed according to established procedures using turkey erythrocytes. 5 , 6
Statistical methods
Data were analyzed using spss version 10.0 (SPSS Inc., Chicago, IL, USA). As for the categorical data, univariate analysis was carried out using the chi‐square test or Fisher’s exact test. Mann–Whitney U‐test was used to compare continuous variables between two groups. To compare continuous variables among three groups, one‐way anlysis of variance (anova) test was performed and multiple comparison test was based on Tukey’s method. To determine discriminative markers between concomitant/secondary bacterial pneumonia and primary influenza pneumonia, receiver‐operator characteristic (ROC) curve analysis was performed. A P‐value of <0.05 was considered to be statistically significant. Using a logistic regression model, multivariate analysis was carried out to compare concomitant/secondary bacterial pneumonia with primary influenza pneumonia.
Results
During the study period, a total of 3400 patients aged ≥15 years were laboratory confirmed to have 2009 A/H1N1 influenza infection. Among them, 150 patients (4·4%) were hospitalized and four of them died (proportion of fatal cases, 0·12%) because of pneumonia (two patients), myocarditis (one patient), or encephalopathy (one patient). The proportion of pneumonia among laboratory‐confirmed 2009 pandemic influenza A/H1N1 was 1·59% (54 among 3400 patients). Among the 54 patients with pneumonia, 39 patients (72·2%) were primary influenza pneumonia with no evidence of concomitant bacterial pneumonia at presentation. Among 39 patients with primary influenza pneumonia, influenza was confirmed by RT‐PCR with nasopharynx/throat specimens in 37 patients and by RT‐PCR with bronchoalveolar lavage (BAL) fluid after a second negative RT‐PCR with nasopharyngeal specimens in two patients. BAL was performed in 16 patients with pneumonia after patients’ consent, and five (31·3%) of them had concomitant/secondary bacterial pneumonia based on standard criteria (quantitative culture). Thirteen patients were compatible with concomitant bacterial pneumonia based on both positive RT‐PCR results and bacterial isolation from respiratory specimens. We experienced two cases of post‐influenza secondary bacterial pneumonia. One patient initially presented with typical influenza symptoms without pneumonia, with the influenza infection confirmed by RT‐PCR. Treatment of this patient with an antiviral agent resulted in symptomatic improvement after several days, but he revisited our hospital with secondary bacterial pneumonia. The second patient presented with bacterial pneumonia but had negative RT‐PCR results for the influenza virus. This patient had a history of ILI in the previous 2 weeks, and a recent 2009 A/H1N1 influenza infection was diagnosed by the HI test with a titer of 1:2560 at the convalescent stage (3 weeks later from ILI onset).
In 15 patients with concomitant/secondary bacterial pneumonia, the causative pathogens were as follows: methicillin‐sensitive Staphylococcus aureus (n = 3, from sputum culture), methicillin‐resistant S. aureus (n = 2, from sputum culture), Streptococcus pneumoniae (n = 6, from sputum culture), Klebsiella pneumoniae (n = 1, from sputum culture), Streptococcus oralis (n = 1, from blood culture), Hemophilus influenza (n = 1, from sputum culture), Moraxella catarrhalis (n = 1, from sputum culture), and serogroup‐5 Legionella pneumophila (n = 1, serological diagnosis). S. pneumoniae and H. influenza were isolated concurrently in one patient.
Although laboratory‐confirmed influenza was found predominantly among young adults aged 15–39 years, 2009 pandemic influenza A/H1N1 pneumonia and hospitalization occurred in patients in all age groups (Figure 1). All four fatalities involved patients aged ≥50 years.
Figure 1.
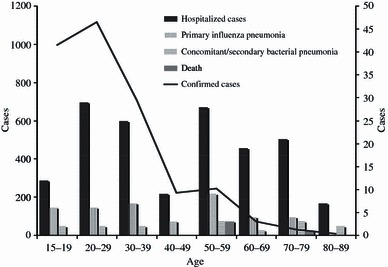
Age‐related differences in the number of laboratory‐confirmed 2009 pandemic influenza A/H1N1 cases, hospitalization, pneumonia, and death; the broken line graph follows the left‐sided gradation, while bar graphs are drawn following the right‐sided gradation.
Clinical characteristics of influenza pneumonia
The demographic and clinical characteristics of patients with 2009 pandemic influenza A/H1N1 pneumonia are presented in Table 1. The majority of influenza patients without pneumonia was female (77·8%) compared with those with pneumonic influenza (P = 0.05).
Table 1.
Demographic and clinical characteristics of patients with influenza pneumonia compared to patients with influenza but without pneumonia
| Influenza without pneumonia (n = 27) | Primary influenza pneumonia (n = 39) | Concomitant/secondary bacterial pneumonia (n = 15) | P‐value | |
|---|---|---|---|---|
| Age, mean ± SD | 36·6 ± 16·3 | 43·1 ± 19·6 | 51·1 ± 23·8 | 0.08 |
| Sex (male), no. (%) | 6 (22·2) | 16 (41·0) | 9 (64·3) | 0.05 |
| Influenza vaccination during 2008–2009 season, no. (%) | 7 (25·9) | 4 (10·3) | 2 (13·3) | 0.22 |
| Body mass index, mean ± SD | 23·2 ± 3·2 | 23·8 ± 3·1 | 22·6 ± 3·7 | 0.48 |
| CURB‐65 score ≥2, no. (%) | – | 7 (17·9) | 8 (53·3) | 0.02 |
| APACHE II score, mean ± SD | – | 12·7 ± 3·7 | 12·4 ± 3·9 | 0.82 |
| Hypoxia (PO2 < 60 mmHg at room air) | – | 7 (17·9) | 5 (33·3) | 0.28 |
| Comorbidities, no. (%) | 7 (25·9) | 11 (28·2) | 8 (53·3) | 0.15 |
| Asthma | 2 (7·4) | 4 (10·3) | 3 (20·0) | 0.45 |
| Chronic obstructive pulmonary diseases | 0 (0) | 0 (0) | 2 (13·3) | 0.01 |
| Interstitial lung diseases | 0 (0) | 0 (0) | 1 (6·7) | 0.11 |
| Cardiovascular diseases | 1 (3·7) | 3 (7·7) | 1 (6·7) | 0.8 |
| Diabetes | 2 (7·4) | 5 (12·8) | 3 (20·0) | 0.49 |
| Chronic renal failure | 0 (0) | 2 (5·1) | 1 (6·7) | 0.44 |
| Liver cirrhosis | 0 (0) | 1 (2·6) | 0 (0) | 0.58 |
| Malignancy | 0 (0) | 2 (5·1) | 2 (13·3) | 0.44 |
| Transplantation | 1 (3·7) | 0 (0) | 0 (0) | 0.36 |
| Neurologic diseases | 1 (3·7) | 0 (0) | 1 (6·7) | 0.32 |
| Immunosuppressant use | 2 (7·4) | 1 (2·6) | 0 (0) | 0.42 |
| Duration of symptoms before hospital visit, mean ± SD | 2·5 ± 1·2a | 3·6 ± 2·2a | 5·0 ± 3·5b | <0.01 |
| Initial symptoms and signs, no. (%) | ||||
| Fever | 25 (92·6) | 35 (89·7) | 12 (80·0) | 0.45 |
| Myalgia | 20 (74·1) | 20 (51·3) | 4 (26·7) | 0.01 |
| Headache | 14 (51·9) | 12 (30·8) | 1 (6·7) | 0.01 |
| Sore throat | 19 (70·4) | 20 (51·3) | 6 (40·0) | 0.13 |
| Cough | 26 (96·3) | 39 (100) | 15 (100) | 0.36 |
| Sputum | 6 (22·2) | 19 (48·7) | 11 (73·3) | 0.01 |
| Hemoptysis | 0 (0) | 1 (2·6) | 1 (6·7) | 0.41 |
| Dyspnea | 10 (37·0) | 21 (53·8) | 10 (66·7) | 0.16 |
| Chest pain | 1 (3·7) | 8 (20·5) | 3 (20·0) | 0.14 |
| Nasal symptoms | 17 (63·0) | 17 (43·6) | 3 (20·0) | 0.03 |
| Nausea/vomiting | 7 (25·9) | 6 (15·4) | 1 (6·7) | 0.26 |
| Diarrhea | 5 (18·5) | 2 (5·1) | 1 (6·7) | 0.18 |
| Gross hematuria | 1 (3·7) | 1 (2·6) | 0 (0) | 0.76 |
| Crackle | 0 (0) | 15 (38·5) | 8 (53·3) | <0.01 |
| Wheezing | 9 (33·3) | 8 (20·5) | 7 (46·7) | 0.15 |
| Death in hospital, no. (%) | 0 (0) | 0 (0) | 2 (13·3) | 0.01 |
| Treatment defervescence interval (days), mean ± SD | 1·1 ± 0·4 | 1·6 ± 1·0 | 1·7 ± 1·2 | 0.07 |
| Duration of hospitalization, mean ± SD | 4·1 ± 1·3a | 8·4 ± 6·7a | 14·5 ± 14·2b | <0.01 |
| ICU admission, no. (%) | – | 10 (25·6) | 7 (46·7) | 0.19 |
| Duration of ICU admission, mean ± SD | – | 2·0 ± 4·0 | 4·3 ± 5·9 | 0.12 |
| Mechanical ventilation, no. (%) | – | 3 (7·7) | 4 (26·7) | 0.09 |
| Shock requiring vasopressors or inotropes, no. (%) | – | 0 (0) | 3 (20·0) | 0.02 |
The same superscript letters indicate a non‐significant difference between groups based on Tukey’s multiple comparison test.
APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit.
Symptomatic duration before the hospital visit and hospital stay was longer in patients with concomitant/secondary bacterial pneumonia than those with non‐pneumonic influenza and primary influenza pneumonia (P < 0.01). Fever (≥37·8°C) was noted in 92·6% of patients with non‐pneumonic influenza, 89·7% of patients with primary influenza pneumonia, and 80·0% of patients with concomitant/secondary bacterial pneumonia. Patients with pneumonic influenza were less likely to have myalgia, headache, and nasal symptoms on presentation than patients with non‐pneumonic influenza. Diarrhea was noted in <10% of patients with pneumonic influenza. Productive cough was more common in patients with concomitant/secondary bacterial pneumonia (73·3%) than patients with non‐pneumonic influenza (22·2%) or primary influenza pneumonia (48·7%) (P = 0.01). Clinically, bronchiolitis was accompanied in 25·9% (seven among 27) of patients with non‐pneumonic influenza. There were no significant differences in age, previous influenza vaccinations, or body mass index (BMI) among the three groups. Underlying co‐morbidities appeared to be more common in patients with concomitant/secondary bacterial pneumonia (53·3%), although this difference was not statistically significant.
When we compared clinical scores between patients with primary influenza pneumonia and concomitant/secondary bacterial pneumonia, we found that patients with concomitant/secondary bacterial pneumonia were more likely to have a CURB‐65 score ≥2 (53·3% versus 17·9%, P = 0.02), and present with shock requiring vasopressor/inotropes (20·0% versus 0%, P = 0.02). On physical examination, crackle was audible in just about half of the patients with pneumonic influenza (38·5% of patients with primary influenza pneumonia and 53·3% of patients with concomitant/secondary bacterial pneumonia). There were no differences in APACHE II score, ICU admission, hypoxia (PO2 < 60 mmHg at room air), or mechanical ventilation between the two pneumonia patient groups.
Laboratory results of influenza pneumonia
In terms of laboratory results, leukocyte count, blood urea nitrogen, erythrocyte sedimentation rate (ESR), and lactate dehydrogenase (LDH) were elevated in 2009 pandemic influenza A/H1N1‐infected patients with pneumonia compared with those without pneumonia, but these parameters could not reliably discriminate between primary influenza pneumonia and concomitant/secondary bacterial pneumonia (Table 2). Lymphopenia (<1000 lymphocytes per cubic millimeter) was found in more than half of the patients irrespective of pneumonia development: 51·9% of patients with non‐pneumonic influenza, 64·1% of patients with primary influenza pneumonia, and 53·3% of patients with concomitant/secondary bacterial pneumonia (P = 0.48). Initial procalcitonin and C‐reactive protein (CRP) values were significantly different among the three groups and could discriminate between primary influenza pneumonia and concomitant/secondary bacterial pneumonia (P < 0.01). A procalcitonin cutoff of >0·35 ng/ml differentiated best between concomitant/secondary bacterial pneumonia and primary influenza pneumonia (sensitivity 81·8% and specificity 66·7%). With respect to CRP values, a cutoff of 86·5 mg/IU best differentiated concomitant/secondary bacterial pneumonia from primary influenza pneumonia (sensitivity 81·8% and specificity 59·3%). The ROC curves for procalcitonin and CRP values are shown in Figure 2; the area under the ROC curve was 0·78 for procalcitonin and 0·75 for CRP. On multivariate analysis, none was independently predictive of concomitant/secondary bacterial pneumonia; in addition to procalcitonin/CRP, age, comorbidity, symptomatic duration, mechanical ventilation, and CURB‐65 score were included in the analysis.
Table 2.
Characteristic laboratory findings of patients with influenza pneumonia compared with those with influenza without pneumonia
| Influenza without pneumonia (n = 27) | Primary influenza pneumonia (n = 39) | Concomitant/secondary bacterial pneumonia (n = 15) | P‐value | |
|---|---|---|---|---|
| WBC (count/μl) | 5·892 ± 2·176a | 7·056 ± 3·586a,b | 9·750 ± 5·442b | 0.01 |
| Lymphopenia (<1000 cells/μl), no (%) | 14 (51·9) | 25 (64·1) | 8 (53·3) | 0.48 |
| Hemoglobin (g/dl) | 13·2 ± 1·1 | 12·7 ± 1·9 | 12·5 ± 1·9 | 0.53 |
| Platelet count, 103 cells/μl | 179 ± 36 | 206 ± 111 | 205 ± 61 | 0.27 |
| AST (IU/l) | 29 ± 21 | 51 ± 47 | 44 ± 32 | 0.14 |
| ALT (IU/l) | 36 ± 43 | 44 ± 44 | 35 ± 325 | 0.63 |
| Bilirubin, total (mg/dl) | 0·53 ± 0·35 | 0·54 ± 0·27 | 0·60 ± 0·42 | 0.79 |
| INR | 1·10 ± 0·09 | 1·11 ± 0·12 | 1·13 ± 0·13 | 0.79 |
| BUN (mg/dl) | 10·7 ± 3·3a | 14·2 ± 10·1a,b | 19·1 ± 13·9b | 0.04 |
| Creatinine (mg/dl) | 0·69 ± 0·15 | 0·91 ± 1·05 | 0·99 ± 0·48 | 0.55 |
| LDH (IU/l) | 365 ± 86a | 567 ± 181b | 502 ± 166b | <0.01 |
| CPK (IU/l) | 102 ± 124 | 247 ± 348 | 159 ± 133 | 0.13 |
| ESR (mm/hour) | 25·1 ± 13·4a | 52·5 ± 29·5b | 69·9 ± 28·2b | <0.01 |
| CRP (mg/l) | 26·5 ± 29·0a | 82·4 ± 65·6b | 183·5 ± 134·4c | <0.01 |
| Procalitonin (IU/l) | 0·07 ± 0·05a | 0·75 ± 1·66b | 5·32 ± 9·23c | <0.01 |
The same superscript letters indicate a non‐significant difference between groups based on Tukey’s multiple comparison test.
WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein.
Figure 2.
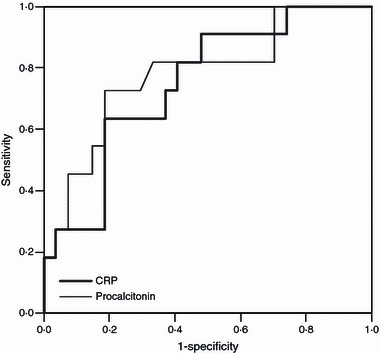
Receiver‐operator characteristic curves of procalcitonin and CRP showing that these can discriminate concomitant/secondary bacterial pneumonia from primary influenza pneumonia.
Radiologic findings of influenza pneumonia
Initial chest X‐ray images were available for all 54 patients with 2009 pandemic influenza A/H1N1 pneumonia. Among them, three patients (5·6%) had normal chest X‐rays, but these patients had abnormal chests (compatible with pneumonia) on CT. On chest X‐rays, the lung abnormalities were bilateral in 69·4% (25 of 36) of primary influenza pneumonia cases and 80% (12 of 15) of concomitant/secondary bacterial pneumonia cases (P = 0.49).
Chest CT images were taken in 43 patients: 30 patients with primary influenza pneumonia and 13 patients with concomitant/secondary bacterial pneumonia (Table 3). The predominant chest CT finding was a mixture of consolidation and ground‐glass opacity detected in 50% of primary influenza pneumonia cases and 76·9% of concomitant/secondary bacterial pneumonia cases. Ground‐glass opacity alone was more common in primary influenza pneumonia cases than concomitant/secondary bacterial pneumonia cases (50% versus 15·4%). Nodular lesions were more common in concomitant/secondary bacterial pneumonia cases than primary influenza pneumonia cases (46·2% versus 20%). Multifocal/diffuse lung involvement was common, predominantly in the peripheral zone (lower lobe). Pleural effusion was only found in patients with concomitant/secondary bacterial pneumonia (four of 13, 30·8%), whereas pneumomediastinum showed the opposite trend (three of 30, 10%); after conservative management, the pneumomediastinum resolved in all three cases. Pulmonary embolism was not found in this study.
Table 3.
Comparison of CT findings between primary influenza pneumonia cases and concomitant/secondary bacterial pneumonia cases
| Primary influenza pneumonia (n = 30) | Concomitant/secondary bacterial pneumonia (n = 13) | P‐value | |
|---|---|---|---|
| Patterns of radiographic abnormality | |||
| Consolidation | 0 (0) | 1 (7·7) | 0.08 |
| GGO | 15 (50·0) | 2 (15·4) | |
| Consolidation + GGO | 15 (50·0) | 10 (76·9) | |
| Nodular lesion | 6 (20·0) | 6 (46·2) | 0.08 |
| Extent of lung involvement | |||
| Unifocal (1–2 lesions) | 8 (26·7) | 2 (15·4) | 0.61 |
| Multifocal (3–5 lesions) | 16 (53·3) | 9 (69·2) | |
| Diffuse (≥6 lesions) | 6 (20·0) | 2 (15·4) | |
| Predominance | |||
| Central zone (upper and middle lobe) | 6 (20·0) | 1 (7·7) | 0.6 |
| Peripheral zone (lower lobe) | 12 (40·0) | 6 (46·2) | |
| Both | 12 (40·0) | 6 (46·2) | |
| Pleural effusion | 0 (0) | 4 (30·8) | 0.01 |
| Lymph node enlargement | 3 (10·0) | 4 (30·8) | 0.09 |
| Pneumomediastinum | 3 (10·0) | 0 (0) | 0.24 |
GGO, ground‐glass opacity.
Treatment and clinical outcome
Fifty‐two of 54 patients with 2009 pandemic influenza A/H1N1 pneumonia were hospitalized, and antiviral agents were given to all with the exception of one serologically diagnosed patient with secondary bacterial pneumonia. For the patients with non‐pneumonic influenza, 25 were treated with oseltamivir (75 mg bid, 5 days), while two patients received zanamivir. For the patients with 2009 pandemic influenza A/H1N1 pneumonia, antiviral agents were given to 53 patients for 7–10 days based on clinician’s preference: high‐dose oseltamivir (150 mg bid, n = 37), high‐dose oseltamivir plus amantadine 100 mg bid (n = 15), or high‐dose oseltamivir plus amantadine plus ribavirin 500 mg bid (n = 1). Fever usually disappeared within 2 days after antiviral treatment irrespective of combined pneumonia (P = 0.07). Antibacterial agents were administered to all the patients with 2009 pandemic influenza A/H1N1 pneumonia: ceftriaxone (n = 28), levofloxacin (n = 25), vancomycin (n = 1), teicoplanin (n = 1), or azithromycin (n = 2). Two fatal cases with 2009 pandemic influenza A/H1N1 pneumonia were observed (3·7%, two of 54 cases).
Discussion
The clinical presentation of influenza ranges from a self‐limiting upper respiratory tract infection to severe pneumonia. The incidence of influenza pneumonia has been reported to range from 0·1% to ≥10% depending on levels of antigenic variation. 7 In this study, the proportion of pneumonia development among patients with 2009 pandemic influenza A/H1N1 was 1·59%, which is higher than the reports of the inter‐pandemic seasonal influenza. 7 Although the 2009 pandemic A/H1N1 virus was initially reported to have a 2‐6 sialic acid (Sia) receptor binding preference similar to human seasonal influenza viruses, recent glycan array data indicate that the virus can bind to both Sia α2‐6 and Sia α2‐3 and can thereby readily infect the alveolar epithelium. 8 , 9 , 10 Using a ferret influenza model, van den Brand et al. 11 showed that the 2009 pandemic A/H1N1 virus replicated well throughout the lower respiratory tract and to a greater extent than the seasonal H1N1 virus, especially at the level of the bronchiole, which may explain why pneumonia and bronchiolitis were more common in patients infected with the 2009 pandemic A/H1N1 virus. Given the more extensive viral replication in the columnar epithelial cells of the lower respiratory tract, some reports recommend BAL in cases of rapidly progressive, highly suspicious lower respiratory tract infections because of 2009 pandemic A/H1N1 virus if the RT‐PCR result is negative with nasopharyngeal specimens. 12 , 13
Despite the relatively frequent development of pneumonia in the 2009 A/H1N1 pandemic, the overall proportion of fatal cases was not high (0·12%). Considering that the mild 2009 pandemic influenza A/H1N1 cases were not laboratory confirmed, the real proportion of fatal cases might be lower than that of this study. Although Mexico and Brazil reported a high case fatality rate (0·4–11·2%) in the early pandemic, 14 , 15 many western countries, including the United States, reported a fatality rate of <0·1%. 16 , 17 , 18 Several possible reasons for this low fatality rate have been proposed. First, according to several recent reports, the 2009 pandemic A/H1N1 virus does not appear to induce pro‐inflammatory cytokine dysregulation unlike the highly pathogenic H5N1 or 1918 H1N1 viruses. 19 , 20 Seasonal influenza viruses are known to cause tissue damage mainly through virus‐induced cell death, whereas H5N1 infection in humans is associated with a massive cytokine storm, resulting in a high number of deaths. 21 , 22 Second, the 2009 pandemic A/H1N1 virus is known to express a truncated PB1‐F2 protein. The PB1‐F2 protein has been implicated as a virulence factor that specifically targets and destroys alveolar macrophages, thereby increasing the likelihood of secondary bacterial infections. 23 , 24 In addition, there are several possible additional reasons for the low case fatality observed in our study. First, obesity is considered to be another risk factor for poor prognosis; according to reports from western countries, about 90% of mechanically ventilated patients had a BMI ≥30, and pulmonary embolism was also present in 36% of patients. 25 However, in our study, the mean BMI was 20–25 and pulmonary embolism was not found. Second, early aggressive antiviral treatment is a policy of the national health department of Korea; antiviral agents were supplied free of charge.
Compared to the patients with 2009 pandemic influenza A/H1N1 pneumonia, those without pneumonia were younger and more likely to complain of constitutional symptoms (fever, myalgia, and headache). Such differences might be related to the reason of admission; 20 among 27 patients without pneumonia (74·1%) were hospitalized just because of severe subjective symptoms. In terms of clinical manifestations, patients with 2009 pandemic influenza A/H1N1 pneumonia were less likely to have extra‐pulmonary symptoms such as myalgia, headache, and diarrhea. In the early 2009 pandemic in Mexico, Perez‐Padilla et al. 26 reported that 22% of influenza patients with pneumonia complained of diarrhea, but <10% of patients with 2009 pandemic influenza A/H1N1 pneumonia presented with diarrhea in the present study. Considering that crackle was audible in only about 50% of patients with 2009 pandemic influenza A/H1N1 pneumonia, high clinical vigilance is required to identify pneumonia; some patients showed rapid progression with dyspnea, tachypnea, and cyanosis over a period of 2–3 days. In terms of laboratory results, a high LDH level and lymphopenia are considered characteristic findings of 2009 pandemic influenza A/H1N1 pneumonia, but we found that lymphopenia was common in patients with influenza at early stages, irrespective of pneumonia development. 26 In addition to high LDH levels, procalcitonin, CRP, and ESR were markedly increased in patients with 2009 pandemic influenza A/H1N1 pneumonia.
Early detection of concomitant bacterial infection can reduce the need of unnecessary antibiotic therapy, but microbiological isolation usually requires 3–5 days. Procalcitonin, CRP, ESR, and leukocyte count have been widely used to distinguish severe bacterial infections from viral infections. 27 , 28 Of note, procalcitonin and CRP could discriminate between concomitant/secondary bacterial pneumonia and primary influenza pneumonia in this study. Recently, Ingram et al. 29 reported that 2009 H1N1‐infected patients with high procalcitonin levels had a poor prognosis. Radiologically, consolidation with pleural effusion was also a discriminative finding suggesting concomitant/secondary bacterial pneumonia. Pneumomediastinum was found only in patients with primary influenza pneumonia and may be related to accompanying bronchiolitis. 30 Currently, it is recommended that suspected influenza patients with lung infiltrates on chest X‐ray receive both antibiotics and antiviral agents; in this study, all patients with 2009 pandemic influenza A/H1N1 pneumonia were treated with antibiotics. 31 Allowing for the current tendency to prescribe antibiotics to all patients with 2009 pandemic influenza A/H1N1 pneumonia, the above‐mentioned clinical, laboratory, and radiographic findings may be very useful in real clinical settings. In accordance with previous reports, S. aureus and S. pneumoniae were the most common concomitant/secondary bacterial pathogens, 7 but atypical pathogens including Legionella spp. and Mycoplasma pneumoniae should also be considered.
Antiviral agents were usually given for 5 days, but for the patients with 2009 pandemic influenza A/H1N1 pneumonia, the antiviral agents were administered for 7–10 days according to the CDC recommendations. 32 In some cases, amantadine was co‐administered because of its possible synergistic effect and its effect on dilatation of the distal bronchioles. 33 , 34
This study has several limitations. First, this study was performed with retrospective design and small sample size. Because we confined this study to microbiologically confirmed pneumonia, there was a chance that mild, silent cases were not included; the traditional definition of ILI is poorly sensitive to detect influenza among hospitaized adults, and nasopharyngeal swab is known to miss about 20% of influenza pneumonia. 12 , 35 Moreover, some potential confounders, such as age and comorbidities, might affect on the differences between primary influenza pneumonia and concomitant/secondary bacterial pneumonia. Although statistically insignificant, patients with concomitant/secondary bacterial pneumonia were rather older and more likely to have comorbidities compared with those with primary influenza pneumonia. Second, the results might be rather variable according to the medical setting; some patients would eventually develop bacterial pneumonia without adequate antiviral/antibacterial treatment.
In conclusion, considering that 2009 pandemic influenza A/H1N1 pneumonia manifests subtly in the early stage, high clinical suspicion is required to detect this type of pneumonia. Both procalcitonin and CRP would be helpful to differentiate primary influenza pneumonia from concomitant/secondary bacterial pneumonia. To better clarify the clinical usefullness of procalcitonin/CRP in the treatment of influenza pneumonia, well‐designed prospective randomized studies are required.
Acknowledgements
This research was supported by a Korea University Grant.
References
- 1. Prevention KCfDCa . Korean Influenza Surveillance Report, 2008–2009. 2009.
- 2. Jia N, de Vlas SJ, Liu YX et al. Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J Clin Virol 2009; 44:225–229. [DOI] [PubMed] [Google Scholar]
- 3. Organization WH . Recommendations and laboratory procedures for detection of avian influenza A(H5N1) virus in specimens from suspected human cases. 2007.
- 4. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133–138. [DOI] [PubMed] [Google Scholar]
- 6. Kendal AP, Pereira MS, Skehel JJ. Concepts and Procedures for Laboratory Based Influenza Surveillance. Geneva: Viral Disease Unit, World Health Organization, 1982. [Google Scholar]
- 7. Nicholson KG. Textbook of Influenza; in: Nicholson KG, Webster RG, Hay AJ. (eds): Human Influenza. London: Blackwell Science Ltd, 1998; 219–264. [Google Scholar]
- 8. Childs RA, Palma AS, Wharton S et al. Receptor‐binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nat Biotechnol 2009; 27:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature 2006; 440:435–436. [DOI] [PubMed] [Google Scholar]
- 10. Shinya K, Kawaoka Y. [Influenza virus receptors in the human airway]. Uirusu 2006; 56:85–89. [DOI] [PubMed] [Google Scholar]
- 11. van den Brand JM, Stittelaar KJ, van Amerongen G et al. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J Infect Dis 2010; 201:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blyth CC, Iredell JR, Dwyer DE. Rapid‐test sensitivity for novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 361:2493. [DOI] [PubMed] [Google Scholar]
- 13. Singh K, Vasoo S, Stevens J, Schreckenberger P, Trenholme G. Pitfalls in the diagnosis of pandemic (Novel) A/H1N1 2009 influenza. J Clin Microbiol 2010; 48:1501–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraser C, Donnelly CA, Cauchemez S et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009; 324:1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira W, Carmo E, Penna G et al. Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza‐like illness with severe acute respiratory infection (SARI). Euro Surveill 2009; 14:1–6. [DOI] [PubMed] [Google Scholar]
- 16. Donaldson LJ, Rutter PD, Ellis BM et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009; 339:b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamigaki T, Oshitani H. Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr 2009; 1:RRN1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Presanis AM, De AngelisD, Hagy A et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med 2009; 6:e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan MC, Chan RW, Yu WC et al. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol 2010; 176:1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo PC, Tung ET, Chan KH, Lau CC, Lau SK, Yuen KY. Cytokine profiles induced by the novel swine‐origin influenza A/H1N1 virus: implications for treatment strategies. J Infect Dis 2010; 201:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beigel JH, Farrar J, Han AM et al. Avian influenza A (H5N1) infection in humans. N Engl J Med 2005; 353:1374–1385. [DOI] [PubMed] [Google Scholar]
- 22. Cheung CY, Poon LL, Lau AS et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 2002; 360:1831–1837. [DOI] [PubMed] [Google Scholar]
- 23. Coleman JR. The PB1‐F2 protein of influenza A virus: increasing pathogenicity by disrupting alveolar macrophages. Virol J 2007; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAuley JL, Zhang K, McCullers JA. The effects of influenza A virus PB1‐F2 protein on polymerase activity are strain specific and do not impact pathogenesis. J Virol 2010; 84:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine‐origin influenza A (H1N1) virus (S‐OIV) infection. AJR Am J Roentgenol 2009; 193:1488–1493. [DOI] [PubMed] [Google Scholar]
- 26. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]
- 27. Diez‐Padrisa N, Bassat Q, Machevo S et al. Procalcitonin and C‐reactive protein for invasive bacterial pneumonia diagnosis among children in Mozambique, a malaria‐endemic area. PLoS ONE 2010; 5:e13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai CC, Tan CK, Chen SY et al. Diagnostic performance of procalcitonin for bacteremia in patients with bacterial infection at the emergency department. J Infect 2010; 61:512–515. [DOI] [PubMed] [Google Scholar]
- 29. Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C‐reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Given K, Schultz A, Douglas TA, Martin AC. Air leaks in children with acute bronchiolitis. J Paediatr Child Health 2008; 44:604–606. [DOI] [PubMed] [Google Scholar]
- 31. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009; 361:1935–1944. [DOI] [PubMed] [Google Scholar]
- 32. Prevention CfDCa . Intensive‐care patients with severe novel influenza A (H1N1) virus infection – Michigan, June 2009. MMWR Morb Mortal Wkly Rep 2009; 58:749–752. [PubMed] [Google Scholar]
- 33. Cunha BA. Swine Influenza (H1N1) pneumonia: clinical considerations. Infect Dis Clin North Am 2010; 24:203–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob Agents Chemother 2009; 53:2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babcock HM, Merz LR, Dubberke ER, Fraser VJ. Case–control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:921–926. [DOI] [PubMed] [Google Scholar]


